Abstract
Cochlear hair cells are essential for the mechanotransduction of hearing. Sensorineural hearing loss can be irreversible because hair cells have a minimal ability to repair or regenerate themselves once damaged. In order to develop therapeutic interventions to prevent hair cell loss, it is necessary to understand the signaling pathway operating in cochlear hair cells and its alteration upon damage. Diacylglycerol kinase (DGK) regulates intracellular signal transduction through phosphorylation of lipidic second messenger diacylglycerol. We have previously reported characteristic expression and localization patterns of DGKs in various organs under pathophysiological conditions. Nevertheless, little is known about morphological and functional aspects of this enzyme family in the cochlea. First RT-PCR analysis reveals predominant mRNA expression of DGKα, DGKε and DGKζ. Immunohistochemical analysis shows that DGKζ localizes to the nuclei of inner hair cells (IHCs), outer hair cells (OHCs), supporting cells and spiral ganglion neurons in guinea pig cochlea under normal conditions. It is well known that loud noise exposure induces cochlear damage, thereby resulting in hair cell loss. In particular, OHCs are highly vulnerable to noise exposure than IHCs. We found that after 1 week of noise exposure DGKζ translocates from the nucleus to the cytoplasm in damage-sensitive OHCs and gradually disappears thereafter. In sharp contrast, DGKζ remains to the nucleus in damage-resistant IHCs. These results suggest that DGKζ cytoplasmic translocation is well correlated with cellular damage under noise-exposure stress conditions and is involved in delayed cell death in cochlear outer hair cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sensorineural hearing loss can be caused by multiple factors such as genetics, aminoglycosides, anticancer agents including cisplatin, loud noise or aging. Some of these factors cause damage to the cochlear hair cells which are essential for the mechanotransduction of hearing. In mammals, sound waves received by the tympanic membrane produce vibrations in the middle ear ossicles which are transmitted to the cochlea. Hair cells in the organ of Corti respond to subsequent vibrations of the basilar membrane of the cochlea and transmit information along the auditory pathway via spiral ganglion neurons to the auditory cortex where impulses are perceived as sound. Noise-induced hair cell damage is one of the key pathologies contributing to sensorineural hearing loss. While our understanding of the pathomechanisms behind sensorineural hearing loss is advancing, treatments have yet to be developed that can restore hearing function (Forge et al. 1993; Warchol et al. 1993). Hair cells are terminally differentiated and thus have minimal ability to repair or regenerate themselves once damaged. Oshima reported on the ability to induce otic progenitors from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) and succeeded in producing hair cell-like cells with stereociliary bundles (Oshima et al. 2010). Inhibition of Notch signaling has also been shown to induce cochlear hair cell formation after acoustic trauma, although hearing thresholds failed to show significant recovery (Mizutari et al. 2013). Despite extensive efforts towards regenerating hair cells, little or no success has been achieved in the clinical setting. Thus, a likely more fruitful avenue of research would be to elucidate the physiological factors and subcellular mechanisms behind hair cell loss. Identification of the signaling pathways regulating hair cell loss would facilitate the development of novel strategies for therapeutic interventions to prevent such loss.
Many researchers have looked into the molecular mechanisms of hair cell loss due to loud noise. Several studies suggest that noise exposure induces ischemia–reperfusion injury, which leads to an increase in reactive oxygen species (Miller et al. 2003; Quirk and Seidman 1995; Yamashita et al. 2004), and inflammatory cytokines (Tan et al. 2016; Fujioka et al. 2006) together with stress-responsive MAP kinase signaling (Jamesdaniel et al. 2011; Maeda et al. 2013).
Lipid metabolism is closely associated with intracellular signaling. It is worthy of note that the phosphoinositide (PI) signal transduction cascade is active in a wide variety of cells to yield a lipidic second messenger diacylglycerol (DG) (Rhee et al. 1989). DG activates proteins containing a DG-binding C1 domain, such as protein kinase C (PKC), which phosphorylates various proteins, thereby contributing to diverse cellular responses including growth, differentiation and apoptosis (Ron and Kazanietz 1999; Nishizuka 1992; Martelli et al. 2004). In this system, diacylglycerol kinase (DGK) phosphorylates DG to produce phosphatidic acid (PA) (Kanoh et al. 1990). PA binds and activates several proteins, such as mTOR (mammalian target of rapamycin), a master regulator of cell growth (Merida et al. 2008). Therefore, DGK regulates many kinds of intracellular signal transduction by metabolizing DG and PA. To date, ten mammalian DGK isozymes have been reported, DGKα (Sakane et al. 1990; Schaap et al. 1990), DGKβ (Goto and Kondo 1993), DGKγ (Goto et al. 1994; Kai et al. 1994), DGKδ (Sakane et al. 2002), DGKε (Tang et al. 1996), DGKζ (Bunting et al. 1996; Goto and Kondo 1996), DGKη (Murakami et al. 2003), DGKθ (Houssa et al. 1997), DGKι (Ding et al. 1998; Ito et al. 2004) and DGKκ (Imai et al. 2005). We and others have previously reported the functional implications of the DGK family in various tissues and cells under pathophysiological conditions (Goto et al. 2007; Topham 2006; Sakane et al. 2007). Intriguingly, each DGK isozyme exhibits distinct properties in terms of expression, enzymatic characteristics, physiological functions and regulatory mechanisms depending on tissue and cell type. Moreover, recent studies have suggested the involvement of the DGK family in various diseases (Sakane et al. 2016). However, little is known about the expression and localization of the DGK family, as well as their functional roles, in the cochlea.
In this study, we investigated the expression and localization of the DGK family in the cochlea. Using RT-PCR assays, abundant mRNA expression of DGKα, DGKε and DGKζ was detected. Immunohistochemistry using currently available antibodies for the various DGKs revealed that DGKζ localizes to the nuclei of inner hair cells (IHC), outer hair cells (OHC) and supporting cells (SC) in the cochlea, and to the nuclei of spiral ganglion neurons (SGNs), but not glial cells. Interestingly, we found that in a noise-induced damage model, DGKζ translocates from the nucleus to the cytoplasm of outer hair cells, which are vulnerable to noise exposure. This suggests that DGKζ is involved in the vulnerability of OHCs after noise exposure.
Materials and methods
Animals
Approximately 250–350 g, 4-week-old male Hartley strain guinea pigs were purchased from Kumagai-Shigeyasu Co., Ltd. and analyzed to investigate the expression of DGK isozymes. Three animals were assigned to the normal hearing group and three animals were assigned to the noise-exposure groups at three different time points: 24 h, 1 week and 2 weeks after noise exposure. For immunohistochemical analysis, three animals from the normal group and nine animals from the noise-exposure subgroups were investigated. The research protocol was approved by the Animal Research Committee of Yamagata University.
Anesthesia protocol
Animals were anesthetized with an intraperitoneal injection of a mixture of hydrochloric acid medetomidine (0.3 mg/kg), midazolam (4 mg/kg) and butorphanol tartrate (5 mg/kg). Animals were anesthetized prior to auditory brain stem response (ABR) recordings and tissue collection for both the normal and noise-exposed groups, as well as prior to noise exposure in the noise-exposed group.
Auditory brain stem response
All animals had their baseline ABR thresholds measured before tissue examination, and 24 h, 1 week and 2 weeks after noise exposure to confirm the threshold shifts in each subgroup. Tone burst stimuli with of 1 ms duration at frequencies of 4 kHz and 8 kHz were generated using a Tucker-Davis Technologies system (USA). Electrical responses were sampled via a needle electrode inserted subcutaneously on each postauricular side with a ground electrode placed on the back. Responses to 250 stimuli were averaged at each sound pressure level with PowerLab® software (AD Instruments, New South Wales, Australia). The ABR waveforms were recorded in 5 dB steps and the lowest stimulus level at which wave II could be identified reproducibly was defined as the hearing threshold.
Noise exposure
Animals were exposed to 4–8 kHz octave-band noise at 125 dB sound pressure level (SPL) for 3 h in a sound proof box under general anesthesia using a slightly modified protocol (Yamashita et al. 2004). The noise was generated by LabVIEW software® (National Instruments, Texas, USA) and amplified with an A100a power amplifier (Yamaha, Hamamatsu, Japan). The loudness of noise intensity was routinely reconfirmed using a noise level meter (Rion, Tokyo, Japan) prior to noise exposure.
Immunoblotting
Brains of adult guinea pigs and rats were homogenized with 4 volumes of a buffer containing 10 mM Tris–HCl (pH 7.4), 20 mM KCl, 0.1 mM EDTA and 0.25 M sucrose, and centrifuged at 1000g for 10 min at 4 °C to remove debris. Protein concentration was determined using BCA Protein Assay Reagent (Thermo Scientific, Rockford, USA). The resulting supernatants (20 µg) were boiled for 5 min in sodium dodecylsulfate (SDS) sample buffer (New England Biolabs, Inc., Beverly, USA) and subjected to 10% SDS–polyacrylamide gel electrophoresis (PAGE). The proteins were then electrophoretically transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, USA). After blocking the non-specific binding sites with 4% non-fat dry milk (w/v) in phosphate-buffered saline (PBS) containing 0.02% sodium azide and 0.2% Tween 20, the membrane was incubated for 1 h at room temperature with rabbit anti-DGKζ antibody (0.5 µg/ml) (Hozumi et al. 2003) in 2% non-fat dry milk (w/v) in PBS containing 0.02% sodium azide and 0.1% Tween 20. Sites of antigen–antibody reaction were visualized using the chemiluminescent Immobilon Western blotting detection system (Millipore).
Tissue and section preparation and cell count
Cochleae were harvested at 24 h, 1 week and 2 weeks after noise exposure. Transcardial perfusion was performed using 4% paraformaldehyde (PFA) in phosphate buffer saline (pH 7.4) under deep anesthesia followed by immediate decapitation. Both left and right cochleae were collected and immersed in 4% PFA overnight. The cochleae were incubated in 0.25 M EDTA (pH 7.4) using a microwave-based rapid decalcification device (Azumaya, Tokyo, Japan) for 3 days. After decalcification, cochleae were prepared for two different methods of analysis: cryosection and whole-mount. For analysis of cross-sections, cochleae from the right side were cryoprotected in 30% sucrose and cut at a thickness of 8 µm using a cryostat. The left cochleae were microdissected into eight pieces for whole-mount analysis. Cochlear frequency mapping was performed using an ImageJ plugin to identify the tonotopic placement. Two frequency regions corresponding to 4 and 8 kHz were analyzed to evaluate the changes in DGKζ expression. Approximately 30–40 OHCs are present per 10 IHCs in the cochleae. Using this reference value, the number of OHCs remaining after noise exposure was counted per 10 IHCs and subtracted from 35 to determine the rate of OHC loss. The rate of OHC loss was calculated by dividing the loss of OHCs by 35 to evaluate the severity of the damage.
Immunohistochemistry
Methanol treatment has been used to enhance the immunoreactivity of proteins in the PI signaling pathway (Hozumi et al. 2008). Thus, sections were treated with 100% methanol for 10 min at room temperature followed by blocking and permeabilization with 10% donkey serum and 0.3% triton X-100 in PBS. After blocking and permeabilization for 30 min at room temperature, the sections were incubated with primary antibodies overnight at 4 °C. The following primary antibodies were diluted with 5% donkey serum and 0.1% TritonX-100 in PBS: rabbit anti-DGKζ (1 µl/ml) (Hozumi et al. 2003), mouse monoclonal anti-Calmodulin (Sigma Aldrich, St Louis, USA; 1:100), goat polyclonal anti-Sox2 (Santa Cruz, Texas, USA; 1:200) and mouse monoclonal anti-Parvalbumin (Sigma Aldrich, St Louis, USA; 1:1000). The primary antibodies were visualized by incubation for 2 h at room temperature with species-specific secondary antibodies (Invitrogen, Oregon, USA; 1:500). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Dojindo, Mashiki, Japan). Specimens were imaged by confocal laser microscopy (LSM-700, Carl Zeiss, Gottinen, Germany).
Rt-pcr
Total RNA was extracted from the dissected cochleae using the TRIzol® Plus RNA purification Kit (Invitrogen, California, USA). Reverse transcription was carried out using the PrimeScript™ II 1st strand cDNA Synthesis Kit (TakaRa Bio Inc., Kusatsu, Japan). DGK isozymes were detected by PCR with Tks Gflex™ DNA polymerase (TakaRa Bio Inc., Kusatsu, Japan). The primers for guinea pig DGK isozymes are described in Table 1. The following PCR thermal cycling conditions were used: initial denaturation at 95 °C for 2 min, denaturation at 95 °C for 2 min, annealing and extension at 60 °C for 30 s for 30 cycles. PCR products were separated by electrophoresis using a 2% agarose gel stained with ethidium bromide.
Results
Expression and localization of DGKζ in normal guinea pig cochlea
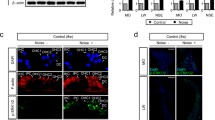
We first examined the expression profile of DGK isozymes at the mRNA level in normal guinea pig cochleae. RT-PCR analysis showed abundant expression of DGKα, DGKε and DGKζ in the cochlea (Fig. 1a). In contrast, DGKι was only weakly detected, whereas DGKβ and DGKγ signals were below the detection limit.
RT-PCR of DGK isozymes in normal guinea pig cochlea (a) and immunoblot of DGKζ (b). a Bands for DGK isozymes were amplified using specific primer sets (Table 1) for each isozyme and electrophoresed. Primers for β-actin and GAPDH were also used as controls. The mRNA signals are detected intensely for DGKα, DGKε, DGKζ and weakly for DGKι, whereas DGKβ and DGKγ are below the detection limit. The position of each band amplified and DNA size marker (M) are indicated in bp. b Immunoblot analysis of rabbit anti-DGKζ antibody in lysates (20 µg/lane) of guinea pig and rat brains. The position of standard protein markers is indicated to the left (kDa)
We next examined protein localization of the DGKs detected in the cochlea. To date, we have generated several DGK isozyme-specific antibodies (Hozumi et al. 2013) and performed immunohistochemical analysis on rat tissues and cells (Hozumi et al. 2010, 2015, 2017). Among these DGK isozymes, DGKζ is reported to be associated with cell death under stress conditions; thus we specifically focused on DGKζ for the detailed morphological analysis. In immunoblot analysis, the antibody recognized a single band of DGKζ at an estimated molecular mass (~ 104 kDa) in lysates of guinea pig and rat brains (Fig. 1b). Results show that this antibody specifically recognizes DGKζ protein in guinea pig and rat tissues and cells, although we failed to detect a band in guinea pig cochlea because of difficulties in obtaining adequate amounts of proteins from cochlea that is embedded in the temporal bone.
For the immunostaining, we analyzed two types of cochlear sample preparations: cryosections and whole-mount tissues. Cryosections were cut vertically relative to the basilar membrane, then adhered to glass slides, while whole-mount cochlear tissues, in which the organ of Corti was well preserved, were directly incubated in solution for immunostaining. Confocal images from whole-mount samples were oriented horizontal to the basilar membrane, which provided clear views of the IHC and OHC arrays in the same location and plane.
Figure 2 shows an image of a vertical cryosection from normal guinea pig organ of Corti. The plasma membranes and cuticular plates of both the IHCs and OHCs are clearly delineated by immunostaining for the hair cell marker calmodulin (Slepecky and Ulfendahl 1993). DGKζ-immunoreactivity was principally observed in the nuclei of IHCs, OHCs and SCs (Fig. 2). Horizontal images of whole-mount organ of Corti preparations provided well-preserved and clear images (Fig. 3a–c). IHC nuclei exhibited intense DGKζ-immunoreactivity (Fig. 3a), whereas OHC nuclei showed moderate staining (Fig. 3b, c). SCs, which are labeled by Sox2 and are situated beneath the hair cell layer, also exhibited nuclear DGKζ-immunoreactivity (Fig. 4). In addition, the nuclei of parvalbumin-positive SGNs showed DGKζ immunoreactivity, although the glial cells did not (Fig. 5).
Localization of DGKζ in the organ of Corti under normal conditions. Vertical cryosections made from normal guinea pig cochleae were subjected to immunohistochemical analysis. Note that DGKζ-immunoreactivity is detected in the nuclei of inner hair cells (IHCs) and outer hair cells (OHCs), both of which are labeled with calmodulin staining. Supporting cells (SCs) are also immunoreactive for D DGKζ. Nuclei were stained by DAPI. A schematic representation of the immunohistochemical results in the organ of Corti is shown above. O1, O2, O3; three rows of OHCs. Scale bar: 10 µm
DGKζ localizes to the nuclei of inner and outer hair cells. Horizontal images were taken at the levels a–c of normal guinea pig whole-mount organ of Corti immunostained for DGKζ and calmodulin. Note that DGKζ-immunoreactivity is observed in the nuclei of calmodulin-positive IHCs and OHCs. A schematic representation of the immunohistochemical results is shown above. Scale bar: 10 µm
DGKζ localizes to the nuclei of supporting cells (SCs) of the organ of Corti. Horizontal image was taken at the level of SC nucleus layer of normal guinea pig whole-mount organ of Corti immunostained for DGKζ and Sox2 (a marker for SCs). A schematic representation of the immunohistochemical results is shown above. Scale bar: 10 µm
Subcellular localization of DGKζ within the cochlea after noise exposure
We next investigated how cochlear expression and localization of DGKζ changes after exposure to loud noise. The noise-exposure group showed elevated hearing thresholds over 60 dB SPL and the rate of OHC loss was 60–70% after 24 h and tended to increase slightly (~ 80%) 2 weeks after noise exposure at two tonotopic regions (4 and 8 kHz; Fig. 6), which is consistent with the previous study (Yamashita et al. 2004).
Rate of OHC loss at each time point after noise exposure. 24 h after noise exposure, 59.3% of OHCs in the 4 kHz region and 74.3% in the 8 kHz region were degenerated. 2 weeks after noise exposure, the rate of OHC loss was increased to 76.5% in the 4 kHz region and 80.6% in the 8 kHz region. Error bars indicate standard error (n = 4)
DGKζ-immunoreactivity remained unchanged in the nuclei of IHCs and OHCs at 24 h (Fig. 7); however, distinct changes in immunoreactivity were detected in these cells at 1 week post-noise (Fig. 8). In IHCs, DGKζ-immunoreactivity remained in the nucleus. In most of the OHCs, however, DGKζ-immunoreactivity was detected as particulate aggregates in the cytoplasm (arrows in Fig. 8c), suggesting cytoplasmic translocation of DGKζ in these cells. At 2 weeks post-noise, the immunoreactivity almost disappeared in the remaining calmodulin-positive OHCs (Fig. 9c). On the other hand, DGKζ-immunoreactivity remained intense in the nuclei of IHCs after post-noise (Fig. 9a, b), comparable to that observed under normal conditions.
Localization of DGKζ in the organ of Corti 24 h after noise exposure. Horizontal images were taken at the levels a–c of guinea pig whole-mount organ of Corti immunostained for DGKζ and calmodulin after 24 h of noise exposure. Note that DGKζ-immunoreactivity remains in the nuclei of calmodulin-positive IHCs and OHCs, to an extent similar to that observed under normal conditions in Fig. 3. A schematic representation of the immunohistochemical results is shown above. Scale bar: 10 µm
Localization of DGKζ in the organ of Corti 1 week after noise exposure. Horizontal images were taken at the levels a–c of guinea pig whole-mount organ of Corti immunostained for DGKζ and calmodulin after 1 week of noise exposure. DGKζ-immunoreactivity remains in the nuclei of calmodulin-positive IHCs. By contrast, in most of the OHCs the immunoreactivity is detected as aggregates in the cytoplasm (arrows). Higher magnification image of the square is also shown in the inset. A schematic representation of the immunohistochemical results is shown above. Scale bar: 10 µm
Localization of DGKζ in the organ of Corti 2 weeks after noise exposure. Horizontal images were taken at the levels a–c of guinea pig whole-mount organ of Corti immunostained for DGKζ and calmodulin after 2 weeks of noise exposure. DGKζ-immunoreactivity is still observed in the nuclei of calmodulin-positive IHCs. However, the immunoreactivity is significantly attenuated or almost disappears from the remaining calmodulin-positive OHCs (arrowheads). A schematic representation of the immunohistochemical results is shown above. Scale bar: 10 µm
Taken together, these results suggest that loud noise exposure induces significant changes in the subcellular localization of DGKζ within OHCs, which are particularly vulnerable to noise-induced stress. In response to this stress, DGKζ may be translocated from the nucleus to the cytoplasm of OHCs then gradually disappear over time. In contrast, IHCs are much more resistant to noise-induced stress and their nuclear DGKζ-immunoreactivity remained mostly unchanged after noise exposure.
Discussion
This study is the first report to investigate the expression and localization of DGK isozymes in the cochlea. We found that the mRNA transcripts for DGKα, DGKε and DGKζ are abundantly expressed, whereas DGKι mRNA expression level is weak and DGKβ and DGKγ levels are below the detection limit in normal guinea pig cochlea. Subsequent immunohistochemical investigations found that DGKζ-immunoreactivity is observed principally in the nuclei of IHCs and OHCs, along with SC and SGN nuclei under normal conditions. These findings are consistent with previous studies that showed that DGKζ localizes to the nuclei of many cell types under normal conditions, including postmitotic neurons (Goto and Kondo 1996). In terms of subcellular localization, previous studies also show that DGKζ shuttles between the nucleus and the cytoplasm under some pathological conditions (Goto et al. 2014), which may be based on the presence of both a nuclear localization signal (NLS) and nuclear export signal (NES) in its primary structure (Evangelisti et al. 2010).
It is well known that loud noise exposure induces cochlear damage, thereby resulting in hair cell loss. In particular, OHCs are highly vulnerable to noise exposure than IHCs. Under the noise-exposure conditions used in this study, 60–70% of OHCs were lost by 24 h after noise exposure, and 80% were lost by 2 weeks after noise exposure, as indicated by the disappearance of immunostaining for the hair cell marker calmodulin. This suggests that an additional 10–20% of cells gradually die within the 2 weeks following noise exposure. Our result is compatible with that of the previous study (Yamashita et al. 2004), which suggests that immediate OHC loss by 24 h may primarily reflect direct mechanical damage, while slowly progressing OHC loss is caused by continued formation of reactive oxygen species in response to noise exposure.
In this regard, we found that 24 h after noise exposure, DGKζ-immunoreactivity remains in the nuclei of inner and outer hair cells that were immunoreactive for calmodulin. It should be noted, however, that 1 week after noise exposure the subcellular localization of DGKζ-immunoreactivity shifts from the nucleus to the cytoplasm in damage-sensitive OHCs, whereas DGKζ-immunoreactivity remains in the nuclei of damage-resistant IHCs that were intensely immunoreactive for calmodulin. These results suggest that the nucleocytoplasmic translocation of DGKζ occurs gradually in OHCs during the first week after noise exposure. The retention of DGKζ in the nucleus of intact IHC and the cytoplasmic translocation in damaged OHCs raise the possibility that the subcellular localization of DGKζ after noise exposure is correlated with cellular viability.
The correlation between DGKζ translocation and cellular damage in the present model of cochlear noise exposure resembles that observed in the hippocampus in a model of transient ischemia. In that model, DGKζ translocates from the nucleus to the cytoplasm in hippocampal CA1 neurons immediately after a 20-min ischemic induction (Ali et al. 2004). This is specifically observed in CA1 neurons, but not in other hippocampal areas. In addition, the nucleocytoplasmic translocation of DGKζ is also reported in CA1 neurons after 2 h of limbic seizure induced by kainate injection (Saino-Saito et al. 2011). In this regard, it is reported that CA1 neurons show selective vulnerability to stress, such as transient ischemia, and experience a delayed cell death within a couple of days (Kirino 1982). All these models, including noise-induced and ischemic stress conditions, are shown to engender glutamate excitotoxicity, which leads to overstimulation of glutamate receptors and a resultant massive influx of calcium that culminates in catastrophic consequences (Lau and Tymianski 2010). Furthermore, hippocampal slice culture experiments using oxygen–glucose deprivation (OGD)-reperfusion conditions, which mimic ischemia–reperfusion in animals, recapitulates this DGKζ nucleocytoplasmic translocation (Suzuki et al. 2012).
Pharmacological approaches further revealed that DGKζ nucleocytoplasmic translocation is triggered by NMDA-type glutamate receptor activation and subsequent calcium influx and that the NMDA receptor antagonist AP4 inhibits its nucleocytoplasmic translocation under OGD-reperfusion conditions. Following transient ischemia-induced cytoplasmic translocation in CA1 neurons, DGKζ never relocates to the nucleus, which results in neuronal apoptosis within several hours to days following excessive calcium influx.
A similar phenomenon has also been reported in other cell types besides neurons. β cells of the pancreatic islets of Langerhans are responsible for insulin production in response to high blood glucose levels to facilitate glucose uptake. Dysregulation of this homeostasis results in diabetes mellitus, which is caused by β cell degeneration or insulin signal resistance. Streptozotocin is an agent that causes selective degeneration of β cells and is often used to induce experimental diabetes (Schnedl et al. 1994). Interestingly, under streptozotocin-induced stress conditions, β cells gradually degenerate with increasing levels of blood glucose, during which DGKζ is found to translocate from the nucleus to the cytoplasm and then subsequently disappear (Hozumi et al. 2016).
The present study revealed that DGKζ cytoplasmic translocation also occurs in cochlear OHCs that are vulnerable to noise-induced stress. DGKζ was translocated from the nucleus to the cytoplasm in remaining OHCs 1 week after noise exposure when the rate of OHC loss continued to increase. Some OHCs then lost their DGKζ-immunoreactivity 2 weeks after noise exposure. Thus, in all three stress models (hippocampus, pancreas and cochlea), DGKζ cytoplasmic translocation is observed prior to cell death, even though the input stress may differ between these models. How exactly is cytoplasmic translocation of DGKζ implicated in the stress responses of cells?
What is the functional significance of cytoplasmic translocation of DGKζ? Previously, we have reported that cytoplasmic DGKζ anchors p53 and facilitates its degradation through the ubiquitin–proteasome system in the cytoplasm, thereby attenuating p53-mediated cytotoxicity (Tanaka et al. 2013; Goto et al. 2014). This suggests that cytoplasmic translocation of DGKζ exerts cytoprotective effect under stress conditions. On the other hand, it is also shown that nuclear DGKζ interacts with retinoblastoma protein (pRB), a tumor suppressor and cell cycle regulator (Los et al. 2006). Dephosphorylation of pRB facilitated by nuclear DGKζ causes direct binding to E2F, thereby repressing the transcription of genes required for cell cycle progression (Evangelisti et al. 2009, 2010). Prolonged absence of nuclear DGKζ caused by cytoplasmic translocation under stress conditions leads to increased pRB phosphorylation, thereby liberating E2F from its complex. E2F then upregulates the expression of type D and E cyclins which facilitate cell cycle progression, while cytoplasmic DGKζ is degraded via the cytoplasmic ubiquitin–proteasome system (Okada et al. 2012). In this regard, cell cycle reentry in postmitotic neurons, which is referred to as aberrant cell cycle reentry, leads to DNA replication without entering M phase, resulting in unusual conditions in which cells have twice the amount of DNA content (Herrup and Yang 2007). Mammalian hair cells are also postmitotic and a previous study reported that forced cell cycle reentry induces OHC death (Sulg et al. 2010). Thus, cytoplasmic translocation of DGKζ may also induce aberrant cell cycle reentry in noise-exposed OHCs, thereby culminating in cell death. Further studies are needed to investigate and confirm this molecular mechanism. Interventions aimed at transiently suppressing DGKζ cytoplasmic translocation could be therapeutically efficacious for preventing the delayed hair cell loss that occurs after various kinds of stress, including exposure to loud noise.
Abbreviations
- DGK:
-
Diacylglycerol kinase
- IHC:
-
Inner hair call
- OHC:
-
Outer hair cell
- SC:
-
Supporting cell
- SGN:
-
Spiral ganglion neuron
References
Ali H, Nakano T, Saino-Saito S, Hozumi Y, Katagiri Y, Kamii H, Sato S, Kayama T, Kondo H, Goto K (2004) Selective translocation of diacylglycerol kinase zeta in hippocampal neurons under transient forebrain ischemia. Neurosci Lett 372:190–195. https://doi.org/10.1016/j.neulet.2004.09.052
Bunting M, Tang W, Zimmerman GA, McIntyre TM, Prescott SM (1996) Molecular cloning and characterization of a novel human diacylglycerol kinase zeta. J Biol Chem 271:10230–10236
Ding L, Traer E, McIntyre TM, Zimmerman GA, Prescott SM (1998) The cloning and characterization of a novel human diacylglycerol kinase, DGKiota. J Biol Chem 273:32746–32752
Evangelisti C, Astolfi A, Gaboardi GC, Tazzari P, Pession A, Goto K, Martelli AM (2009) TIS21/BTG2/PC3 and cyclin D1 are key determinants of nuclear diacylglycerol kinase-zeta-dependent cell cycle arrest. Cell Signal 21:801–809
Evangelisti C, Gaboardi GC, Billi AM, Ognibene A, Goto K, Tazzari PL, McCubrey JA, Martelli AM (2010) Identification of a functional nuclear export sequence in diacylglycerol kinase-zeta. Cell Cycle 9:384–388. https://doi.org/10.4161/cc.9.2.10469
Forge A, Li L, Corwin JT, Nevill G (1993) Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 259:1616–1619
Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H (2006) Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res 83:575–583. https://doi.org/10.1002/jnr.20764
Goto K, Kondo H (1993) Molecular cloning and expression of a 90-kDa diacylglycerol kinase that predominantly localizes in neurons. Proc Natl Acad Sci USA 90:7598–7602
Goto K, Kondo H (1996) A 104-kDa diacylglycerol kinase containing ankyrin-like repeats localizes in the cell nucleus. Proc Natl Acad Sci USA 93:11196–11201
Goto K, Funayama M, Kondo H (1994) Cloning and expression of a cytoskeleton-associated diacylglycerol kinase that is dominantly expressed in cerebellum. Proc Natl Acad Sci USA 91:13042–13046
Goto K, Hozumi Y, Nakano T, Saino SS, Kondo H (2007) Cell biology and pathophysiology of the diacylglycerol kinase family: morphological aspects in tissues and organs. Int Rev Cytol 264:25–63. https://doi.org/10.1016/s0074-7696(07)64002-9
Goto K, Tanaka T, Nakano T, Okada M, Hozumi Y, Topham MK, Martelli AM (2014) DGKzeta under stress conditions: “To be nuclear or cytoplasmic, that is the question”. Adv Biol Regul 54:242–253. https://doi.org/10.1016/j.jbior.2013.08.007
Herrup K, Yang Y (2007) Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8:368–378. https://doi.org/10.1038/nrn2124
Houssa B, Schaap D, van der Wal J, Goto K, Kondo H, Yamakawa A, Shibata M, Takenawa T, van Blitterswijk WJ (1997) Cloning of a novel human diacylglycerol kinase (DGKtheta) containing three cysteine-rich domains, a proline-rich region, and a pleckstrin homology domain with an overlapping Ras-associating domain. J Biol Chem 272:10422–10428
Hozumi Y, Ito T, Nakano T, Nakagawa T, Aoyagi M, Kondo H, Goto K (2003) Nuclear localization of diacylglycerol kinase zeta in neurons. Eur J Neurosci 18:1448–1457
Hozumi Y, Fukaya M, Adachi N, Saito N, Otani K, Kondo H, Watanabe M, Goto K (2008) Diacylglycerol kinase beta accumulates on the perisynaptic site of medium spiny neurons in the striatum. Eur J Neurosci 28:2409–2422. https://doi.org/10.1111/j.1460-9568.2008.06547.x
Hozumi Y, Watanabe M, Goto K (2010) Signaling cascade of diacylglycerol kinase beta in the pituitary intermediate lobe: dopamine D2 receptor/phospholipase Cbeta4/diacylglycerol kinase beta/protein kinase Calpha. J Histochem Cytochem 58:119–129. https://doi.org/10.1369/jhc.2009.954347
Hozumi Y, Matsui H, Sakane F, Watanabe M, Goto K (2013) Distinct expression and localization of diacylglycerol kinase isozymes in rat retina. J Histochem Cytochem 61:462–476. https://doi.org/10.1369/0022155413483574
Hozumi Y, Akimoto R, Suzuki A, Otani K, Watanabe M, Goto K (2015) Expression and localization of the diacylglycerol kinase family and of phosphoinositide signaling molecules in adrenal gland. Cell Tissue Res 362:295–305. https://doi.org/10.1007/s00441-015-2199-3
Hozumi Y, Nakano T, Tanaka T, Goto K (2016) Localization of diacylglycerol kinase zeta in rat pancreatic islet cells under normal and streptozotocin-induced stress conditions. Arch Histol Cytol 76:23–33. https://doi.org/10.1679/aohc.76.23
Hozumi Y, Fujiwara H, Kaneko K, Fujii S, Topham MK, Watanabe M, Goto K (2017) Diacylglycerol kinase epsilon localizes to subsurface cisterns of cerebellar Purkinje cells. Cell Tissue Res 368:441–458. https://doi.org/10.1007/s00441-017-2579-y
Imai S, Kai M, Yasuda S, Kanoh H, Sakane F (2005) Identification and characterization of a novel human type II diacylglycerol kinase, DGK kappa. J Biol Chem 280:39870–39881. https://doi.org/10.1074/jbc.M500669200
Ito T, Hozumi Y, Sakane F, Saino-Saito S, Kanoh H, Aoyagi M, Kondo H, Goto K (2004) Cloning and characterization of diacylglycerol kinase iota splice variants in rat brain. J Biol Chem 279:23317–23326. https://doi.org/10.1074/jbc.M312976200
Jamesdaniel S, Hu B, Kermany MH, Jiang H, Ding D, Coling D, Salvi R (2011) Noise induced changes in the expression of p38/MAPK signaling proteins in the sensory epithelium of the inner ear. J Proteomics 75:410–424. https://doi.org/10.1016/j.jprot.2011.08.007
Kai M, Sakane F, Imai S, Wada I, Kanoh H (1994) Molecular cloning of a diacylglycerol kinase isozyme predominantly expressed in human retina with a truncated and inactive enzyme expression in most other human cells. J Biol Chem 269:18492–18498
Kanoh H, Yamada K, Sakane F (1990) Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci 15:47–50
Kirino T (1982) Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res 239:57–69
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542. https://doi.org/10.1007/s00424-010-0809-1
Los AP, Vinke FP, de Widt J, Topham MK, van Blitterswijk WJ, Divecha N (2006) The retinoblastoma family proteins bind to and activate diacylglycerol kinase zeta. J Biol Chem 281:858–866. https://doi.org/10.1074/jbc.M502693200
Maeda Y, Fukushima K, Omichi R, Kariya S, Nishizaki K (2013) Time courses of changes in phospho- and total- MAP kinases in the cochlea after intense noise exposure. PLoS One 8:e58775. https://doi.org/10.1371/journal.pone.0058775
Martelli AM, Fala F, Faenza I, Billi AM, Cappellini A, Manzoli L, Cocco L (2004) Metabolism and signaling activities of nuclear lipids. Cell Mol Life Sci 61:1143–1156. https://doi.org/10.1007/s00018-004-3414-7
Merida I, Avila-Flores A, Merino E (2008) Diacylglycerol kinases: at the hub of cell signalling. Biochem J 409:1–18. https://doi.org/10.1042/BJ20071040
Miller JM, Brown JN, Schacht J (2003) 8-Iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neurootol 8:207–221. https://doi.org/10.1159/000071061
Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, Okano H, Edge AS (2013) Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77:58–69. https://doi.org/10.1016/j.neuron.2012.10.032
Murakami T, Sakane F, Imai S, Houkin K, Kanoh H (2003) Identification and characterization of two splice variants of human diacylglycerol kinase eta. J Biol Chem 278:34364–34372. https://doi.org/10.1074/jbc.M301542200
Nishizuka Y (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258:607–614
Okada M, Hozumi Y, Tanaka T, Suzuki Y, Yanagida M, Araki Y, Evangelisti C, Yagisawa H, Topham MK, Martelli AM, Goto K (2012) DGKzeta is degraded through the cytoplasmic ubiquitin-proteasome system under excitotoxic conditions, which causes neuronal apoptosis because of aberrant cell cycle reentry. Cell Signal 24:1573–1582. https://doi.org/10.1016/j.cellsig.2012.03.021
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S (2010) Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141:704–716. https://doi.org/10.1016/j.cell.2010.03.035
Quirk WS, Seidman MD (1995) Cochlear vascular changes in response to loud noise. Am J Otol 16:322–325
Rhee SG, Suh PG, Ryu SH, Lee SY (1989) Studies of inositol phospholipid-specific phospholipase C. Science 244:546–550
Ron D, Kazanietz MG (1999) New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB journal: FASEB J 13:1658–1676
Saino-Saito S, Hozumi Y, Goto K (2011) Excitotoxicity by kainate-induced seizure causes diacylglycerol kinase zeta to shuttle from the nucleus to the cytoplasm in hippocampal neurons. Neurosci Lett 494:185–189. https://doi.org/10.1016/j.neulet.2011.02.062
Sakane F, Kanoh H, Yokoyama C, Tanabe T (1990) Porcine diaglycerol kinase sequence has zinc finger and E-F hand motifs. Nature 344:345–348
Sakane F, Imai S, Yamada K, Murakami T, Tsushima S, Kanoh H (2002) Alternative splicing of the human diacylglycerol kinase delta gene generates two isoforms differing in their expression patterns and in regulatory functions. J Biol Chem 277:43519–43526. https://doi.org/10.1074/jbc.M206895200
Sakane F, Imai S, Kai M, Yasuda S, Kanoh H (2007) Diacylglycerol kinases: why so many of them? Biochim Biophys Acta 1771:793–806. https://doi.org/10.1016/j.bbalip.2007.04.006
Sakane F, Mizuno S, Komenoi S (2016) Diacylglycerol kinases as emerging potential drug targets for a variety of diseases: an update. Front Cell Dev Biol 4:82. https://doi.org/10.3389/fcell.2016.00082
Schaap D, de Widt J, van der Wal J, Vandekerckhove J, van Damme J, Gussow D, Ploegh HL, van Blitterswijk WJ, van der Bend RL (1990) Purification, cDNA-cloning and expression of human diacylglycerol kinase. FEBS Lett 275:151–158. https://doi.org/10.1016/0014-5793(90)81461-V
Schnedl WJ, Ferber S, Johnson JH, Newgard CB (1994) STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes 43:1326–1333
Slepecky NB, Ulfendahl M (1993) Evidence for calcium-binding proteins and calcium-dependent regulatory proteins in sensory cells of the organ of Corti. Hear Res 70:73–84
Sulg M, Kirjavainen A, Pajusola K, Bueler H, Ylikoski J, Laiho M, Pirvola U (2010) Differential sensitivity of the inner ear sensory cell populations to forced cell cycle re-entry and p53 induction. J Neurochem 112:1513–1526. https://doi.org/10.1111/j.1471-4159.2009.06563.x
Suzuki Y, Yamazaki Y, Hozumi Y, Okada M, Tanaka T, Iseki K, Ohta N, Aoyagi M, Fujii S, Goto K (2012) NMDA receptor-mediated Ca(2+) influx triggers nucleocytoplasmic translocation of diacylglycerol kinase zeta under oxygen-glucose deprivation conditions, an in vitro model of ischemia, in rat hippocampal slices. Histochem Cell Biol 137:499–511. https://doi.org/10.1007/s00418-011-0907-y
Tan WJ, Thorne PR, Vlajkovic SM (2016) Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem Cell Biol 146:219–230. https://doi.org/10.1007/s00418-016-1436-5
Tanaka T, Okada M, Hozumi Y, Tachibana K, Kitanaka C, Hamamoto Y, Martelli AM, Topham MK, Iino M, Goto K (2013) Cytoplasmic localization of DGKzeta exerts a protective effect against p53-mediated cytotoxicity. J Cell Sci 126:2785–2797. https://doi.org/10.1242/jcs.118711
Tang W, Bunting M, Zimmerman GA, McIntyre TM, Prescott SM (1996) Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J Biol Chem 271:10237–10241
Topham MK (2006) Signaling roles of diacylglycerol kinases. J Cell Biochem 97:474–484. https://doi.org/10.1002/jcb.20704
Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT (1993) Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 259:1619–1622
Yamashita D, Jiang HY, Schacht J, Miller JM (2004) Delayed production of free radicals following noise exposure. Brain Res 1019:201–209. https://doi.org/10.1016/j.brainres.2004.05.104
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP15K20178 to HM, JP15K20179 to CS and JP17K11313 to TI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shinkawa, C., Ito, T., Hozumi, Y. et al. Expression and localization of diacylglycerol kinase ζ in guinea pig cochlea and its functional implication under noise-exposure stress conditions. Histochem Cell Biol 151, 461–474 (2019). https://doi.org/10.1007/s00418-019-01781-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-019-01781-9