Abstract
Aberrant glycosylation is an important factor in facilitating tumor progression and therapeutic resistance. In this study, using Wisteria floribunda agglutinin (WFA), we examined the expression of WFA-binding glycans (WFAG) in cholangiocarcinoma (CCA). The results showed that WFAG was highly detected in precancerous and cancerous lesions of human CCA tissues, although it was rarely detected in normal bile ducts. The positive signal of WFAG in the cancerous lesion accounted for 96.2% (50/52) of the cases. Overexpression of WFAG was significantly associated with lymph node and distant metastasis (P < 0.05). The study using the CCA hamster model showed that WFAG is elevated in preneoplastic and neoplastic bile ducts as early as 1 month after being infected with liver fluke and exposed to N-nitrosodimethylamine. Functional analysis was performed to reveal the role of WFAG in CCA. The CCA cell lines KKU-213A and KKU-213B were treated with WFA, followed by migration assay. Our data suggested that WFAG facilitates the migration of CCA cells via the activation of the Akt and ERK signaling pathways. In conclusion, we have demonstrated the association of WFAG with carcinogenesis and metastasis of CCA, suggesting its potential as a target for the treatment of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma (CCA), an aggressive malignant tumor of bile duct epithelia, is rare worldwide; however, it is highly prevalent in East Asia, especially in the countries along the Mekong River (Sripa and Pairojkul 2008; Shin et al. 2010). One of the main risk factors for CCA in Thailand is infection with a liver fluke—Opisthorchis viverrini (OV) (Sripa and Pairojkul 2008; Shin et al. 2010). Most CCA patients visit the hospital at a late stage and are difficult to receive curative treatment. The curative surgery for CCA is possible only when diagnosis is successfully performed at the early stage of the disease (Titapun et al. 2015).
In CCA, abnormal glycosylation and the expression of CCA-associated glycans have been reported; the glycans play roles in tumor formation, progression, and therapeutic resistance (Silsirivanit 2021; Silsirivanit et al. 2021). These glycans could be a target for the diagnosis and treatment of CCA (Silsirivanit 2021; Silsirivanit et al. 2021). Various plant lectins such as soybean agglutin (SBA), Sophora japonica agglutinin (SJA), Ulex europaeus agglutinin-I (UEA-1), Vicia villosa lectin (VVL), and Wisteria floribunda agglutinin (WFA) have been used to detect CCA-associated glycans (Silsirivanit et al. 2011; Saentaweesuk et al. 2018; Indramanee et al. 2019; Detarya et al. 2020; Matsuda et al. 2010).
Previously, WFA was used to reveal the elevation of CCA-associated glycoform of mucin-1 (MUC1) in patients’ tissue and serum (Matsuda et al. 2010; Matsuda et al. 2017; Matsuda et al. 2015). The WFA-positive MUC1 could be used as a serum glycobiomarker for diagnosis and prognostic prediction of CCA (Matsuda et al. 2010; Silsirivanit 2021; Shoda et al. 2017). Moreover, WFA was found to be a useful tool for the detection of glycobiomarkers in several human diseases (Narimatsu and Sato 2018). WFA binds preferentially to the terminal galactose (Gal) and N-acetylgalactosamine (GalNAc), particularly to LacdiNAc (β-GalNAc-1,4-GlcNAc) (Sato et al. 2017; Poiroux et al. 2017; Haji-Ghassemi et al. 2016; Bojar et al. 2022; Kuno et al. 2013; Narimatsu et al. 2014). LacdiNAcylated glycan is over-expressed in many types of cancers, such as prostate, colon, and pancreatic cancers, and is considered to play an important role in cancer progression (Hirano and Furukawa 2022).
In this study, using WFA, we examined the expression of WFA-binding glycans (WFAG) in human and hamster CCA tissues and explored its association with carcinogenesis and progression of CCA. The in vitro functional analysis was performed using CCA cell lines. In silico analysis was used to predict the glycosyltransferase enzyme responsible for WFAG synthesis in CCA. Our study suggested that WFAG can be a potential target for the treatment of CCA.
Materials and methods
Human and hamster tissues
Paraffin-embedded human CCA tissues (N = 52) were obtained from the specimen bank of the Cholangiocarcinoma Research Institute, Khon Kaen University, Thailand. All cases were histologically proven to be CCA. Informed consent was obtained from each subject. The research protocol was approved by the Ethics Committee for Human Research of Khon Kaen University based on the Declaration of Helsinki and the International Council for Harmonisation (ICH) Good Clinical Practice Guidelines (HE641574). Hamster liver tissues were the same as those used in the previous study (Indramanee et al. 2019). The animal study was performed under the approval of the Ethics Committee for Animal Research of Khon Kaen University (AEMDKKU001/2558), based on the Ethics of Animal Experimentation of the National Research Council of Thailand. The male golden hamsters were divided into four experimental groups: (1) untreated control, (2) OV-infected, (3) N-nitrosodimethylamine (NDMA)-treated, and (4) OV-infected + NDMA-treated, as described previously (Prakobwong et al. 2010). In this study, we used five representative tissue sections per group at 1, 3, and 6 months after treatment.
Cell lines
The human CCA cell lines, KKU-055, KKU-213A, and KKU-213B (Panawan et al. 2023; Sripa et al. 2020), and an immortalized human cholangiocyte cell line, MMNK-1 (Maruyama et al. 2004), were obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank, Osaka, Japan, through the Cholangiocarcinoma Research Institute, Khon Kaen University, Thailand. The cells were maintained in DMEM (Gibco, Life Technologies Corporation, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Life Technologies Limited, Paisley, UK) and 1% antibiotic–antimycotic (Gibco, Life Technologies Corporation, Grand Island, NY, USA). All cell lines were cultured in a 37 °C incubator with 5% CO2.
Lectin histochemistry
All paraffin-embedded tissues were processed as previously described (Detarya et al. 2020; Indramanee et al. 2012). Briefly, after deparaffinization and rehydration, antigen retrieval was performed by boiling under pressure in 0.01 M citrate buffer pH 6.0 and cooled down in phosphate-buffered saline (PBS). Endogenous peroxidase activity was eliminated using 0.3% (v/v) H2O2 in methanol for 30 min. The non-specific binding of lectin to proteins was blocked using 0.5% (w/v) bovine serum albumin (BSA) in PBS for 30 min in a humidified chamber at room temperature. Sections were incubated with 5 μg/ml of biotinylated WFA (Vector Laboratories, Burlingame, CA, USA) for 2 h followed by incubation with 12.5 μg/ml streptavidin-conjugated horseradish peroxidase (Invitrogen, Camarillo, CA, USA) for 40 min. The signal was developed using 25 mg/ml diaminobenzidine (Dako, Glostrup, Denmark) and counterstained with Mayer’s hematoxylin (Bio-Optica, Milano, Italy). PBS was used instead of lectin as a negative control. Each section was scored under a light microscope. The expression level of WFAG, represented by WFAG score (0–300), was calculated from the multiplication of the staining intensity (negative, 0; weak, 1; intermediate, 2; strong, 3) with the percentage of positive cells (0–100%) (Fedchenko and Reifenrath 2014).
Lectin cytofluorescence
The 5 × 103 cells of CCA cell lines were seeded into each well of a 24-well plate and cultured for 72 h. After washing with PBS, the cells were fixed with 4% paraformaldehyde for 30 min, followed by non-specific reactivity blockage with 3% (w/v) BSA in PBS for 30 min at room temperature. WFAG was detected by overnight incubation with 5 μg/ml biotinylated-WFA at 4 °C, followed by 1 h incubation with 4 μg/ml streptavidin conjugated Alexa FluorTM®488 (Thermo Fisher Scientific, Eugene, OR, USA). The nucleus was stained using Hoechst33342 (Thermo Fisher Scientific, Eugene, OR, USA). For negative control, instead of biotinylated WFA, the cells were probed with PBS or biotinylated WFA that was preincubated with 10 mM and 50 mM galactose (Gal) for 30 min. The experiments were repeated at least three times. All fluorescent images were examined using an Eclipse Ti fluorescence microscope (Nikon, Tokyo, Japan). The fluorescence filter cubes consisted of GFP/FITC, TRITC/CY3, and DAPI at 200× magnification (S plan Fluor ELWD 20X Ph1 ADM objective, NA 0.45, refractive index: 1, binning: 3 × 3, gain: 16.0×, sharpness: high, brightness: 0.00, hue: 0.00, saturation: 0.00, WB red: 1.43, and WB blue: 2.66.). The images were captured on a digital color camera, Nikon DS-Ri2 with 1636 × 1088 pixels, 96 dpi, and 24-bit depth (Nikon, Tokyo, Japan). NIS-Elements D 4.30.00 (64bit) software (Nikon, Tokyo, Japan) was used for image analysis.
Cell migration assay
The KKU213-A and KKU213-B CCA cell lines (1×106 cells) were cultured for 72 h and harvested by trypsinization. After washing, the cells were counted and resuspended in a serum-free medium containing 0.1 and 1 μg/ml unconjugated WFA (Vector Laboratories, Burlingame, CA, USA). Cell migration ability was measured using the Boyden chamber assay described previously (Sripa et al. 2020). The cells (20,000 cells) were seeded into the upper chamber of transwell cell culture inserts (8.0-μm pore size, Corning Incorporated, Corning, NY, USA) and allowed to migrate for 12–24 h. Cells treated with a serum-free medium instead of WFA were used as a control. The migrated cells were counted under a microscope and presented as a percentage of control. For sugar neutralization control, the unconjugated WFA was preincubated with 10 mM and 50 mM galactose (Gal) for 30 min before being subjected to treat the cells. Three independent experiments were performed, and the presented quantitative data were averaged from three experiments.
Cell proliferation assay
CCA cells were seeded into 96-well plates at 1 × 103 cells per well and cultured overnight. The conditioned medium was replaced by a medium containing 0.1 and 1 μg/ml WFA, and the culture was continued for 72 h. In the control condition, PBS was used instead of WFA. Cell number was measured using MTT assay (Molecular probes, Eugene, OR, USA) at 0 and 72 h after treatment, according to the manufacturer’s guidelines. Dimethyl sulfoxide (DMSO) was used to solubilize the formazan complexes, and the absorbance was measured at 540 nm. Cell proliferation was calculated as a percentage of control by [optical density (OD) of treatment ×100]/mean OD of control. The presented data was the average from three experiments.
SDS–PAGE and western blot
After overnight seeding, the CCA cell lines were treated with 0.1 and 1 μg/ml unconjugated WFA for 24 h. After complete treatment, the cellular proteins were harvested using cell lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris-HCl pH 7.4) containing phosphatase and protease inhibitors (Roche, Mannheim, Germany). The protein concentration was determined using the Bradford assay (Quick StartTM Bradford, Biorad, USA). Proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore; Darmstadt, Germany) as described previously (Teeravirote et al. 2022). Immunodetection was performed using the desired concentration of specific monoclonal antibodies (mAbs) and polyclonal antibodies (pAbs) as follows: 1:1000 anti-Akt (rabbit pAb, #9272S, Cell Signaling Technology, Danvers, MA, USA), anti-pAkt (S473; rabbit pAb, #9271S, Cell Signaling Technology), anti-ERK (rabbit pAb, #9101S, Cell Signaling Technology), anti-pERK (T202/Y204; rabbit pAb, #9102S, Cell Signaling Technology), anti-STAT3 (mouse mAb, clone B-7, sc-8059, Santa Cruz Biotechnology, Santa Cruz, CA), anti-pSTAT3 (S727) (rabbit pAb, sc-8001, Santa Cruz Biotechnology), and 1:10,000 anti-β-actin (mouse mAb, clone AC-15, A5441, Sigma Aldrich, St. Louis, MO, USA). The signal was detected using the ECLTM Prime Western Blotting Detection Reagent (AmershamTM, Buckinghamshire, UK) and analyzed using an ImageQuant LAS4000 image analyzer with ImageQuantTM TL analysis software (GE Healthcare, Buckinghamshire, UK). Three independent experiments were performed, and the quantitative data presented in the graphs were the average values from three experiments.
In silico gene expression analysis
To explore the potential glycosyltransferase enzymes that are responsible for the synthesis of WFAG, the mRNA expression level of LacdiNAc-related enzymes β-1,4-N-acetylgalactosaminyltransferase-3 (B4GALNT3) and B4GALNT4 in CCA tissues (N=36) were compared with normal tissues (N=9) using data available in the online web server–GEPIA (http://gepia.cancer-pku.cn). By “Expression DIY/Box plot” under “Cancer Type Analysis/ Differential Expression Analysis” section, the mRNA expression of B4GALNT3 and B4GALNT4 was compared using “CHOL” dataset with the default parameter setting (|Log2FC| cutoff = 1, p-value cutoff = 0.01, jitter size = 0.4, matched normal data = match TCGA normal and GTEx data).
Survival analysis was compared between CCA patients with high versus low glycosyltransferase enzymes (B4GALNT3 and B4GALNT4) using “Survival/Survival plot” tab under “Cancer Type Analysis/ Differential Expression Analysis” section. The analysis was performed using the “CHOL” dataset with the default parameter setting [Methods = Overall Survival, Group Cutoff = Median (High 50%, Low 50%), Hazards Ratio (HR)= Yes, 95% Confidence Interval = Yes, Axis Units = Months].
Statistical analysis
All bar and line graphs were generated using GraphPad Prism 9.5.1 software (GraphPad, Inc., La Jolla, CA, USA). Statistical analysis was performed using SPSS Statistics 27.0 software (SPSS, Inc., Chicago, IL, USA). The chi-squared (χ2) test was used to analyze the association between WFAG score and clinical data of CCA patients. WFAG score in each group was presented as mean ± standard deviation (SD) and compared by student’s t-test. Kaplan–Meier plot and log-rank test were used for survival analysis. The P < 0.05 was considered statistically significant.
Results
WFA-binding glycan is elevated in neoplastic bile ducts and associated with metastasis of CCA.
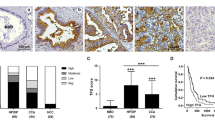
The expression of WFA-binding glycan (WFAG) was determined in 52 histologically proven CCA tissues using lectin histochemistry. WFAG was highly detected in hyperplastic/dysplastic (HP/DP) and cancerous bile ducts, but it was rarely detected in normal bile ducts (NBD) in the adjacent non-cancerous tissues (Fig. 1a–b). WFAG staining was mainly seen as a diffuse cytoplasmic/membranous pattern (Fig. 1a). The WFAG positive rate was 96.2% (50/52) among CCA patients recruited in this study. When the WFAG expression level was semi-quantified using the WFAG score based on the staining frequency and intensity, it was significantly higher in CCA (145.8 ± 67.2) and HP/DP (140.2 ± 55.64) than in NBD (P < 0.05, student’s t-test, WFAG-score = 0.3). This data illustrated the association between WFAG expression level and the abnormalities of biliary epithelia.
Lectin histochemistry staining of WFAG in human CCA tissues. a Lectin histochemistry was used to determine the expression of WFAG in human CCA tissues (N = 52). NBD normal bile ducts, HP/DP hyperplasia/dysplasia, CCA cholangiocarcinoma. Scale bar = 100 μm. b Expression of WFAG was semi-quantified as WFAG score based on the intensity (0–3) × frequency (%) of lectin histochemistry staining. Star (*) represents a significant difference (P < 0.05 by student’s t-test) compared with NBD. A number sign (#) represents a significant difference (P < 0.05 by student’s t-test) compared with HP/DP
To analyze the correlation between WFAG expression level and the clinical features of the CCA patients, the patients were subcategorized into high WFAG (≥ 145.8) and low WFAG (< 145.8) groups using the mean WFAG score of the patients as a cutoff value. Chi-squared (χ2) test analysis revealed that high WFAG expression in cancerous tissues was closely associated with lymph node (N)/distance (M) metastasis in the CCA patients (*P < 0.05, Table 1). The expression of WFAG in patients with lymph node metastasis (N1) or distance metastasis (M1) was higher than those without lymph node metastasis (N0)/without distance metastasis (M0). These results suggested the involvement of WFAG in the metastasis of CCA.
WFAG is aberrantly expressed during the carcinogenesis of CCA
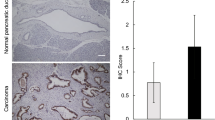
The role of WFAG in the carcinogenesis of CCA was investigated using the paraffin-embedded liver tissues of the hamster CCA model. Similar to the observation in human tissues, the normal bile ducts of hamsters were negative for WFAG staining, while precancerous (HP/DP) and cancerous tissues were positive (Fig. 2a). HP/DP was developed after OV infection and/or NDMA treatment. In OV-infected hamsters, the HP/DP was observed 3 and 6 months after infection, while it was found only 6 months after treatment in NDMA-treated hamsters. In the OV infection + NDMA treatment group, the HP/DP lesion was observed as early as 1 month after the treatment. The cancerous lesion was observed in all hamsters at 3 months (N = 5, WFAG score of 90.0 ± 41.8) and 6 months (N=5, WFAG-score = 110.0 ± 22.4) after OV infection + NDMA treatment. Notably, CCA was not developed in hamsters of the non-treated, OV-infected alone, and NDMA-treated alone groups. WFAG staining of cancerous lesions (WFAG score of 100.0 ± 33.3) was significantly higher than HP/DP lesions [WFAG score of 16.3 ± 18.6 (P < 0.05, student’s t-test, Fig. 2b)]. Comparing between groups, at the corresponding time point (3 and 6 months), WFAG expression in OV infection + NDMA-treated group was significantly higher than other groups (P < 0.05, student’s t-test). This data indicated the close association between WFAG expression and carcinogenesis of CCA.
Lectin histochemistry staining of WFAG in hamster tissues. a Lectin histochemistry was used to determine the expression of WFAG in hamster liver tissues. NBD normal bile ducts, OV Opisthorchis viverrini, NDMA N-nitrosodimethylamine, CCA cholangiocarcinoma. Scale bar, 100 μm. b Expression of WFAG was semi-quantified as WFAG score based on the intensity (0–3) × frequency (%) of lectin histochemistry staining. Star (*) represents a significant difference (P < 0.05 by student’s t-test) compared with the non-treated group. A number sign (#) represents a significant difference (P < 0.05 by student’s t-test) compared with the OV-infected group. Dollar sign ($) represents a significant difference (P < 0.05 by student’s t-test) compared with the NDMA-treated group. Small a represents significant differences (P < 0.05 by student’s t-test) compared with 1 month after OV-infected + NDMA-treatment.
WFAG promotes CCA cell migration via activation of Akt and ERK signaling pathways
To explore the roles of WFAG in CCA, functional analyses were performed using CCA cell lines. The expression of WFAG was determined in three CCA cell lines (KKU-055, KKU-213A, and KKU-213B) compared with MMNK1—an immortalized human cholangiocyte cell line. Lectin fluorescence cytochemistry showed that WFAG was faintly expressed in MMNK-1 and KKU-055 (low metastatic cell line), but it was highly expressed in the high metastatic CCA cell lines KKU-213A and KKU-213B (Fig. 3a). This result suggests the association of WFAG expression with the metastatic potential of CCA cells. To confirm the sugar preferential of WFA, Gal was used to preincubate with WFA before proceeding to the fluorescent staining. The result showed that WFA binding activity was drastically suppressed by 10 mM and 50 mM Gal (Fig. 3b). We, therefore, investigated further the effects of WFA treatment on the biological functions of CCA cell lines. First, the cell agglutination assay was performed to determine the subagglutinating concentration of WFA for CCA cells (up to 10 μg/ml, data not shown). Then, we examined the effects of WFA treatment (0.1 and 1.0 μg/ml WFA) on the migration ability of KKU-213A and KKU-214A cells. The results show that both doses of WFA could significantly enhance the migration ability of both CCA cell lines (P < 0.05, student’s t-test, Fig. 4a–b) without affecting their proliferation ability (Fig. 4c). Sugar neutralization assay showed that Gal (10 mM and 50 mM) significantly suppresses the ability of WFA to facilitate the migration of KKU-213A CCA cells (P < 0.05, student’s t-test, Fig. 4b).
WFAG expression in CCA cell lines. a Lectin cytofluorescence was used to determine the expression of WFAG in immortalized cholangiocytes (MMNK1) and CCA cell lines (KKU-055, KKU-213A, and KKU-213B). b Negative control conditions were performed by probing the cell with PBS or neutralized WFA using 10 mM and 50 mM galactose (Gal). Alexa-488 (green) represents WFAG, and Hoecht-33342 (blue) represents the nucleus. Scale bar, 50 μm
Functional analysis of WFAG in CCA cell lines. a Cell migration was analyzed by Boyden chamber assay after treatment with WFA, cells were allowed to migrate through the transwell cell culture insert for 12–24 h. b Galactose (10 mM and 50 mM) was used to neutralize the function of WFA before treating the cells. c Cell proliferation was measured using MTT assay at 72 h after WFA treatment. d Western blot analysis was used to explore the level of p-Akt (S473), Akt, p-ERK (T202/Y204), ERK, p-STAT, and STAT at 24 h after WFA treatment. β-actin was used as an internal control to normalize the band intensity measured by ImageJ software. Three independent experiments were performed, and the data presented in the graphs were averaged from all experiments. Star (*) represents a significant difference (P < 0.05 by student’s t-test) compared with control. A number sign (#) represents a significant difference (P < 0.05 by student’s t-test) compared with WFA treated condition
To investigate further the possible mechanism(s) by which WFA facilitates CCA cell migration, the KKU-213A and KKU-214A cells were treated with 0.1 and 1.0 μg/ml WFA for 24 h, and the degree of phosphorylation of the signal molecules involved in cell migration was examined. The results show that the proportion of phosphorylated Akt (S473) and ERK (T202, Y204) were significantly increased (P < 0.05, student’s t-test, Fig. 4d), while STAT phosphorylation was not affected by the WFA treatment. These data suggest that WFAG may facilitate the migration of cancer cells via the activation of Akt and ERK signaling pathways.
In silico analysis suggested the elevation of WFAG-associated glycosyltransferase enzyme in CCA and its association with poor prognosis of CCA patients
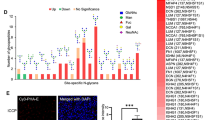
The specify the potential glycosyltransferases that are responsible for the synthesis of WFAG, the mRNA level of B4GALNT3 and B4GALNT4 were compared between CCA tissues (N = 36) and normal tissues (N = 9) using data available in the online web server, GEPIA (http://gepia.cancer-pku.cn). The data showed that B4GALNT3 and B4GALNT4 mRNA expression levels were significantly higher in CCA than in control (P < 0.001, student’s t-test, Fig. 5a). In addition, a high expression level of B4GALNT3 mRNA in CCA tissues tended to associate with the shorter survival of CCA patients with the higher hazard ratio of 2.1, whereas the high expression level of B4GALNT4 mRNA tended to associate with better prognosis of CCA (Fig. 5b). Taking this information together, B4GALNT3 is assumed to be the enzyme responsible for WFAG synthesis and contributes to the metastasis of CCA cells.
Expression of B4GALNT3 and B4GALNT4 in CCA tissues. a The mRNA expression of LacdiNAc-associated glycosyltransferase enzymes, B4GALNT3 and B4GALNT4, was determined in human CCA tissues (N = 9) compared with normal tissues (N = 36) using the data available in GEPIA. Three stars (***) represent a significant difference with P < 0.001 using the student’s t-test. b Survival analysis of B4GALNT3 and B4GALNT4 in CCA tissues
Discussion
Glycosylation is an essential posttranslational modification involved in many biological processes (Ohtsubo and Marth 2006; Sperandio et al. 2009). Aberrant glycosylation resulted in the expression of tumor-associated glycans in cancer cells that could be a biomarker or serve as a target for cancer chemotherapy (Kailemia et al. 2017; Munkley and Elliott 2016). In this study, we used WFA, a lectin isolated from seeds of a flowering plant, Wisteria floribunda, to detect the LacdiNAcylated glycans in CCA. The increase of WFA-binding glycans (WFAG) was associated with the carcinogenesis of CCA, and it was found to promote CCA metastasis via Akt and ERK signaling pathways. The in silico analysis in this study suggested the possible role of B4GALNT3 in the WFAG synthesis in CCA.
WFAG was practically undetectable in the normal bile ducts but elevated in the hyperplastic/dysplastic bile ducts and CCA. The hyperplastic and dysplastic bile ducts found in the liver of hamsters exposed to NDMA without liver fluke infection also showed positive staining for WFAG (Fig. 2). These results suggest the association between WFAG expression and the pathological changes of the bile duct, regardless of liver fluke infection. A previous study by Matsuda et al. showed that WFAG was detected in the preneoplastic and neoplastic bile ducts of Japanese CCA patients, who were assumed not to have liver fluke infection (Matsuda et al. 2010). Moreover, the study in Chinese CCA patients using mass spectrometry analysis also showed that LacdiNAc is elevated in CCA tissues compared with normal adjacent tissues (Li et al. 2022). This information suggested the association between WFAG expression and CCA development notwithstanding the etiology and patients’ ethnicity.
Previously, the roles of glycosylation in facilitating tumor progression and metastasis were elucidated for CCA (Detarya et al. 2020; Detarya et al. 2022; Indramanee et al. 2019; Silsirivanit 2021). The present results suggested the association between WFAG expression and metastasis of CCA. It is suggested that activation of WFAG by WFA may facilitate CCA cell migration via activation of Akt and ERK signaling pathways. This finding suggests the potential of WFAG to be a biomarker for poor prognosis and possibly be a target for the treatment of CCA. WFAG is known to modify mucin-1 (MUC1) glycoprotein, which could be detected in the sera of CCA patients (Matsuda et al. 2010). A higher degree of serum WFAG-modified MUC1 level was associated with shorter survival of CCA patients (Silsirivanit 2021). Not only MUC1, but also other N-linked and O-linked glycoproteins are possibly modified by WFAG. Li et al. showed that many glycoproteins were identified to express LacdiNAc moiety (Li et al. 2022). It has been demonstrated that EGFR and β1-integrin, the upstream receptors for Akt and ERK signaling pathways, could be modified and functionally regulated by LacdiNAc modification (Hirano and Furukawa 2022). LacdiNAc plays an important role in tumor progression and metastasis in many types of cancer (Hirano and Furukawa 2022; Li et al. 2022). Taken together, WFAG possibly modifies many cell surface glycoproteins in CCA, at least EGFR or β1-integrin are speculated, and facilitates cancer cell migration via activation of Akt and ERK signaling pathways (Hirano and Furukawa 2022; Detarya et al. 2022; Wang et al. 2021). To understand the mechanisms by which WFAG regulates CCA metastasis via Akt and ERK signaling pathways, the real carrier glycoproteins for WFAG need to be identified.
Aberrant glycosylation in cancer cells could be triggered by alteration of nucleotide sugar biosynthesis and expression of glycosylation transferases or glycosidases (Silsirivanit 2019). Synthesis of LacdiNAc in human cells was regulated by the β-1,4-N-acetylgalactosaminyltransferases (B4GALNTs): B4GALNT3 and B4GALNT4 (Hirano and Furukawa 2022). Our in-silico analysis suggested B4GALNT3 as a responsible enzyme for WFAG synthesis in CCA cells. By the survival analysis, although it was not statistically significant, high expression of B4GALNT3 tended to be associated with shorter survival of CCA patients. This in silico information from GEPIA agrees with the results obtained from lectin histochemistry staining of human CCA tissues and functional analysis using CCA cell lines. There is a controversy about the role of B4GALNT3 in different types of cancer. In colon cancer, B4GALNT3 plays an important role in malignant phenotype and cancer stemness via the EGFR signaling pathway (Che et al. 2014; Huang et al. 2007). Unlike colon cancer, B4GALNT3 was found to be associated with favorable outcomes of neuroblastoma (Hsu et al. 2011). The study by Hsu et al. showed that overexpression of B4GALNT3 in neuroblastoma cells significantly suppresses their malignant phenotypes via decreasing β1-integrin signaling (Hsu et al. 2011). Therefore, actual functional analysis of B4GALNT3 is essential to confirm its role in the biosynthesis of WFAG and its possibility to be a target for the treatment of CCA.
In conclusion, we have demonstrated the association between the expression of WFA-binding glycans (WFAG) and carcinogenesis and metastasis of CCA. WFAG was involved in CCA cell migration through Akt and ERK signaling pathways. Our study suggests WFAG as a potential therapeutic target of CCA.
Reference
Bojar D, Meche L, Meng G, Eng W, Smith DF, Cummings RD, Mahal LK (2022) A useful guide to lectin binding: machine-learning directed annotation of 57 unique lectin specificities. ACS Chem Biol 17(11):2993–3012. https://doi.org/10.1021/acschembio.1c00689
Che MI, Huang J, Hung JS, Lin YC, Huang MJ, Lai HS, Hsu WM, Liang JT, Huang MC (2014) beta1, 4-N-acetylgalactosaminyltransferase III modulates cancer stemness through EGFR signaling pathway in colon cancer cells. Oncotarget 5(11):3673–3684. https://doi.org/10.18632/oncotarget.1981
Detarya M, Sawanyawisuth K, Aphivatanasiri C, Chuangchaiya S, Saranaruk P, Sukprasert L, Silsirivanit A, Araki N, Wongkham S, Wongkham C (2020) The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology 30(5):312–324. https://doi.org/10.1093/glycob/cwz098
Detarya M, Lert-Itthiporn W, Mahalapbutr P, Klaewkla M, Sorin S, Sawanyawisuth K, Silsirivanit A, Seubwai W, Wongkham C, Araki N, Wongkham S (2022) Emerging roles of GALNT5 on promoting EGFR activation in cholangiocarcinoma: a mechanistic insight. Am J Cancer Res 12(9):4140–4159
Fedchenko N, Reifenrath J (2014) Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn Pathol 9:221. https://doi.org/10.1186/s13000-014-0221-9
Haji-Ghassemi O, Gilbert M, Spence J, Schur MJ, Parker MJ, Jenkins ML, Burke JE, van Faassen H, Young NM, Evans SV (2016) Molecular basis for recognition of the cancer glycobiomarker, LacdiNAc (GalNAc[beta1–>4]GlcNAc), by Wisteria floribunda Agglutinin. J Biol Chem 291(46):24085–24095. https://doi.org/10.1074/jbc.M116.750463
Hirano K, Furukawa K (2022) Biosynthesis and biological significances of LacdiNAc group on N- and O-Glycans in human cancer cells. Biomolecules. https://doi.org/10.3390/biom12020195
Hsu WM, Che MI, Liao YF, Chang HH, Chen CH, Huang YM, Jeng YM, Huang J, Quon MJ, Lee H, Huang HC, Huang MC (2011) B4GALNT3 expression predicts a favorable prognosis and suppresses cell migration and invasion via beta(1) integrin signaling in neuroblastoma. Am J Pathol 179(3):1394–1404. https://doi.org/10.1016/j.ajpath.2011.05.025
Huang J, Liang JT, Huang HC, Shen TL, Chen HY, Lin NY, Che MI, Lin WC, Huang MC (2007) Beta 1,4-N-acetylgalactosaminyltransferase III enhances malignant phenotypes of colon cancer cells. Mol Cancer Res 5(6):543–552. https://doi.org/10.1158/1541-7786.MCR-06-0431
Indramanee S, Silsirivanit A, Pairojkul C, Wongkham C, Wongkham S (2012) Aberrant glycosylation in cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac J Cancer Prev 13(Suppl):119–124
Indramanee S, Sawanyawisuth K, Silsirivanit A, Dana P, Phoomak C, Kariya R, Klinhom-On N, Sorin S, Wongkham C, Okada S, Wongkham S (2019) Terminal fucose mediates progression of human cholangiocarcinoma through EGF/EGFR activation and the Akt/Erk signaling pathway. Sci Rep 9(1):17266. https://doi.org/10.1038/s41598-019-53601-8
Kailemia MJ, Park D, Lebrilla CB (2017) Glycans and glycoproteins as specific biomarkers for cancer. Anal Bioanal Chem 409(2):395–410. https://doi.org/10.1007/s00216-016-9880-6
Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, Narimatsu H (2013) A serum “sweet-doughnut” protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep 3(1):1065. https://doi.org/10.1038/srep01065
Li J, Zhao T, Li J, Shen J, Jia L, Zhu B, Dang L, Ma C, Liu D, Mu F, Hu L, Sun S (2022) Precision N-glycoproteomics reveals elevated LacdiNAc as a novel signature of intrahepatic cholangiocarcinoma. Mol Oncol 16(11):2135–2152. https://doi.org/10.1002/1878-0261.13147
Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB, Leboulch P, Tanaka N (2004) Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation 77(3):446–451. https://doi.org/10.1097/01.Tp.0000110292.73873.25
Matsuda A, Kuno A, Kawamoto T, Matsuzaki H, Irimura T, Ikehara Y, Zen Y, Nakanuma Y, Yamamoto M, Ohkohchi N, Shoda J, Hirabayashi J, Narimatsu H (2010) Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology 52(1):174–182. https://doi.org/10.1002/hep.23654
Matsuda A, Kuno A, Nakagawa T, Ikehara Y, Irimura T, Yamamoto M, Nakanuma Y, Miyoshi E, Nakamori S, Nakanishi H, Viwatthanasittiphong C, Srivatanakul P, Miwa M, Shoda J, Narimatsu H (2015) Lectin microarray-based sero-Biomarker verification targeting aberrant O-linked glycosylation on mucin 1. Anal Chem 87(14):7274–7281. https://doi.org/10.1021/acs.analchem.5b01329
Matsuda A, Higashi M, Nakagawa T, Yokoyama S, Kuno A, Yonezawa S, Narimatsu H (2017) Assessment of tumor characteristics based on glycoform analysis of membrane-tethered MUC1. Lab Invest 97(9):1103–1113. https://doi.org/10.1038/labinvest.2017.53
Munkley J, Elliott DJ (2016) Hallmarks of glycosylation in cancer. Oncotarget 7(23):35478–35489. https://doi.org/10.18632/oncotarget.8155
Narimatsu H, Sato T (2018) Wisteria floribunda agglutinin positive glycobiomarkers: a unique lectin as a serum biomarker probe in various diseases. Expert Rev Proteomics 15(2):183–190. https://doi.org/10.1080/14789450.2018.1419066
Narimatsu Y, Kuno A, Ito H, Kaji H, Kaneko S, Usui J, Yamagata K, Narimatsu H (2014) IgA nephropathy caused by unusual polymerization of IgA1 with aberrant N-Glycosylation in a patient with monoclonal immunoglobulin deposition disease. PloS One 9(3):e91079. https://doi.org/10.1371/journal.pone.0091079
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126(5):855–867. https://doi.org/10.1016/j.cell.2006.08.019
Panawan O, Silsirivanit A, Chang CH, Putthisen S, Boonnate P, Yokota T, Nishisyama-Ikeda Y, Detarya M, Sawanyawisuth K, Kaewkong W, Muisuk K, Luang S, Vaeteewoottacharn K, Kariya R, Yano H, Komohara Y, Ohta K, Okada S, Wongkham S, Araki N (2023) Establishment and characterization of a novel cancer stem-like cell of cholangiocarcinoma. Cancer Sci. https://doi.org/10.1111/cas.15812
Poiroux G, Barre A, van Damme EJM, Benoist H, Rouge P (2017) Plant lectins targeting o-glycans at the cell surface as tools for cancer diagnosis, prognosis and therapy. Int J Mol Sci. https://doi.org/10.3390/ijms18061232
Prakobwong S, Yongvanit P, Hiraku Y, Pairojkul C, Sithithaworn P, Pinlaor P, Pinlaor S (2010) Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int J Cancer 127(11):2576–2587. https://doi.org/10.1002/ijc.25266
Saentaweesuk W, Silsirivanit A, Vaeteewoottacharn K, Sawanyawisuth K, Pairojkul C, Cha’on U, Indramanee S, Pinlaor S, Boonmars T, Araki N, Wongkham C (2018) Clinical significance of GalNAcylated glycans in cholangiocarcinoma: Values for diagnosis and prognosis. Clin Chim Acta 477:66–71. https://doi.org/10.1016/j.cca.2017.12.005
Sato T, Tateno H, Kaji H, Chiba Y, Kubota T, Hirabayashi J, Narimatsu H (2017) Engineering of recombinant Wisteria floribunda agglutinin specifically binding to GalNAcbeta1,4GlcNAc (LacdiNAc). Glycobiology 27(8):743–754. https://doi.org/10.1093/glycob/cwx038
Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST (2010) Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci 101(3):579–585. https://doi.org/10.1111/j.1349-7006.2009.01458.x
Shoda J, Matsuda A, Shida T, Yamamoto M, Nagino M, Tsuyuguchi T, Yasaka T, Tazuma S, Uchiyama K, Unno M, Ohkohchi N, Nakanuma Y, Kuno A, Narimatsu H (2017) Wisteria floribunda agglutinin-sialylated mucin core polypeptide 1 is a sensitive biomarker for biliary tract carcinoma and intrahepatic cholangiocarcinoma: a multicenter study. J Gastroenterol 52(2):218–228. https://doi.org/10.1007/s00535-016-1230-0
Silsirivanit A (2019) Glycosylation markers in cancer. Adv Clin Chem 89:189–213. https://doi.org/10.1016/bs.acc.2018.12.005
Silsirivanit A (2021) Glycans: potential therapeutic targets for cholangiocarcinoma and their therapeutic and diagnostic implications. Expert Opin Ther Targets 25(1):1–4. https://doi.org/10.1080/14728222.2021.1861250
Silsirivanit A, Araki N, Wongkham C, Pairojkul C, Narimatsu Y, Kuwahara K, Narimatsu H, Wongkham S, Sakaguchi N (2011) A novel serum carbohydrate marker on mucin 5AC: values for diagnostic and prognostic indicators for cholangiocarcinoma. Cancer 117(15):3393–3403. https://doi.org/10.1002/cncr.25912
Silsirivanit A, Phoomak C, Wongkham S (2021) Glycosylation in cholangiocarcinoma development and metastasis: diagnostic and therapeutic considerations. In: Tabibian, J.H. (eds) Diagnosis and management of cholangiocarcinoma: a multidisciplinary approach. Springer, Cham, pp 527–553. https://doi.org/10.1007/978-3-030-70936-5_25
Sperandio M, Gleissner CA, Ley K (2009) Glycosylation in immune cell trafficking. Immunol Rev 230(1):97–113. https://doi.org/10.1111/j.1600-065X.2009.00795.x
Sripa B, Pairojkul C (2008) Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 24(3):349–356. https://doi.org/10.1097/MOG.0b013e3282fbf9b3
Sripa B, Seubwai W, Vaeteewoottacharn K, Sawanyawisuth K, Silsirivanit A, Kaewkong W, Muisuk K, Dana P, Phoomak C, Lert-Itthiporn W, Luvira V, Pairojkul C, Teh BT, Wongkham S, Okada S, Chamgramol Y (2020) Functional and genetic characterization of three cell lines derived from a single tumor of an Opisthorchis viverrini-associated cholangiocarcinoma patient. Hum Cell 33(3):695–708. https://doi.org/10.1007/s13577-020-00334-w
Teeravirote K, Sutthanut K, Thonsri U, Mahalapbutr P, Seubwai W, Luang S, Tippayawat P, Kanthawong S, Pipattanaboon C, Duangjinda M, Chankitisakul V, Silsirivanit A (2022) Anserine/Carnosine-rich extract from Thai Native chicken suppresses melanogenesis via activation of ERK signaling pathway. Molecules. https://doi.org/10.3390/molecules27217440
Titapun A, Pugkhem A, Luvira V, Srisuk T, Somintara O, Saeseow OT, Sripanuskul A, Nimboriboonporn A, Thinkhamrop B, Khuntikeo N (2015) Outcome of curative resection for perihilar cholangiocarcinoma in Northeast Thailand. World J Gastrointest Oncol 7(12):503–512. https://doi.org/10.4251/wjgo.v7.i12.503
Wang Y, Li K, Zhao W, Liu Z, Liu J, Shi A, Chen T, Mu W, Xu Y, Pan C, Zhang Z (2021) Aldehyde dehydrogenase 3B2 promotes the proliferation and invasion of cholangiocarcinoma by increasing Integrin Beta 1 expression. Cell Death Dis 12(12):1158. https://doi.org/10.1038/s41419-021-04451-8
Acknowledgments
This project was funded by Kasetsart University through the Graduate School Fellowship Program and Faculty of Public Health, Kasetsart University, Thailand, to W.P. and S.C. and by the Fundamental Fund of Khon Kaen University (FF65) to A.S. We thank Prof. Yukifumi Nawa for English editing via the KKU publication clinic.
Funding
Fundamental Fund of Khon Kaen University (FF65). Kasetsart University through the Graduate School Fellowship Program
Author information
Authors and Affiliations
Contributions
W.P., S.P., O.P., P.M., K.T., and M.D. performed experiments and analyzed data; W.P., S.P, and P.M. prepared Figs. 1–2; W.P., O.P., P.S., and S.U. prepared Figs. 3–4; T.S., A.K., P.M., and S.L. supervised experiment handling and data analysis; S.C. and A.S. designed the study and funding acquisition; and W.P., S.P., S.C., and A.S. wrote and edited manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Phuyathip, W., Putthisen, S., Panawan, O. et al. Role of Wisteria floribunda agglutinin binding glycans in carcinogenesis and metastasis of cholangiocarcinoma. Histochem Cell Biol 161, 423–434 (2024). https://doi.org/10.1007/s00418-024-02270-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-024-02270-4