Abstract
Lipolysis-stimulated lipoprotein receptor (LSR)/angulin-1 is a crucial molecule of tricellular contacts in the epithelial barrier of normal cells and the malignancy of cancer cells. To investigate whether LSR/angulin-1 affects the epithelial barrier and malignancy in human pancreatic cancer, human pancreatic cancer cell line HPAC was used. Treatment with EGF or TGF-β increased the expression of LSR, but not tricellulin (TRIC), and induced the localization of LSR and TRIC to bicellular tight junctions from tricellular tight junctions. TGF-β receptor type-1 inhibitor EW-7197 prevented changes of the distribution and the barrier function of LSR by TGF-β. Knockdown of LSR increased cell migration, invasion, proliferation and EGF ligand amphiregulin expression and decreased the epithelial barrier. Treatment with amphiregulin induced cell migration and invasion and knockdown of amphiregulin prevented the increases of cell migration, invasion and proliferation caused by knockdown of LSR. Treatment with LSR ligand peptide angubindin-1 decreased the epithelial barrier and the expression of LSR, but not TRIC, and increased cell invasion. Knockdown of TRIC decreased cell migration and the epithelial barrier. In immunohistochemical analysis of human pancreatic cancer tissues, LSR and TRIC were found to be localized at the cell membranes of normal pancreatic ducts and well-differentiated pancreatic ductal adenocarcinomas (PDAC), whereas in poorly differentiated PDAC, LSR was weakly detected in the cytoplasm. Amphiregulin was highly expressed in the cytoplasm of well- and poorly differentiated PDAC. In pancreatic cancer, LSR contributes to the epithelial barrier and malignancy via growth factors and may be a potential targeting molecule in the therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancer, which has a strong invasive ability with frequent metastasis and recurrence, is one of the most malignant diseases and its cancer-associated mortality has not decreased worldwide for decades. Thus, novel diagnostic and therapeutic strategies must be developed urgently to improve the poor outcome of pancreatic cancer patients.
Tight junctions (TJ) are epithelial and endothelial cell–cell junctions that regulate the flow of solutes through paracelluar pathways and maintain cell polarity, thereby functioning as a barrier of epithelial and endothelial cellular sheets (Angelow et al. 2008; Furuse and Tsukita 2006). Tricellular tight junctions (tTJs) form at the convergence of bicellular tight junctions (bTJs) where three epithelial cells meet in polarized epithelia (Ikenouchi et al. 2005; Furuse et al. 2014).
Several studies have reported that loss of bTJ proteins, including claudins and occludin, enhances tumor progression (Tsukita et al. 2008; Martin and Jiang 2009; Martin 2014; Martin et al. 2016). The tTJ protein tricellulin TRIC is expressed in both normal and neoplastic pancreatic exocrine tissues and it has a significant negative correlation with the degree of differentiation in pancreatic ductal adenocarcinomas (Korompay et al. 2012). Furthermore, nuclear localization of tricellulin in part promotes the malignancy of pancreatic cancer (Takasawa et al. 2016).
Lipolysis-stimulated lipoprotein receptor (LSR) is a novel molecular constituent of tricellular contacts localized in most epithelial tissues and has a barrier function (Masuda et al. 2011). LSR is also angulin-1 and is one of angulin family proteins (angulin-1/LSR, angulin-2/ILDR1 and angulin-3/ILDR2) (Higashi et al. 2013). LSR recruits TRIC, which is the first molecular component of tTJs (Ikenouchi et al. 2005), and the interaction between the cytoplasmic domain of LSR and the C-terminal cytoplasmic domain of TRIC is required for this recruitment (Masuda et al. 2011). The LSR-related proteins immunoglobulin-like domain containing receptors ILDR1 and ILDR2 are also expressed at tricellular contacts of many epithelial cells and recruit tricellulin, and ILDR1 is responsible for the barrier function (Higashi et al. 2013). Loss of LSR affects the malignancy of various cancers, including bladder cancer, colon cancer, endometrial cancer and head and neck cancer (Czulkies et al. 2017; Shimada et al. 2016, 2017b; Takano et al. 2016). Furthermore, downregulation of LSR promotes cell invasion via claudin-1-mediated MMPs in endometrial cancer cells (Shimada et al. 2017a). However, the role and regulation of LSR in pancreatic cancer remain unknown.
Amphiregulin (AREG) is a ligand of the epidermal growth factor receptor (EGFR) and EGFR binding AREG activates the main intracellular signaling cascades of cell survival, proliferation and motility (Berasain and Avila 2014). The oncogenic activity of AREG is known in lung, breast, colorectal, ovary, prostate and pancreas cancers (Ebert et al. 1994; Busser et al. 2011). AREG regulates the activation of ERK and Akt through EGFR and HER3 signals involved in the progression of pancreatic cancer (Yotsumoto et al. 2010). The YAP signaling network correlates with poor survival of pancreatic cancer patients (Rozengurt et al. 2018). In human endometrial cancer cells, loss of LSR induces YAP/TEAD-regulated gene AREG expression and promotes cell invasion and cell migration via upregulation of TEAD1/AREG dependent on YAP/pYAP (Shimada et al. 2017b).
Angubindin-1 is a novel binder to angulin-1 and -3 that was developed from Clostridium perfringens iota-toxin, which is a binary toxin comprising an enzymatic component (Ia) and a receptor-binding component (Ib) (Nagahama et al. 2011; Krug et al. 2017). A fragment of the domain corresponding to amino acids 421–664 (angubindin-1) modulates the tTJ without causing cytotoxicity, increases intestinal absorption of large molecules and opens the blood–brain barrier via the paracellular route by disrupting tricellulin recruitment (Krug et al. 2017; Zeniya et al. 2018; Hashimoto et al. 2019). However, the effects of angubindin-1 on cell migration, invasion, proliferation and the epithelial barrier of cancer cells are unclear.
In the present study, we investigated the role and regulation of the tTJ protein LSR compared to TRIC in pancreatic cancer. In human pancreatic cancer cells, LSR was regulated via growth factors and the loss of LSR promoted cell invasion, migration and proliferation via AREG. These results are important for understanding the behavior and role of tTJ proteins in cancers and developing novel therapeutic strategies for pancreatic cancer.
Materials and methods
Ethics statement
The protocol for human study was reviewed and approved by the ethics committee of the Sapporo Medical University School of Medicine. Written informed consent was obtained from each patient who participated in the investigation. All experiments were carried out in accordance with the approved guidelines and the Declaration of Helsinki.
Reagents and antibodies
A rabbit polyclonal anti-actin antibody and EGFR inhibitor (AG1478) were obtained from Sigma-Aldrich (St. Louis, MO). A rabbit polyclonal anti-lipolysis-stimulated receptor (LSR) antibody was obtained from Novus Biological, LLC (Centennial, CO). A rabbit polyclonal anti-tricellulin (TRIC) antibody was obtained from Zymed Laboratories (San Francisco, CA). A mouse monoclonal anti-LSR antibody was obtained from Abnova (Yaipei, Taiwan). A mouse monoclonal anti-amphiregulin antibody (G-4) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). EGF, TGF-β1 and AREG were purchased from PeproTech EC (London, UK). A TGF-β receptor type 1 inhibitor (EW-7197) was obtained from Cayman Chemical (Ann Arbor, MI). Alexa 488 (green)-conjugated anti-rabbit IgG and Alexa 594 (red)-conjugated anti-mouse IgG antibodies were purchased from Molecular Probes, Inc. (Eugene, OR).
Preparation of angubindin-1
Angubindin-1 was a kind gift from the Drug Discovery Center, Graduate School of Pharmaceutical Sciences, Osaka University, Osaka, Japan as part of our joint research (Krug et al. 2017; Zeniya et al. 2018). The plasmid encoding angubindin-1 (pGEX-Ib421–664) was expressed as a fusion protein with glutathione S-transferase (GST) in Escherichia coli strain BL21 (DE3). After growth at 37 °C and induction with isopropyl β-d-thiogalactopyranoside (Nacalai Tesque, Kyoto, Japan) of a 1000-mL culture, cells were harvested, resuspended in buffer F [50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 2 mM CaCl2], and then lysed by sonication. The lysates were applied to glutathione Sepharose 4B beads (GE Healthcare, Buckinghamshire, UK) and purified GST-tagged angubindin-1 was cleaved with thrombin. After the removal of thrombin using Benzamidine Sepharose 4 Fast Flow (GE Healthcare), the solvent for angubindin-1 was changed to phosphate-buffered saline (PBS) by gel filtration using a PD-10 column (GE Healthcare), and the purified proteins were stored at − 80 °C until use. The concentration of the purified proteins was determined using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA) with bovine serum albumin (BSA) as the standard. Purification of the recombinant proteins was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). To investigate the distribution of angubindin-1 in the brain, a DyLight 550 antibody labeling kit (Thermo Scientific) was used to label the angubindin-1 with DyLight 550.
Cultures of cell lines and treatment
The human pancreatic cancer cell line HPAC was purchased from ATCC (Manassas, VA). HPAC cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) supplemented with 10% dialyzed fetal bovine serum (FBS, Invitrogen; Carlsbad, CA). The medium contained 100 U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml amphotericin-B. The cells were plated on 60-mm culture dishes (Corning Glass Works, Corning, NY) that were coated with rat tail collagen (500 μg of dried tendon/ml in 0.1% acetic acid), and incubated in a humidified 5% CO2 incubator at 37 °C. HPAC cells were treated with 100 ng/ml EGF, 100 ng/ml TGF-β1, 1 μM EW-7197, 100 ng/ml AREG, 10 μM AG1478 and 2.5 μg/ml angubindin-1.
RNA interference and transfection
siRNA duplex oligonucleotides against LSR (forward sense 5′-CCCACGCAACCCAUCGUCAUCUGGA-3′, reverse sense 5′-UCCAGAUGACGAUGGGUUGCGUGGG-3′) and TRIC (forward sense 5′-GACAGACAAAGAGACTCAGAAGTTA-3′, reverse sense 5′-UTTCUUCUGTGUCUCUUUGUCUGUC-3′) were synthesized by Thermo Fisher Scientific (Waltham, MA). siRNA duplex oligonucleotides against AREG (sc-39412) were synthesized by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). A scrambled siRNA sequence (BLOCK-iT Alexa Fluor fluorescent, Invitrogen) was employed as control siRNA. At 24 h after plating, HPAC cells were transfected with 100 nM siRNAs of LSR, TRIC and AREG using Lipofectamine™ RNAiMAX Reagent (Invitrogen).
Immunohistochemical analysis
TNM histologic grading system of pancreatic ductal adenocarcinomas (PDAC) recommended by pathologists is based on the extent of glandular differentiation: G1 = well differentiated, (> 95% tumor composed of glands), G2 = moderately differentiated (50–95% glands), G3 = poorly differentiated (< 50% glands), G4 = no or minimal differentiation. Immunohistochemical analysis was performed to evaluate the expression and distribution of LSR, TRIC and AREG in each six cases of well-differentiated (G1) and poorly differentiated (G3) PDAC tissues and the normal pancreatic tissue areas. Deparaffinized tissue sections were immersed in 10 mmol/L sodium citrate (pH 6.0) and boiled for antigen retrieval by microwave (95 °C, 30 min). Endogenous peroxidase activity was blocked using 3% hydrogen peroxidase for 10 min. The sections were incubated with rabbit polyclonal anti-LSR, rabbit polyclonal anti-TRIC (1:100 dilution) and mouse monoclonal anti-AREG (1: 300 dilution) antibodies overnight at 4 °C. The sections were incubated with a Dako REAL™ EnVision™/HRP, Rabbit/Mouse [Dako REAL™ EnVision™ Detection System; Dako, code K5007, (Glostrup, Denmark)] for 1 h at room temperature. After washing with PBS, the labeled secondary antibody was visualized by adding Dako REAL™ Substrate Buffer (Dako REAL™ EnVision™ Detection System: Dako, code K5007) containing Dako REAL™ DAB + Chromogen (Dako REAL™ EnVision™ Detection System: Dako, code K5007). The sections were counterstained with hematoxylin. A negative control was performed by replacing the first antibodies with normal serum. The extent of positively stained area was scored from 0 to 3 according to the percentage of atypical cells showing positive IHC staining in the observed area: 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–100%). The intensity of staining was graded 0–3 according to the intensity of immunohistochemical staining: 0 (none), 1 (low), 2 (middle), and 3 (high).
Immunofluorescence
Cells cultured in 35-mm glass-coated wells (Iwaki, Chiba, Japan) were fixed with cold acetone and ethanol (1: 1) at − 20 °C for 10 min. After rinsing in PBS, the cells were incubated with rabbit polyclonal or mouse monoclonal anti-LSR (1:100) and rabbit polyclonal anti-TRIC (1:100) antibodies at room temperature for 1 h. Alexa Fluor 488 (green)-conjugated anti-rabbit IgG and Alexa Fluor 594 (red)-conjugated anti-mouse IgG (Invitrogen) were used as secondary antibodies. The specimens were examined using an epifluorescence microscope (Olympus, Tokyo, Japan) and a confocal laser scanning microscope (LSM5; Carl Zeiss, Jena, Germany).
Transmission electron microscopy analysis
The cells were cultured to confluence in eight chambers of CultureSlides (Falcon). For transmission electron microscopy (TEM), the cultured cells were fixed in 2.5% glutaraldehyde in PBS overnight at 4 °C, followed by post-fixing in 2% osmium tetroxide in the same buffer. Then, the cells were dehydrated with a graded ethanol series and embedded in Epon 812. Following this, ultrathin sections were cut on a Sovall Ultramicrotome MT-5000. The sections were stained with uranyl acetate followed by lead citrate and examined at 80 kV with a transmission electron microscope (H7500; Hitachi, Tokyo, Japan).
Measurement of transepithelial electrical resistance (TEER)
The cells were cultured to confluence in the inner chambers of 12-mm Transwell inserts with 0.4-µm pore-size filters (Corning Life Sciences). TEER was measured using an EVOM voltameter with an ENDOHM-12 (World Precision Instruments, Sarasota, FL) on a heating plate (Fine, Tokyo, Japan) adjusted to 37 °C. The values were expressed in standard units of ohms per square centimeter and presented as mean ± S.D. For calculation, the resistance of blank filters was subtracted from that of filters covered with cells.
Western blot analysis
The cultured cells were scraped from 60 mm dishes containing 400 μl of buffer (1 mM NaHCO3 and 2 mM phenylmethylsulfonyl fluoride), collected in microcentrifuge tubes, and then sonicated for 10 s. The protein concentrations of the samples were determined using a BCA protein assay regent kit (Pierce Chemical Co.; Rockford, IL, USA). Aliquots of 15 μl of protein/lane for each sample were separated by electrophoresis in 5–20% SDS polyacrylamide gels (Wako, Osaka, Japan), and electrophoretically transfered to a nitrocellulose membrane (Immobilon; Millipore Co.; Bedford, UK). The membrane was saturated with blocking buffer (25 mM Tris, pH 8.0, 125 mM NaCl, 0.1% Tween 20, and 4% skim milk) for over 30 min at room temperature and incubated with rabbit polyclonal anti-LSR (1: 1000), rabbit polyclonal anti-tricellulin (1:1000) and rabbit polyclonal anti-actin (1: 1000) antibodies at room temperature for over 1 h. Then, it was incubated with HRP-conjugated anti-mouse and anti-rabbit IgG antibodies at room temperature for 1 h. The immunoreactive bands were detected using an ECL Western blotting system. The bands were quantitated by densitometry and the data normalized to actin. The corresponding expression levels were shown as bar graphs.
Matrigel invasion assay
For the invasion assay, we used Matrigel (Becton–Dickinson Labware, Bedford, MA) and Cell Culture Insert (pore size 8 μm; Becton–Dickinson Labware). HPAC cells were plated onto the upper chamber coated with Matrigel and the lower chamber of the Transwell was filled with human fibroblast conditioned medium containing 10 nM EGF as an adhesive substrate. Then, the cells were incubated for 36 h, after which the upper chamber was fixed with 100% methanol for 10 min and stained with Giemsa for 20 min. The areas of invading cells were measured using a microscope imaging system (Olympus, Tokyo, Japan).
Migration assay
After the cells were plated onto the 35 mm dishes, they were cultured to confluence. At 24 h, we wounded the cell layer using a plastic pipette tip (P200), and measured the length of the wound using a microscope imaging system (Olympus, Tokyo, Japan).
Data analysis
Each set of results shown is representative of at least three separate experiments. Results are given as mean ± SEM. Statistical analysis was by one-way analysis of variance (ANOVA) with the Tukey–Kramer method. Statistical significance was set at *p < 0.05 and **p < 0.01.
Results
Effects of EGF and TGF-β on the expression and distribution of LSR and TRIC in HPAC cells
To investigate effects of EGF and TGF-β on the expression and distribution of LSR and TRIC during the carcinogenesis of pancreatic cancer cells, cells of human pancreatic cancer cell line HPAC were treated with 100 ng/ml EGF or 100 ng/ml TGF-β for 24 h. In Western blot analysis, treatment with EGF and TGF-β was found to increase the expression of LSR, but not TRIC (Fig. 1a). In Immunofluorescence, LSR and TRIC were strongly observed not only at tTJs, but also at bTJs in the cells treated with EGF and TGF-β, whereas in the control, LSR and TRIC were strongly observed at tTJs (Fig. 1b).
Effects of TGF-β receptor type 1 inhibitor EW-7197 on the expression and distribution of LSR and TRIC and the epithelial barrier function in HPAC cells
To investigate effects of a TGF-β receptor type 1 inhibitor on the expression and distribution of LSR and TRIC and the epithelial barrier function of pancreatic cancer cells, HPAC cells were pretreated with 10 μM EW-7197 before treatment with or without 100 ng/ml TGF-β for 24 h. Immunofluorescence revealed that treatment with EW-7197 increased the expression of LSR and TRIC at tTJs in the cells pretreated with or without TGF-β (Fig. 2a). In TEM analysis, the borders of the cells treated with EW-7197 were linear and electron dense compared to the control cells (Fig. 2b). Western blot analysis showed that treatment with EW-7197 increased the expression of LSR but not TRIC-like phosphorylated bands in the cells pretreated with or without TGF-β (Fig. 2c). In the epithelial barrier function, treatment with EW-7197 significantly increased TEER values in the cells pretreated with or without TGF-β (Fig. 2d).
Effects of TGF-β receptor type 1 inhibitor EW-7197 on expression and distribution of LSR and TRIC and the epithelial barrier function in HPAC cells. a Immunofluorescence for LSR and TRIC in HPAC cells treated with EW-7197 with or without TGF-β. Scale bar: 20 μm. b TEM analysis of the cell borders of HPAC cells treated with EW-7197. Scale bar: 1 µm. c Western blotting for LSR and TRIC in HPAC cells treated with EW-7197 with or without TGF-β. A bar graph of bands in c. d A bar graph of TEER values in HPAC cells treated with EW-7197 with or without TGF-β. Control vs. EW-7197: *p < 0.05. Control vs. TGF-β: *p < 0.05. Control vs. TGF-β and EW-7197: #p < 0.05
Knockdown of LSR increased cell migration, cell invasion, cell proliferation and AREG expression and decreased the epithelial barrier in HPAC cells
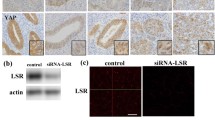
To investigate whether knockdown of LSR affected cell migration, invasion and proliferation or the epithelial barrier function in human pancreatic cancer cells, HPAC cells were transfected with the siRNA of LSR. The expression of LSR, but not TRIC, was found to be deceased by the siRNA of LSR in Western blot and Immunofluorescence (Fig. 3a, b). Knockdown of LSR significantly increased cell migration, invasion and proliferation (Fig. 3c, e) and reduced TEER values compared to the control (Fig. 3f). On the other hand, in HPAC cells, knockdown of LSR increased the mRNA of EGFR ligand amphiregulin (AREG), as in Sawano cells (Shimada et al. 2016) (Fig. 3g).
Knockdown of LSR induces malignancy and increases the expression of AREG mRNA in HPAC cells. a Western blotting for LSR and TRIC in LSR-knockdown HPAC cells. A bar graph of bands in a. b Immunofluorescence for LSR and TRIC in LSR-knockdown HPAC cells. Scale bar: 20 μm. c Matrigel invasion assay of LSR-knockdown HPAC cells. The result is shown as a bar graph. Control vs. siRNA-LSR: *p < 0.05. Scale bar: 100 μm. d Migration assay of LSR-knockdown HPAC cells. The result is shown as a bar graph. Control vs. siRNA-LSR: *p < 0.05. Scale bar: 400 μm. e A bar graph of cell proliferation assay of LSR-knockdown HPAC cells. Control vs. siRNA-LSR: *p < 0.05. f A bar graph of TEER values in LSR-knockdown HPAC cells. Control vs. siRNA-LSR: *p < 0.05. g Real-time PCR for AREG mRNA in LSR-knockdown HPAC cells. The result is shown as a bar graph. Control vs. siRNA-LSR: *p < 0.05
Treatment with AREG induced cell migration and cell invasion in HPAC cells
To investigate whether AREG alone affected the cell migration, invasion and proliferation or the epithelial barrier in human pancreatic cancer cells, HPAC cells were treated with 100 ng/ml AREG for 24 h after pretreatment with or without 10 μM EGFR inhibitor AG1478. Treatment with AREG significantly increased cell migration, invasion and proliferation compared to the control and reduced TEER values, whereas pretreatment with AG1478 prevented all the effects of AREG treatment (Fig. 4a–d).
Effects of AREG on the malignancy of HPAC cells. a Matrigel invasion assay of HPAC cells treated with AREG with and without AG1478. Scale bar: 100 μm. The results are shown as bar graphs. Control vs. AREG: *p < 0.05. AREG vs. AREG with AG1478: #p < 0.01. b Migration assay of HPAC cells treated with AREG with and without AG1478. Scale bar: 400 μm. The results are shown as bar graphs. Control vs. AREG: *p < 0.01. AREG vs. AREG with AG1478: #p < 0.01. c A bar graph of cell proliferation assay of HPAC cells treated with AREG with and without AG1478. Control vs. AREG: *p < 0.05. AREG vs. AREG with AG1478: #p < 0.01. d A bar graph of TEER values in HPAC cells treated with AREG with and without AG1478. Control vs. AREG: *p < 0.05. AREG vs. AREG with AG1478: #p < 0.01. e Matrigel invasion assay of HPAC cells transfected with siRNAs of LSR and/or AREG. Scale bar: 100 μm. The results are shown as bar graphs. Control vs. siRNA-LSR: *p < 0.01. siRNA-LSR vs. siRNA-LSR and siRNA-AREG: #p < 0.01. Control vs. siRNA-AREG: not significant. f Migration assay of HPAC cells transfected with siRNAs of LSR and/or AREG. Scale bar: 400 μm. The results are shown as bar graphs. Control vs. siRNA-LSR: *p < 0.01. siRNA-LSR vs. siRNA-LSR and siRNA-AREG: #p < 0.01. Control vs. siRNA-AREG: *p < 0.01. g A bar graph of cell proliferation assay of HPAC cells transfected with siRNAs of LSR and/or AREG. Control vs. siRNA-LSR: *p < 0.05. siRNA-LSR vs. siRNA-LSR and siRNA-AREG: #p < 0.01. Control vs. siRNA-AREG: not significant
Effects of knockdown of LSR via AREG in HPAC cells
To investigate the effects of LSR via AREG in human pancreatic cancer cells, HPAC cells were co-transfected with siRNAs of LSR and AREG. Knockdown of AREG prevented the increases of cell migration, invasion and proliferation caused by knockdown of LSR (Fig. 4e–g).
Effects of LSR ligand peptide angubindin-1 on the malignancy and barrier function in HPAC cells
The LSR ligand peptide angubindin-1 reduces TEER values in epithelial cells (Krug et al. 2017). To investigate the effects of angubindin-1 on cell migration, cell invasion, cell proliferation and the epithelial barrier in human pancreatic cancer cells, HPAC cells were treated with 2.5 μg/ml angubindin-1 for 24 h. The molecular weight of the angubindin-1 peptide was about 35 kDa (Fig. 5a). Treatment with angubindin-1 decreased the expression of LSR, but not TRIC, while no change in the distribution of LSR was observed (Fig. 5b, c). Treatment with angubindin-1 significantly increased cell invasion and decreased TEER values, whereas no changes in cell migration and proliferation were observed (Fig. 5d–g).
Effects of LSR ligand peptide angubindin-1 on the malignancy and barrier function in HPAC cells. a The molecular weight of angubindin-1 peptide. b Western blotting for LSR and TRIC in HPAC cell treated with angubindin-1. A bar graph of bands in b. c Immunocytochemical staining for LSR in HPAC cells treated with angubindin-1. Scale bar: 20 μm. d Matrigel invasion assay of HPAC cells treated with angubindin-1. Scale bar: 100 μm. The result is shown as a bar graph. Control vs. Angubindin-1: *p < 0.01. e Migration assay of HPAC cells treated with Angubindin-1. Scale bar: 400 μm. The result is shown as a bar graph. Control vs. Angubindin-1: not significant. f A bar graph of cell proliferation assay of HPAC cells treated with Angubindin-1. Control vs. Angubindin-1: not significant. g A bar graph of TEER values in HPAC cells treated with Angubindin-1. Control vs. Angubindin-1: *p < 0.01
Knockdown of TRIC decreased cell migration and the epithelial cell barrier in HPAC cells
To investigate whether knockdown of TRIC affected cell migration, invasion and proliferation or the epithelial barrier function in human pancreatic cancer cells, HPAC cells were transfected with the siRNA of TRIC. The expression of TRIC, but not LSR, was found to be decreased by the siRNA of TRIC in Western blot and immunocytochemical analyses (Fig. 6a, b). Knockdown of TRIC significantly decreased cell migration and TEER values compared to the control, whereas no changes of cell invasion and proliferation were observed (Fig. 6c, e, f).
Knockdown of TRIC decreases cell migration and the epithelial cell barrier in HPAC cells. a Western blotting for TRIC and LSR in TRIC-knockdown HPAC cells. A bar graph of bands in A. b Immunofluorescence for TRIC in TRIC-knockdown HPAC cells. c Matrigel invasion assay of TRIC-knockdown HPAC cells. Scale bar: 100 μm. The result is shown as a bar graph. Control vs. siRNA-TRIC: not significant. d Migration assay of TRIC-knockdown HPAC cells. Scale bar: 400 μm. The result is shown as a bar graph. Control vs. siRNA-TRIC: *p < 0.01. e A bar graph of cell proliferation assay of TRIC-knockdown HPAC cells. Control vs. siRNA-TRIC: not significant. f A bar graph of TEER values in TRIC-knockdown HPAC cells. Control vs. siRNA-TRIC: *p < 0.01
The expression and distribution of LSR, TRIC and AREG in human pancreatic cancer tissues
Using immunohistochemical analysis, we examined the expression and distribution of LSR, TRIC and AREG in normal pancreatic tissue, and well- and poorly differentiated pancreatic ductal adenocarcinomas (PDAC) (Fig. 7). In pancreatic acini of normal pancreatic tissues, LSR, TRIC and AREG were positive. LSR and TRIC were localized at the cell membranes of the normal pancreatic ducts and in six of six (100%) cases of well-differentiated PDAC, whereas in six of six (100%) cases of poorly differentiated PDAC, LSR was weakly detected in the cytoplasm and TRIC was not detected. AREG was highly expressed at the cytoplasm in six of six (100%) cases of well- and poorly differentiated PDAC, whereas in the normal pancreatic ducts, it was weakly detected.
Expression and localization of LSR, TRIC and AREG in the tissues of human normal pancreas and pancreatic cancer. Hematoxylin and eosin staining and immunohistochemical staining for LSR, TRIC and AREG in human normal pancreatic ducts (arrows), well- and poorly differentiated pancreatic ductal adenocarcinomas. Scale bar: 100 µm
Discussion
In the present study, we investigated the role and regulation of tTJ protein LSR compared to TRIC in pancreatic cancer. In human pancreatic cancer cells, LSR was regulated via growth factors and the loss of LSR promoted cell invasion, migration and proliferation via AREG.
In pancreatic cancer cells, treatment with EGF or TGF-β increased the expression of LSR, but not TRIC, and induced the localization of LSR and TRIC to bTJs from tTJs. TGF-β receptor type 1 inhibitor EW-7197 prevented the changes of the distribution and the barrier function of LSR induced by TGF-β. TGF-β is a negative modulator of overexpression of bTJ protein claudin-4 expression in pancreatic cancer (Michl et al. 2001). The expression and the distribution of LSR in pancreatic cancer were also regulated via growth factors, including TGF-β.
We previously reported that, in human endometrial cancer cells, loss of LSR induced YAP/TEAD-regulated gene AREG expression and promoted cell invasion and migration via upregulation of TEAD1/AREG (Shimada et al. 2017b).
In the present study of human pancreatic cancer cells, knockdown of LSR increased cell migration, invasion and proliferation, and induced AREG expression and decreased the epithelial barrier. Knockdown of AREG prevented the increases of cell migration, invasion and proliferation caused by knockdown of LSR.
In human endometrial cancer tissues, downregulation of LSR and upregulation of AREG are observed together with malignancy (Shimada et al. 2016). In human pancreatic cancer tissues, LSR was localized at the cell membranes of normal pancreatic ducts and well-differentiated PDAC, whereas in poorly differentiated PDAC, LSR was weakly detected in the cytoplasm. AREG was highly expressed in the cytoplasm of well and poorly differentiated PDAC.
AREG activates the main intracellular signaling cascades of cell survival, proliferation and motility (Berasain and Avila 2014). The oncogenic activity of AREG is known in various cancers, including pancreatic cancer (Ebert et al. 1994; Busser et al. 2011). AREG regulates the activation of ERK and Akt through EGFR and HER3 signals involved in the progression of pancreatic cancer (Yotsumoto et al. 2010).
These findings suggested that the loss of LSR induced AREG expression and then enhanced the malignancy, causing cell invasion, migration and proliferation in pancreatic cancer as in endometrial cancer. However, the detailed mechanisms in the induction of AREG expression caused by loss of LSR remain unknown.
In the present study, to investigate a novel role of LSR in pancreatic cancer, we used the LSR ligand peptide angubindin-1 as a modulator of the tTJs barrier. Treatment with angubindin-1 decreased the epithelial barrier and the expression of LSR and enhanced cell invasion in pancreatic cancer cells. It has been reported that the binding of angubindin-1 leads to removal of LSR and tricellulin from the tTJ, which enhances the permeation of macromolecular solutes (Krug et al. 2017; Hashimoto et al. 2019). These findings indicate that the downregulation of not only LSR expression, but also LSR function may enhance the malignancy of pancreatic cancer.
TRIC is expressed in both normal and neoplastic pancreatic exocrine tissues and it has a significant negative correlation with the degree of differentiation in PDAC (Korompay et al. 2012). We also investigated the roles of TRIC as well as those of LSR in pancreatic cancer cells. Knockdown of TRIC by the siRNA decreased cell migration and the epithelial barrier. In human pancreatic cancer tissues, TRIC and LSR were localized at the cell membranes of normal pancreatic ducts and well-differentiated PDAC, whereas TRIC was not detected in poorly differentiated PDAC. TRIC in part may prevent the malignancy of pancreatic cancer.
In conclusion, our findings indicate that the loss of LSR, which regulates growth factors, promotes the malignancy of human pancreatic cancer cells via upregulation of AREG. Thus, it is possible that LSR may be a potential targeting molecule in therapy for human pancreatic cancer.
References
Angelow S, Ahlstrom R, Yu AS (2008) Biology of claudins. Am J Physiol Renal Physiol 295:867–876
Berasain C, Avila MA (2014) Amphiregulin. Semin Cell Dev Biol 28:31–41
Busser B, Sancey L, Brambilla E, Coll JL, Hurbin A (2011) The multiple roles of amphiregulin in human cancer. Biochim Biophys Acta 1816:119–131
Czulkies BA, Mastroianni J, Lutz L, Lang S, Schwan C, Schmidt G, Lassmann S, Zeiser R, Aktories K, Papatheodorou P (2017) Loss of LSR affects epithelial barrier integrity and tumor xenograft growth of CaCo2 cells. Oncotarget 8:37009–37022
Ebert M, Yokoyama M, Kobrin MS, Friess H, Lopez ME, Büchler MW, Johnson GR, Korc M (1994) Induction and expression of amphiregulin in human pancreatic cancer. Cancer Res 54:3959–3962
Furuse M, Tsukita S (2006) Claudins in occluding junctions of humans and flies. Trends Cell Biol 16:181–188
Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N (2014) Molecular organization of tricellular tight junctions. Tissue Barriers 2:e28960
Hashimoto Y, Tachibana K, Krug SM, Kunisawa J, Fromm M, Kondoh M (2019) Potential for tight junction protein-directed drug development using claudin binders and angubindin-1. Int J Mol Sci 20:E4016
Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, Furuse M (2013) Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci 126:966–977
Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S (2005) Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171:939–945
Korompay A, Borka K, Lotz G, Somorácz A, Törzsök P, Erdélyi-Belle B, Kenessey I, Baranyai Z, Zsoldos F, Kupcsulik P, Bodoky G, Schaff Z, Kiss A (2012) Tricellulin expression in normal and neoplastic human pancreas. Histopathology 60:E76–86
Krug SM, Hayaishi T, Iguchi D, Watari A, Takahashi A, Fromm M, Nagahama M, Takeda H, Okada Y, Sawasaki T, Doi T, Yagi K, Kondon M (2017) Angubindin-1, a novel paracellular absorption enhancer acting at the tricellular tight junction. J Control Release 260:1–11
Martin TA (2014) The role of tight junctions in cancer metastasis. Semin Cell Dev Biol 36:224–231
Martin TA, Jiang WG (2009) Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta 1788:872–891
Martin TA, Jordan N, Davies EL, Jiang WG (2016) Metastasis to bone in human cancer is associated with loss of occludin expression. Anticancer Res 36:1287–1293
Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M (2011) LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci 124:548–555
Michl P, Buchholz M, Rolke M, Kunsch S, Löhr M, McClane B, Tsukita S, Leder G, Adler G, Gress TM (2001) Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology 121:678–684
Nagahama M, Umezaki M, Oda M, Kobayashi K, Tone S, Suda T, Ishidoh K, Sakurai J (2011) Clostridium perfringens iota-toxin b induces rapid cell necrosis. Infect Immun 79:4353–4360
Rozengurt E, Sinnett-Smith J, Eibl G (2018) Yes-associated protein (YAP) in pancreatic cancer: at the epicenter of a targetable signaling network associated with patient survival. Signal Transduct Target Ther 3:11
Shimada H, Satohisa S, Kohno T, Takahashi S, Hatakeyama T, Konno T, Tsujiwaki M, Saito T, Kojima T (2016) The roles of tricellular tight junction protein lipolysis-stimulated lipoprotein receptor in malignancy of human endometrial cancer cells. Oncotarget 7:27735–27752
Shimada H, Satohisa S, Kohno T, Konno T, Takano KI, Takahashi S, Hatakeyama T, Arimoto C, Saito T, Kojima T (2017a) Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer. Oncol Lett 14:6776–6782
Shimada H, Abe S, Kohno T, Satohisa S, Konno T, Takahashi S, Hatakeyama T, Arimoto C, Kakuki T, Kaneko Y, Takano KI, Saito T, Kojima T (2017b) Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer. Sci Rep 7:37049
Takano K, Kakuki T, Obata K, Nomura K, Miyata R, Kondo A, Kurose M, Kakiuchi A, Kaneko Y, Kohno T, Himi T, Kojima T (2016) The behavior and role of lipolysis-stimulated lipoprotein receptor, a component of tricellular tight junctions, in head and neck squamous cell carcinomas. Anticancer Res 36:5895–5904
Takasawa A, Murata M, Takasawa K, Ono Y, Osanai M, Tanaka S, Nojima M, Kono T, Hirata K, Kojima T, Sawada N (2016) Nuclear localization of tricellulin promotes the oncogenic property of pancreatic cancer. Sci Rep 6:33582
Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S (2008) Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 27:6930–6938
Yotsumoto F, Fukami T, Yagi H, Funakoshi A, Yoshizato T, Kuroki M, Miyamoto S (2010) Amphiregulin regulates the activation of ERK and Akt through epidermal growth factor receptor and HER3 signals involved in the progression of pancreatic cancer. Cancer Sci 101:2351–2360
Zeniya S, Kuwahara H, Daizo K, Watari A, Kondoh M, Yoshida-Tanaka K, Kaburagi H, Asada K, Nagata T, Nagahama M, Yagi K, Yokota T (2018) Angubindin-1 opens the blood-brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J Control Release 283:126–134
Acknowledgements
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kyuno, T., Kyuno, D., Kohno, T. et al. Tricellular tight junction protein LSR/angulin-1 contributes to the epithelial barrier and malignancy in human pancreatic cancer cell line. Histochem Cell Biol 153, 5–16 (2020). https://doi.org/10.1007/s00418-019-01821-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-019-01821-4