Abstract
This study aimed to analyze immunohistochemical staining and pathological data in cervical intraepithelial neoplasia (CIN) and squamous cell cervical carcinoma (SCC) with abnormal colposcopic findings. A histopathological evaluation of 45 low-grade squamous lesions (LSILs), 177 high-grade squamous lesions (HSILs) and 16 SCC biopsy materials from existing slides was obtained from blocks obtained from the archive. In addition, SOX-2 immunohistochemical staining was evaluated. The mean age of the HSIL group was 43.20 ± 8.97 years, younger than the mean age of the LSIL group of 51.62 ± 9.64 years (p = 0.000). There was no difference between the groups regarding the method of biopsy (p > 0.05). Endocervical gland involvement was not observed in the LSIL group, but was observed in 66 (37.3%) biopsy materials in the HSIL group (p = 0.000). There was a difference between the groups in terms of the level of CIN at the surgical margin (p = 0.000). Ki-67, SOX-2 staining percentage and p16INK4a positivity were higher in the HSIL group than in the LSIL group (respectively, 67.57 ± 19.10 vs. 14.62 ± 7.11, p = 0.000; 27.72 ± 31.56 vs. 10.09 ± 15.38, p = 0.003; 66 (82.5%) vs. 8 (44.4%), p = 0.001). While there was no difference in SOX-2 intensity between the HSIL and LSIL groups (p > 0.05), it was statistically significantly higher in the SCC group (p = 0.000), as was the percentage of SOX-2 (p = 0.000). We have shown that p16INK4a and SOX-2 staining is useful, in addition to Ki-67 immunostaining, which is widely used for SCC, which is one of the preventable cancer types. In addition, SOX-2 may provide a glimmer of hope in the development of SCC treatment modalities, especially since it is aggressively elevated in SCC rather than CIN lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is usually caused by squamous cell carcinoma, and it usually develops within decades. Although its incidence is decreasing with screening programs, the mortality rate in women is still high (Bray et al. 2018). Although women may encounter human papillomavirus (HPV) throughout their lives, it is usually eliminated by the immune system. Cervical intraepithelial neoplasia (CIN) caused by HPV may progress to squamous cell carcinoma (Kilic et al. 2020). CIN is classified as a low-grade squamous intraepithelial lesion (LSIL; CIN I) or high-grade squamous intraepithelial lesion (HSIL; CIN II and CIN III) according to its potential to transform into cancer (Atigan et al. 2022).
Turkey was one of the first countries in Europe to successfully implement a HPV screening program throughout the country (Maver and Poljak 2020; Atıgan and Eraydın 2020). Co-testing, which includes Pap smear tests and HPV-DNA analysis, is recommended by international associations for cervical cancer screening. It is successfully implemented in Turkey with public participation (Kilic et al. 2020; Atigan et al. 2022; Atıgan and Eraydın 2020; Saslow et al. 2012). The golden rule for the diagnosis of CIN following the results of the co-test is to perform a cervical biopsy with colposcopy (Kingnate et al. 2015). A cervical biopsy can be excised with cold-knife conization or the loop electrosurgical excision procedure (LEEP).
SOX [the sex-determining region on the Y chromosome (SRY)-related high mobility group (HMG)-box] was first identified as a testis-determining gene. However, today 20 different varieties have been detected (Pouremamali et al. 2022). The sex-determining region Y-box 2 (SOX-2) gene is currently being studied because it may be connected to numerous cancers (Zhang et al. 2020). The development of various tissues and maintenance of homeostasis between them are both governed by SOX-2, which also functions in the determination of sexual orientation and growth, proliferation and division of pluripotent cells (Pouremamali et al. 2022).
In our study, we aimed to evaluate the immunohistochemical staining of SOX-2 in squamous intraepithelial lesions (SILs). In addition, endocervical gland involvement and endocervical and ectocervical surgical margin continuity were evaluated. Basic histopathological features were also analyzed.
Materials and methods
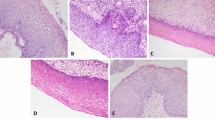
The study included patients with cervical intraepithelial lesions identified from biopsies sent to the pathology department from the colposcopy unit of a tertiary hospital between January 2017 and January 2020. Distributions of cervical lesions were CIN I (n = 45), CIN II (n = 57), CIN III (n = 120) and squamous cell carcinoma (SCC) (n = 16) (Fig. 1a–b). A total of 238 biopsy materials were reevaluated. Evaluation as high and low grade showed LSIL (n = 45) (Fig. 2a) and HSIL (n = 177) (Fig. 2b).
a Low-grade squamous lesions (LSIL). Atypia is observed in the lower 1/3 of the epithelium, and koilocytosis and binucleation findings are observed on the surface, H&E (× 400). b High-grade squamous lesions (HSIL). Full-thickness atypia in the epithelium does not exceed the basement membrane; there is no endocervical gland involvement, H&E (× 200). Scale bars = 25 µm in a and 50 µm in b
The Non-Interventional Clinical Research Ethics Committee of Pamukkale University Medicine Faculty gave ethical approval for the study (decision number: 60116787-020/83871).
Colposcopic examination and abnormal findings were evaluated as described in our recent article (Atigan et al. 2022). The pathology record provided information about how the biopsy sample was taken and the age of the patient. Biopsies were obtained by two methods, conization or LEEP. Afterwards, the ready-made slides were examined. Ki-67 (Ventana, prepkit, 30–9 clone) and hematoxylin-eosin (H&E) staining were available for each biopsy in these ready slides. Some biopsies also had p16INK4a (Ventana, prepkit, E6H4 clone) staining. The degree of the cervical intraepithelial lesion as well as information on endocervical and ectocervical margins was determined by reexamining these preparations. Sections of the lesion’s 3–5-µm-thick paraffin block were made for SOX-2 immunohistochemical staining. Lysine slides were stained with SOX-2 [(SP76) Rabbit Monoclonal Antibody Cell Marque, 1:100 dilution] on the Ventana Benchmark ULTRA™ fully automated stainer using the ultraView Universal DAB detection kit. In the immunohistochemical staining negative control, the primary antibody step was omitted. Cervical lesions were evaluated with a microscope (Nikon Eclipse e200) by at least two researchers, including one pathologist. Tonsil tissues were used in accordance with protocol as the positive control for SOX-2 staining. SOX-2 shows nuclear staining (Sato et al 2019). Hematoxylin-eosin (H&E) staining, some Ki-67 and p16 staining were available for these ready slides. Some biopsies also had staining. The degree of the cervical intraepithelial lesion, as well as information about endocervical and ectocervical margins, was determined by reexamining these preparations.
Whether there was endocervical gland involvement and, if so, its depth, was considered. In addition, both endocervical and ectocervical surgical margins were evaluated (Fig. 3a–b). Surgical margins were divided into three categories as positive, < 1 mm and negative. The lesion’s persistence in surgical margins that were non-negative was investigated. Distance was indicated in patients with both negative endocervical and ectocervical surgical margins.
Statistical analysis
IBM SPSS Statistics (version 21.0, IBM, Chicago, USA) program was used for statistical analysis. Standard descriptive statistical methods were used to analyze the descriptive values of quantitative continuous variables. Normal distribution of the data was examined by using the Kolmogorov-Smirnov test. Pearson χ2 and Mann-Whitney U tests were used for comparisons between groups. p < 0.05 was accepted as significant in all statistical comparisons.
Results
Comparisons of LSIL (n = 45) and HSIL (n = 177) are presented in Table 1. Mean age was statistically significantly greater in the LSIL group than in the HSIL group (51.62 ± 9.64 vs. 43.20 ± 8.97, p = 0.000). While the LEEP method was used more than conization as the biopsy method, there was no statistically significant difference according to the degree of cervical lesion (p = 0.369). There was no difference between the groups in terms of lesion area (4.51 ± 2.72 vs. 3.85 ± 2.26, p = 0.173). Endocervical gland involvement was not observed in the LSIL group. However, there was endocervical gland involvement at a mean depth of 1.27 ± 0.69 mm in 66 (37.3%) (p = 0.000) biopsies in the HSIL group. Endocervical surgical margins were grouped as positive, closer than 1 mm and negative. The number of biopsies with negative endocervical surgical margins was 14 (31.1%) in the LSIL group and 69 (39.0%) in the HSIL group (p = 0.206). The mean distance of those with negative endocervical surgical margins in the LSIL and HSIL groups was 3.71 ± 1.32 vs. 3.57 ± 1.76, respectively (p = 0.526). Endocervical surgical margin positivity was persistent in 30 (66.7%) biopsies in the LSIL group as CIN I. In addition, CIN I 17 (9.6%), CIN II 25 (14.1%) and CIN III 66 (37.3%) were persistent in the HSIL group (p = 0.000). The number of biopsies with negative ectocervical surgical margins was 14 (31.1%) in the LSIL group and 83 (46.9%) in the HSIL group (p = 0.163). The mean distance of those with negative ectocervical surgical margins in the LSIL and HSIL groups was 4.72 ± 1.75 vs. 4.85 ± 2.78, respectively (p = 0.559). Ectocervical surgical margin positivity was persistent in 31 (68.9%) biopsies in the LSIL group as CIN I. In addition, CIN I 34 (19.2%), CIN II 30 (16.9%) and CIN III 30 (16.9%) were persistent in the HSIL group (p = 0.000). The percentage of Ki-67 immunostaining was statistically significantly lower in the LSIL group than in the HSIL group (14.62 ± 7.11 vs. 67.57 ± 19.10, p = 0.000) (Fig. 4a–b). p16INK4a staining was not applied to all biopsies. While 8 (44.4%) biopsies were positive in the LSIL group, 66 (82.5%) biopsies in the HSIL group showed positive staining (p = 0.001). The percentage of SOX-2 immunostaining was 10.09 ± 15.38 in the LSIL group and 27.72 ± 31.56 in the HSIL group (p = 0.003) (Fig. 5a–b). There was no difference between the groups in terms of SOX-2 intensity (0.96 ± 1.18 vs. 1.25 ± 1.21, p = 0.126). The percentage and severity of SOX-2 immunostaining were statistically significantly higher in the SCC group (p = 0.000 for both) (Figs. 5C, 6a–b).
Discussion
Screening recommended by the World Health Organization (WHO) for three types of cancer, namely colorectal, breast and cervical cancer, is also carried out in Turkey. To reduce mortality and morbidity, cancer early diagnosis screening and education centers have been established throughout the country. We believe that our study, which was conducted on patients referred to our tertiary hospital that provides outpatient services to 1 ½ million patients a year, constitutes a quality sample pool.
In the current study, the mean age of the patients in the HSIL group was younger and endocervical gland involvement was more common. In addition, the lack of difference between the lesion area and biopsy method was consistent with our previous studies (Kilic et al. 2020; Atigan et al. 2022). In the study of Güdücü et al. (2013), there was no difference in surgical margin positivity, but endocervical gland involvement was higher in the HSIL group. Similar to our study in the literature, endocervical and ectocervical surgical margin positivity was not different in the LSIL and HSIL groups (Kilic et al. 2020; Costa et al. 2000). Previous studies have suggested that the patient's age, biopsy method, sample size, severity of the disease and persistence of HPV may be factors in surgical margin positivity (Kilic et al. 2020; Costa et al. 2000; Bae et al. 2013; Giray et al. 2020). In our current study, there was a difference between the groups in terms of the level of CIN that continued at the surgical margin. However, it is remarkable that the HSIL group has higher CIN III rate at the endocervical than ectocervical surgical margin.
p16INK4a staining was routinely used in CIN lesions in the study of Sano et al. (1998a). p16, a tumor suppressor gene that is generally decreased in malignant tumors, is increased especially in HSIL group CINs (Sano et al. 1998b). In a review study performed on cervical precancerous lesions, Ki-67 and p16INK4a immunostains were found to stain more aggressively as lesion severity increased (Silva et al. 2017). Consistent with the literature in our study, both stainings were found to be statistically significantly higher in the HSIL group.
SOX-2, which is responsible for tumor formation and drug resistance in cancer cells, has an important role in the cell cycle and DNA repair (Kim et al. 2015). In addition, it has effects on many signaling pathways in cell cycles. For this reason, it has been shown to cause more than a dozen types of cancer (Zhang et al. 2020; Novak et al. 2020; Yuan et al. 2021). SOX-2 has been studied in different tumor types in various studies and is generally associated with poor prognostic factors as in SCC (Stewart and Crook 2018). The literature shows that as SOX-2 expression increases, it causes resistance to treatment and high mortality in cancer patients (Zhang et al. 2020; Kim et al. 2015; Novak et al. 2020; Grimm et al. 2020). A study in which no relationship was found with CA125 level in ovarian cancer showed that SOX-2 expression increased as the stage increased (Ye et al. 2011). In a meta-analysis on cervical cancer, SOX-2 expression was shown to be unrelated to tumor stage. However, it has been shown to be associated with tumor grade (Yuan et al 2021). In our study, SOX-2 was highest in SCC. In addition, it was still higher in the HSIL group than in the LSIL group. Our findings for SOX-2 are in agreement with the study of Moshi et al. (2022). We believe biopsies can be helpful in the histopathological classification of CIN.
Conclusions
Much can be done for SCC, a preventable cancer with over half a million cases, resulting in death for more than half of this number as of 2018 (Bray et al. 2018). Turkey’s handling of the challenge regarding SCC caused by HPV makes it a premise country in Europe (Maver and Poljak 2020). The most helpful procedure in reducing SCC after vaccination is histopathological examination of the biopsies taken. The benefits of new immunostains such as SOX-2 will undoubtedly be revealed as more studies are conducted. We think that the importance of SOX-2 will be better understood as immunotherapy modalities develop.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
References
Atigan A, Güler T, Arman Karakaya Y, Kilic D (2022) Relationship between ımmunohistochemical CD3, CD4, CD5, CD8, and PD1 staining and histopathological diagnosis of cervical lesions in patients with abnormal colposcopic findings. Cureus 14(11):e31399
Atıgan A, Eraydın E (2020) Human papillomavirus (HPV) tiplerinin prevalansinin saptanması. KSÜ Tıp Fak Der 15(2):35–40
Bae HS, Chung YW, Kim T, Lee KW, Song JY (2013) The appropriate cone depth to avoid endocervical margin involvement is dependent on age and disease severity. Acta Obstet Gynecol Scand 92(2):185–192
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Costa S, De Nuzzo M, Terzano P, Santini D, De Simone P, Bovicelli A, Bovicelli L, Bucchi L (2000) Factors associated with cone margin involvement in CIN patients undergoing conization-equivalent electrosurgical procedure. Acta Obstet Gynecol Scand 79(7):586–592
Giray B, Kabaca-Kocakusak C, Guray-Uzun M, Akis S (2020) Post-conization follow-up of patients with CIN 2/3 with different amount of distance to negative cone biopsy margin: a retrospective cohort study. J Obstet Gynaecol 40(3):406–410
Grimm D, Bauer J, Wise P, Krüger M, Simonsen U, Wehland M, Infanger M, Corydon TJ (2020) The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol 67(Pt 1):122–153
Güdücü N, Sidar G, Başsüllü N, Türkmen I, Dünder I (2013) Endocervical glandular involvement, multicentricity, and extent of the disease are features of high-grade cervical intraepithelial neoplasia. Ann Diagn Pathol 17(4):345–346
Kilic D, Guler T, Atigan A, Avsaroglu E, Karakaya YA, Kaleli I, Kaleli B (2020) Predictors of human papillomavirus (HPV) persistence after treatment of high grade cervical lesions; does cervical cytology have any prognostic value in primary HPV screening? Ann Diagn Pathol 49:151626
Kim BW, Cho H, Choi CH, Ylaya K, Chung JY, Kim JH, Hewitt SM (2015) Clinical significance of OCT4 and SOX2 protein expression in cervical cancer. BMC Cancer 15:1015
Kingnate C, Supoken A, Kleebkaow P, Chumworathayi B, Luanratanakorn S, Kietpeerakool C (2015) Is age an independent predictor of high-grade histopathology in women referred for colposcopy after abnormal cervical cytology? Asian Pac J Cancer Prev 16(16):7231–7235
Maver PJ, Poljak M (2020) Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin Microbiol Infect 26(5):579–583
Moshi JM, Ummelen M, Broers JLV, Smedts F, Van de Vijver KK, Cleutjens JPM, Litjens RJNTM, Ramaekers FCS, Hopman AHN (2022) SOX2 expression in the pathogenesis of premalignant lesions of the uterine cervix: its histo-topographical distribution distinguishes between low- and high-grade CIN. Histochem Cell Biol 158(6):545–559
Novak D, Hüser L, Elton JJ, Umansky V, Altevogt P, Utikal J (2020) SOX2 in development and cancer biology. Semin Cancer Biol 67(Pt 1):74–82
Pouremamali F, Vahedian V, Hassani N, Mirzaei S, Pouremamali A, Kazemzadeh H, Faridvand Y, Jafari-Gharabaghlou D, Nouri M, Maroufi NF (2022) The role of SOX family in cancer stem cell maintenance: with a focus on SOX2. Pathol Res Pract 231:153783
Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T (1998a) Immunohistochemical overexpression of p16 protein associated with intact retinoblastoma protein expression in cervical cancer and cervical intraepithelial neoplasia. Pathol Int 48(8):580–585
Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T (1998b) Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol 153:1741–1748
Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, Garcia FA, Moriarty AT, Waxman AG, Wilbur DC, Wentzensen N, Downs LS Jr, Spitzer M, Moscicki AB, Franco EL, Stoler MH, Schiffman M, Castle PE, Myers ER (2012) ACS-ASCCP-ASCP cervical cancer guideline committee. American cancer society, American society for colposcopy and cervical pathology, and American society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin 62(3):147–172
Sato F, Bhawal UK, Tojyo I, Fujita S, Murata SI, Muragaki Y (2019) Differential expression of claudin-4, occludin, SOX2 and proliferating cell nuclear antigen between basaloid squamous cell carcinoma and squamous cell carcinoma. Mol Med Rep 20(2):1977–1985
Silva DC, Gonçalves AK, Cobucci RN, Mendonça RC, Lima PH, Júnior CG (2017) Immunohistochemical expression of p16, Ki-67 and p53 in cervical lesions-A systematic review. Pathol Res Pract 213(7):723–729
Stewart CJR, Crook ML (2018) Podoplanin and SOX2 expression in CIN 3-like squamous cell carcinoma of the cervix. Int J Gynecol Pathol 37(1):59–67
Ye F, Li Y, Hu Y, Zhou C, Hu Y, Chen H (2011) Expression of SOX2 in human ovarian epithelial carcinoma. J Cancer Res Clin Oncol 137(1):131–137
Yuan D, Wang J, Yan M, Xu Y (2021) SOX2 as a prognostic marker and a potential molecular target in cervical cancer: a meta-analysis. Int J Biol Markers 36(4):45–53
Zhang S, Xiong X, Sun Y (2020) Functional characterization of SOX2 as an anticancer target. Signal Transduct Target Ther 5(1):135
Author information
Authors and Affiliations
Contributions
AA, YAK, OTG wrote the main manuscript text, and AA, YAK, DK prepared all figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atıgan, A., Kılıç, D., Karakaya, Y.A. et al. The relationship of immunohistochemical SOX-2 staining with histopathological diagnosis in patients with abnormal colposcopic findings. Histochem Cell Biol 160, 555–561 (2023). https://doi.org/10.1007/s00418-023-02230-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-023-02230-4