Abstract
Aminoglycoside ototoxicity results in permanent loss of the sensory hair cells in the mammalian cochlea. It usually begins at the basal turn causing high-frequency hearing loss. Here we describe previously unreported resistance of hair cells to neomycin ototoxicity in the extreme basal (hook) region of the developing cochlea of the C57BL/6 mouse. Organ of Corti explants from mice at postnatal day 3 were incubated (37 °C, 5% CO2) in normal culture medium for 19.5 h prior to and after exposure to neomycin (1 mM, 3 h). To study neomycin uptake in the hair cells, cochlear explants were incubated with Neomycin Texas-red (NTR) conjugate. As expected, exposure to neomycin significantly reduced the survival of inner (IHC) and outer hair cells (OHC). IHC survival rate was high in the apical segment and low in the basal segment. OHC were well preserved in the apical and hook regions, with substantial OHC loss in the basal segment. The NTR uptake study demonstrated that the high survival rate in the extreme basal turn OHC was associated with low NTR uptake. Treatment with a calcium chelator (BAPTA), which disrupts the opening of mechanoelectrical (MET) transduction channels, abolished or reduced NTR uptake in the hair cells throughout the cochlea. This confirmed the essential role of MET channels in neomycin uptake and implied that the transduction channels could be impaired in the hook region of the developing mouse cochlea, possibly as a result of the cadherin 23 mutation responsible for the progressive deafness in C57BL/6 mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aminoglycosides are broad spectrum antibiotics with a low rate of bacterial resistance that are highly effective against Gram-negative bacteria, including those causing tuberculosis, endocarditis, urinary tract infections and pneumonia (Rybak and Ramkumar 2007). This led to their popular use in the 1940s and 1950s, despite the report of ototoxicity and nephrotoxicity during the initial clinical evaluation (Hinshaw and Feldman 1945). The widespread use of aminoglycosides in the early years saw a large number of patients suffering from auditory and vestibular deficits. Over the next few decades, new aminoglycosides were discovered or synthesised, but the ototoxic side effects remained, which led to the decline in the use of aminoglycosides in most countries in the 1970s (Forge and Schacht 2000). Nevertheless, aminoglycoside antibiotics are still among the most commonly prescribed antibiotics in life-threatening situations. In the developing world, the low cost of production of aminoglycosides and its potent antibacterial activities outrival newer and safer antibiotics, which results in a high incidence of ototoxicity in these countries (Forge and Schacht 2000).
Animal studies demonstrate that aminoglycosides, such as neomycin, gentamycin, kanamycin and tobramycin, primarily damage the sensory hair cells of the cochlea (Forge and Schacht 2000). In mammals, these cells do not regenerate, and their loss leads to permanent hearing disability. Prolonged exposure to aminoglycosides causes progressive inner and outer hair cell loss predominantly in the basal turn (Karasawa and Steyger 2011). The mechanoelectrical transduction channels (MET channels) located at the tip of the hair cell stereocilia are the primary route for aminoglycoside entry into the sensory hair cells (Marcotti et al. 2005; Alharazneh et al. 2011). Additional routes for aminoglycoside trafficking into the hair cells may also involve endocytosis (Hashino and Shero 1995) and transient receptor potential channels (Corey et al. 2004; Lee et al. 2013). The principal mechanism of cochlear injury by aminoglycosides appears to be oxidative stress, which induces sensory cell death by apoptosis or necrosis. Inside the hair cells, aminoglycoside–iron complexes react with electron donors, such as arachidonic acid, to form reactive oxygen species (ROS) (Poirrier et al. 2010). ROS can activate stress-activated protein kinase JNK, which translocates to the nucleus to activate downstream genes involved in the cell death pathways (Poirrier et al. 2010; Karasawa and Steyger 2011). The excessive ROS production will eventually exhaust endogenous antioxidant systems, with downstream pathological effects which include mitochondrial calcium overload, release of cytochrome c and activation of caspase-dependent or caspase-independent cell death pathways (Pinton et al. 2008; Karasawa and Steyger 2011).
There are a number of different animal models of aminoglycoside ototoxicity, but unfortunately there is substantial variability among mammalian species, with mice and rats relatively resistant compared to guinea pigs (Wu et al. 2001), making the choice of animal model challenging. Furthermore, the increasing number of knockout and transgenic mouse models, which enable manipulation of injury pathways, necessitates the development of murine models of ototoxicity to investigate drug-related injury mechanisms and intracellular signalling pathways leading to cell death. The development of organotypic culture models has further improved the reproducibility of ototoxic studies, even though these models can be developed only in pre-hearing animals, and culture conditions remove the natural environment of the sensory hair cells. Despite these limitations, cochlear explant studies using rat and mouse tissues have significantly advanced our understanding of pathological processes affecting the survival of sensory hair cells (Karasawa and Steyger 2011). It is now well-established that the aminoglycoside-induced loss of sensory hair cells occurs in a dose-dependent manner, commencing in the basal turn and progressing towards the apex of the cochlea (Richardson and Russell 1991; Löwenheim et al. 1999). The underlying cause of this turn-specific hair cell susceptibility to aminoglycosides is unclear, but it could be linked to the base-to-apex antioxidant gradient (Sha et al. 2001).
We have recently observed an atypical pattern of neomycin ototoxicity in organotypic cultures of the C57BL/6 mouse cochlea, characterised by the resistance of the hair cells in the extreme basal turn (hook region) to neomycin toxicity. The present study aimed to elucidate the turn-related susceptibility of sensory hair cells to neomycin in this mouse strain using the fluorescently tagged neomycin analogue neomycin-Texas red (NTR). Here, we demonstrate differential NTR uptake in the sensory hair cells of the C57BL/6 mouse cochlea and the strong correlation between NTR uptake and turn-specific differences in hair cell survival.
Materials and methods
Animals
C57BL/6 mice at postnatal day 3 (P3) were used in this study. All experimental procedures were approved by the University of Auckland Animal Ethics Committee. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Organ of corti tissue culture
P3 mouse pups were decapitated and auditory bullae removed. The cochleae were decapsulated in ice-cold dissection solution, a modified artificial perilymph with the following composition: NaCl, 154 mM; KCl, 5.8 mM; HEPES, 10 mM; NaH2PO4; 0.7 mM; d-glucose, 10 mM; MgCl2.6H2O, 0.9 mM; sodium pyruvate, 2 mM; MEM amino acids, 10 mM; MEM vitamins, 1% v/v and CaCl2.2H2O, 1.3 mM. The sensory epithelium was micro-dissected and adhered to the coverslip using rat tail collagen (2.4 mg/mL; Thermo Fisher, MA, USA). The top of the spiral ligament was partially removed and pushed away from the sensory epithelium, Reissner’s membrane was flattened in the opposite direction, whilst the tectorial membrane was gently removed to gain access to the organ of Corti. The culture medium was Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher) and Earle’s Balanced Salt Solution (EBSS, Thermo Fisher) used in a 2:1 ratio with addition of 5% of fetal bovine serum (FBS, Gibco Invitrogen) and 30 units/mL of penicillin G (Sigma Aldrich). The cochlear explants were then transferred to a cell culture incubator and maintained at 37 °C with 5% CO2. Tissue was maintained in culture medium for 42 h, comprising a 19.5 h pre-incubation, 3 h neomycin (1 mM, Sigma Aldrich), followed by a 19.5 h post-incubation period as described previously (Lin 2017; Lin et al. submitted) to maximise hair cell survival in tissue culture conditions, and ensure sufficient level of sensory cell death from neomycin. Neomycin concentrations and exposure times were titrated in a pilot study (data not shown) to obtain a hair cell survival rate of approximately 50%. Pre-incubation was used stabilise tissue preparation in culture conditions (Wang et al. 2003; Lowenheim et al. 1999), and the post-incubation period provided a timeframe for sensory cell death and repair of the reticular lamina.
Hair cell counting
After incubation in normal culture medium with or without neomycin, cochlear explants were fixed with 4% PFA for 30 min at room temperature (RT) and washed with 0.1M PBS. Permeabilisation with 1% Triton X-100 in 0.1M PBS was carried out at RT for 1 h and the cochlear explants were stained with Alexa Fluor 488-phalloidin (1:600, 1 h, RT) to visualise sensory hair cells. Images were captured using an Eclipse 80i Nikon microscope (Japan) with a 20× objective at high resolution (2560 × 1920 pixels). A series of images were taken along the entire length of the cochlea (from the apex to the base) and merged using Photoshop. Every single hair cell presenting stereocilia was counted using markers in the Cell Count plug-in of the ImageJ software (v. 1.46r, NIH, USA). All hair cells were quantified and the results expressed as % of hair cell survival within each segment. All cells with visible stereocilia, regardless of their morphology, were classified as surviving hair cells. The number of missing cells was estimated based on the expected number of cells that would have occupied areas that were devoid of stereocilia bundles, taking into account the average hair cell diameter and the presence of supporting scar tissues.
Neomycin uptake in cochlear hair cells
Neomycin-Texas red conjugate (NTR) is a fluorescently tagged molecule that was used to study the uptake of neomycin in cochlear hair cells. Neomycin sulfate was conjugated with the succinimidyl ester of the Texas red dye using established protocol (Steyger et al. 2003). Neomycin sulfate was dissolved in 125 mM K2CO3, pH 9.0 to a final concentration of (1.08 g/mL). Texas Red sulfonyl chloride (TR, Invitrogen) was dissolved in dimethylformamide (DMF) at 2 mg/mL to produce a final concentration of 2.5 mM. DMF was then slowly mixed with neomycin (12% v/v) at approximately 330:1 molar ratio of neomycin:TR. The high neomycin to TR ratio ensures a binding ratio of one TR per neomycin molecule. The mixture was incubated at 4 °C, protected from light, for 3 days, and the stock solution was aliquoted and stored at − 20 °C. The same batch of NTR stock solution was used in all NTR experiments.
In control experiments, a calcium chelator BAPTA (1,2-bis(o-aminophenoxyl)ethane-N,N,Nʹ,Nʹ-tetraacetic acid), which disrupts the integrity of the stereociliary tip links, was used to prevent the opening of MET channels (Zhao et al. 1996; Vu et al. 2013). BAPTA (5 mM) was added to the culture medium 15 min prior to NTR (1 mM). Cochlear explants were then incubated in NTR + BAPTA in culture medium for a further 3 h, then fixed with 4% PFA for 30 min, and permeabilised for 1 h with 1% Triton X-100. Hair cells were stained with Alexa Fluor 488-phalloidin (1:600, 1 h RT). After washing with PBS, the tissues were mounted using an antifade mounting medium (Citiflour, Leicester, UK).
Fluorescence intensity analysis
Phalloidin and NTR fluorescence were imaged using an epifluorescence microscope (Eclipse 80i, Nikon) or a laser scanning confocal microscope (FluoViewTM FV1000, Olympus). Confocal settings were established using the basal segment of the cochlea where the fluorescence intensity is greatest and the same settings were used for all regions of interest in the cochlea (apical, basal and hook). NTR pixel intensity was quantified using the ImageJ software to estimate neomycin uptake into the hair cells. The cell bodies of IHC and OHC were analysed as separate regions of interest (ROI). Background pixel intensity in a non-labelling region of the cochlea was deducted from the pixel intensity of the ROI to control for inter-cochlear variations.
Statistical analysis
For statistical analysis, the cochlea was divided into three arbitrary segments. The apical segment included the apical 30% of the organ of Corti, the basal segment included the middle 30–85% and the hook region the last 15% of the organ of Corti. To examine the effect of two categorical variables (treatments and cochlear segments) on hair cell survival, a two-way ANOVA was used followed by Tukey’s multiple comparison test or Kruskal–Wallis followed by Dunn’s test, and the α level was set at 0.05. NTR fluorescence intensity was correlated with the hair cell survival using Pearson’s product–moment correlation.
Results
Neomycin-induced hair cell loss
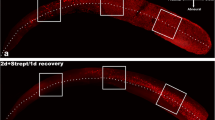
The sensory epithelium of the P3 cochlea was generally well preserved after 42 h of incubation in normal culture medium (Fig. 1a) with just a small amount of sensory cell loss. In the apical and basal segments of the organ of Corti the survival rate of the inner hair cells (IHC) was 97.5 ± 0.87 and 94.2 ± 2.8% respectively (n = 7), but it was slightly reduced in the hook region (to 76.7 ± 6.2%, p < 0.01; Fig. 1b). OHC, on the other hand, showed consistently high survival rate across the length of the sensory epithelium (99.2 ± 0.8%, Fig. 1b).
The effect of neomycin on hair cell survival in the C57BL/6 mouse cochlea at postnatal day 3. a Organ of Corti explants labelled with Alexa Fluor 488-phalloidin were incubated for 42 h in normal culture medium (control) or culture medium containing neomycin (1 mM, 3 h) in the middle of the 42-h timeframe. Incubation in normal culture medium resulted in excellent preservation of the inner and outer hair cells (IHC and OHC) across the entire length of the sensory epithelium. In the neomycin-exposed cochlea hair cell preservation was good in the apical segment, poor in the basal segment and reduced in the hook region. Scale bar 30 µm. b In normal culture medium, OHC demonstrated similar survival rate in the apical, basal and the hook regions, whilst the hook region IHC showed a small reduction in hair cell survival. Exposure to neomycin resulted in significant reduction in IHC survival in the apical and basal segments of the cochlea. OHC show the lowest survival rate in the basal segment, whilst the apical and the hook regions were less affected by neomycin toxicity. Data presented as mean ± SEM; controls n = 7; neomycin-treated, n = 8. *p < 0.05; **p < 0.01; Kruskal–Wallis followed by Dunn’s test
As expected, structural integrity of the organ of Corti was adversely affected by exposure to neomycin. The basal segment of the organ of Corti was most affected by neomycin treatment (Fig. 1a). This was evident from the massive hair cell loss, disorganised rows of the remaining hair cells and extensive scar formation (Fig. 1a). Hair cell counts confirmed a segment-specific sensitivity to neomycin ototoxicity. Quantitative analysis (Fig. 1b) revealed that the IHC preservation in the apical segment following neomycin treatment (77.7 ± 6.3%) was significantly better than the basal (51.0 ± 6.9%, p < 0.05) and the hook regions (43.9 ± 6.4%, p < 0.05, Kruskal–Wallis followed by Dunn’s test). OHC preservation in the apical and the hook regions following neomycin treatment were considerably better than in the basal segment (Fig. 1a). Quantitative analysis of the OHC survival demonstrated a relatively small reduction of OHC survival in the hook region (to 90.4 ± 3.1%, p > 0.05) and the apical segment of the cochlea (to 71.7 ± 9.1%, p < 0.05), whilst a substantial reduction in OHC survival was observed in the basal segment (to 41.4 ± 5.5%, p < 0.05; Fig. 1b). The greater survival of hair cells in the apex has been described before, but this study demonstrated an unexpected resistance to neomycin of the sensory hair cells in the hook region of the developing cochlea.
NTR uptake and hair cell survival
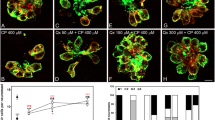
This atypical pattern of neomycin ototoxicity in the sensory epithelium of the C57BL/6 mouse cochlea was further investigated using a fluorescently labelled neomycin conjugate NTR. As expected, NTR fluorescence was detected predominantly in the cell bodies of sensory hair cells, with no apparent staining in the supporting cells (Fig. 2a). However, the study clearly demonstrated segment-specific differences in NTR uptake. The strongest NTR fluorescence was recorded in the basal turn, and the weakest in the hook region (Fig. 2a). The specificity of NTR uptake was shown in control experiments, where the absence of NTR in the culture medium or omission of Texas red from the conjugate abolished fluorescence (Fig. 2a).
Neomycin-Texas red (NTR) uptake in the C57BL/6 mouse cochlea at P3. a Organ of Corti double labelled with NTR (greyscale and red fluorescence) and phalloidin (green fluorescence). NTR labelling was restricted to the sensory hair cells. Low NTR uptake was demonstrated in the apical segment IHC, and stronger in the basal segment. As for the OHC, NTR labelling was the strongest in the basal segment, moderate in the apical and weak in the hook region. NTR staining was abolished when the explants (images showing the basal turn) were incubated with unconjugated neomycin (neo, no TR) or Texas red only (TR control). Scale bars 10 µm. b Cochlear hair cell survival in the neomycin-treated cochlea was displayed as a cochleogram (grey line) and correlated with NTR uptake (red line) in the inner (IHC) and outer (OHC) hair cells. Bar graphs show turn-related differences. Data presented as mean ± SEM; NTR uptake, n = 10; hair cell survival, n = 8
Semi-quantitative analysis of fluorescence intensity demonstrated an inverse correlation between NTR uptake and hair cell survival (Fig. 2b). Thus, the low NTR intensity in the apical segment IHC (13.5 ± 3.6 arbitrary units) corresponded to a high survival rate (77.7 ± 6.3%), whilst the higher NTR intensity in the basal (20.5 ± 2.6) and the hook regions (21.7 ± 3.6) corresponded to a lower IHC survival (51.0 ± 6.9 and 43.9 ± 6.4%, respectively). The NTR fluorescence in the population of OHC along the cochlea was represented by a bell-shaped curve (Fig. 2b), featuring low fluorescence intensity in the apical and hook regions (19.0 ± 4.2 and 17.2 ± 4.0, respectively), and significantly greater fluorescence intensity in the basal segment (35.3 ± 2.7). A high NTR intensity in the basal segment OHC corresponded to a low survival rate of these cells (41.4% ± 5.5). In contrast, the low NTR fluorescence intensity in the apical and the hook regions corresponded to a high survival rate of OHC (71.7 ± 9.1 and 90.4 ± 3.1%, respectively, Fig. 2b). The intensity of NTR fluorescence thus correlated inversely with the hair cell survival (r = − 0.83 in IHC and − 0.91 in OHC; Pearson’s product–moment correlation).
Changes in NTR uptake after BAPTA treatment
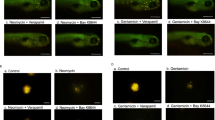
To investigate whether the NTR fluorescence in hair cells was related to the uptake through MET channels, cochlear explants were pre-treated with the calcium chelator BAPTA (5 mM) for 15 min in normal culture medium, followed by incubation with NTR for 3 h in the presence of BAPTA. BAPTA treatment temporarily disrupts stereociliary tip links and thus prevents opening of MET channels (Zhao et al. 1996; Vu et al. 2013). BAPTA treatment reduced NTR fluorescence in all three segments of the cochlea (Fig. 3a, b). This reduction in NTR fluorescence was significant in both IHC (reduced by 83%, averaged across the entire length of the organ of Corti) and OHC (reduced by 82%, Fig. 3b).
The effect of BAPTA treatment on hair cell uptake of Neomycin Texas-red (NTR) conjugate in the P3 mouse cochlea. a NTR uptake was the strongest in the basal segment of the cochlea. Treatment with BAPTA (5 mM) reduced NTR uptake in both inner and outer hair cells. b Semi-quantitative analysis of NTR fluorescence intensity in sensory hair cells. BAPTA substantially reduced NTR uptake in IHC and OHC across the entire length of the sensory epithelium. Data presented as mean ± SEM, n = 4. *p < 0.05; **p < 0.01, two-way ANOVA
Discussion
In this study, organotypic cultures of the developing mouse organ of Corti (P3) demonstrated excellent preservation of the sensory hair cells with the exception of a small reduction in the survival rate of IHC in the hook region. Good survival of the developing organ of Corti in short-term culture conditions is well established in the literature but the survival rates in different cochlear turns are often not reported (Richardson and Russell 1991; Wang et al. 2003) or not compared across the turns (Lowenheim et al. 1999; Mazurek et al. 2006). IHC in the hook region retain their normal stereocilia bundles in explants that were fixed immediately following dissection (data not shown), suggesting that reduced survival rates of IHC in culture likely result from the effect of explantation.
Here, we describe and investigate an unusual pattern of neomycin ototoxicity in organotypic tissue cultures of the developing mouse cochlea. Hair cell counts revealed substantial loss of both IHC and OHC in a region-specific manner. The typical pattern of hair cell loss (high in the basal and low in the apical turn) has been consistently reported across different species, in vitro, in vivo and with different aminoglycosides (Lowenheim et al. 1999; Leake et al. 1997; Wu et al. 2001). This typical pattern of hair cell loss has been attributed to the decremental antioxidant gradient in the apex-to-base direction, at least in OHC (Sha et al. 2001). However, the observed pattern of hair cell loss, particularly their resistance to neomycin ototoxicity in the extreme basal (hook) region, has not been reported previously.
It is well established that increased uptake of neomycin promotes hair cell death (Richardson and Russell 1991; Momiyama et al. 2006; Alharazneh et al. 2011), and our study confirms an inverse correlation between NTR uptake and hair cell survival. However, diminished uptake of NTR in OHC in the extreme basal segment of the developing cochlea and an excellent survival of these cells in the presence of neomycin, have not been reported previously. A similar study in Sprague–Dawley rats reported high gentamicin-Texas red (GTTR) uptake in the basal turn hair cells (Alharazneh et al. 2011). Discrepancies between NTR and GTTR uptake could arise from different properties of the two aminoglycoside antibiotics (neomycin and gentamycin), or as a result of species-specific differences in aminoglycoside uptake.
The cation-selective mechanotransducer (MET) channels located at the tips of the stereocilia are considered to be the main route for aminoglycoside entry into the sensory hair cells (Marcotti et al. 2005; Alharazneh et al. 2011). The gating mechanism of MET channels is controlled by extracellular filaments known as the tip links, consisting of adhesion proteins Cadherin 23 (encoded by Cdh23 gene) and protocadherin 15 (Pcdh15 gene) that span the membrane near a MET channel (possibly a TMC1 protein; Fettiplace 2016) and the adjacent stereocilium (Osborne et al. 1988; Kazmierczak et al. 2007). Differences in maturation of the channels or their opening characteristics along the organ of Corti could provide an explanation for the unusual pattern of aminoglycoside activity. A base-to-apex developmental gradient in the tip links, essential for regulating opening of the transduction channels, has been previously reported in sensory hair cells of Swiss Webster mice (Lelli et al. 2009) and Sprague Dawley rats (Waguespack et al. 2007). In mice, the onset of transduction in the first postnatal week was measured by FM1-43 uptake in sensory hair cells and electrophysiological recordings of transduction currents. FM1-43 uptake started at P0 at the base and was delayed until P7 in the apex (Lelli et al. 2009). Similarly, the onset of mechanosensitivity in Sprague–Dawley rat pups showed a temporal gradient with basal hair cells becoming responsive at P0 and apical cells at P3 (Waguespack et al. 2007). The restricted uptake of neomycin in the hair cells of apical segments and their resistance to neomycin ototoxicity thus may be attributed to the immature transduction channels in the neonatal (P3) mouse pups. However, aminoglycoside resistance observed in the hook region of OHC could not be explained solely by the gradients in tip-link maturation unless there is a reverse maturation gradient from the basal region to the hook region in the developing mouse. The integrity of the tip links is calcium-dependent and the presence of a calcium chelator such as BAPTA results in temporary disruption of tip links and abolition of the transduction currents (Assad et al. 1991; Zhao et al. 1996; Vu et al. 2013). As expected, BAPTA treatment in our study resulted in near-complete block of NTR uptake in IHC and OHC across all cochlear turns, which confirms the essential role of MET channels in aminoglycoside uptake in the C57BL/6 mouse cochlea.
Spontaneous regeneration of tip links has been demonstrated within 24 h following the initial BAPTA treatment (Zhao et al. 1996). This is an unlikely explanation for the incomplete block of NTR uptake observed in our study due to the short culture timeframe. A more probable scenario would be NTR uptake via routes other than the MET channels. Endocytosis has been suggested as a potential route for aminoglycoside uptake in sensory hair cells (Hashino and Shero 1995) but this view has been disputed by others (Alharazneh et al. 2011; Li and Steyger 2011). Emerging evidence has suggested cation permeable transient receptor potential channels (TRPV) as transduction channels in the inner ear (Corey et al. 2004; Marcotti et al. 2014; Stepanyan et al. 2011). This is potentially an alternative route for aminoglycoside uptake in our study.
The inbred C57BL/6 mice used in this study develop early onset age-related hearing loss (AHL) by 3–6 months of age (Kendall and Schacht 2014). At least 8 mapped loci contribute to accelerated AHL in mice (Johnson et al. 2006), and one of them has been identified as a variant of the cadherin 23 gene (Cdh23) expressed on the tip links of the sensory hair cells (Noben-Trauth et al. 2003; Brown et al. 2008). Cdh23 mutations hinder the gating of mechanoelectrical transducer (MET) channels and reduce the transduction currents (Alagramam et al. 2011). The same mutations can also attenuate aminoglycoside uptake through MET channels and thus protect the cochlea from aminoglycoside ototoxicity (Vu et al. 2013). It is unclear, however, whether Cdh23 mutation is present in the C57BL/6 mouse at P3. We postulate that Cdh23 mutation is present at P3 in the hook region and progresses towards the basal and apical turns as the animals age. This developmental gradient of Cdh23 mutation provides a possible explanation for the pattern of neomycin uptake and hair cell loss observed in this study. However, further studies are required to map out developmental expression of Cdh23 mutation in the mouse cochlea and determine its effect on the MET channel opening, to elucidate this pattern of hair cell loss.
Conclusion
Exposure to neomycin (1 mM, 3 h) causes significant loss of sensory hair cells in organotypic cultures of the C57BL/6 mouse organ of Corti at postnatal day 3. As expected, the basal segment IHC (including the hook region) were most susceptible to neomycin, resulting in the standard L-shape cochleogram. Neomycin exposure also induced a substantial loss of OHC in the basal segment, but OHC in the apical and the hook regions were less affected, resulting in a U-shape cochleogram. The uptake of fluorescently tagged neomycin (NTR) was inversely correlated with hair cell survival in this study, however, the low uptake of NTR and high survival rate of OHC in the hook region were unexpected. Significant reduction of NTR uptake following BAPTA treatment demonstrated substantial contribution of MET channels to NTR uptake. We propose that the Cdh23 mutation in the C57BL/6 mouse cochlea affects the opening probability of the OHC MET channels in the extreme basal segment of the cochlea very early during postnatal development and thus improves the OHC survival in the presence of aminoglycosides in the hook region. This atypical pattern of neomycin ototoxicity in C57BL/6 mice represents the caveat for future studies in this widely used animal model of accelerated hearing loss and warrants further investigation of the turn-related differences in hair cell resistance to ototoxic drugs.
References
Alagramam KN, Goodyear RJ, Geng R, Furness DN, van Aken AF, Marcotti W, Kros CJ, Richardson GP (2011) Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS One 6(4):e19183
Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ (2011) Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PloS One 6(7):e22347
Assad JA, Shepherd GM, Corey DP (1991) Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7(6):985–994
Brown SD, Hardisty-Hughes RE, Mburu P (2008) Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet 9(4):277–290
Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung ELM, Derfler BH, Duggan A (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432(7018):723
Fettiplace R (2016) Is TMC1 the hair cell mechanotransducer channel? Biophys J 111:3–9
Forge A, Schacht J (2000) Aminoglycoside antibiotics. Audiol Neurotol 5(1):3–22
Hashino E, Shero M (1995) Endocytosis of aminoglycoside antibiotics in sensory hair cells. Brain Res 704(1):135–140
Hinshaw H, Feldman W (1945) Streptomycin in treatment of clinical tuberculosis: a preliminary report. Proc Staff Meet Mayo Clin 20:313–318
Johnson KR, Zheng QY, Noben-Trauth K (2006) Strain background effects and genetic modifiers of hearing in mice. Brain Res 1091(1):79–88
Karasawa T, Steyger PS (2011) Intracellular mechanisms of aminoglycoside-induced cytotoxicity. Integr Biol 3(9):879–886
Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449:87–91
Kendall A, Schacht J (2014) Disparities in auditory physiology and pathology between C57BL/6J and C57BL/6N substrains. Hear Res 318:18–22
Leake PA, Kuntz AL, Moore CM, Chambers PL (1997) Cochlear pathology induced by aminoglycoside ototoxicity during postnatal maturation in cats. Hear Res 113:117–132
Lee J-H, Park C, Kim S-J, Kim H-J, Oh G-S, Shen A, So H-S, Park R (2013) Different uptake of gentamicin through TRPV1 and TRPV4 channels determines cochlear hair cell vulnerability. Exp Mol Med 45(3):e12
Lelli A, Asai Y, Forge A, Holt JR, Geleoc GS (2009) Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophys 101(6):2961–2973
Li H, Steyger PS (2011) Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci Rep 1:159
Lin CYS (2017) Purinergic signalling and aminoglycoside ototoxicity: the role of P1 and P2 receptors. PhD Thesis, The University of Auckland
Löwenheim H, Kil J, Gültig K, Zenner H (1999) Determination of hair cell degeneration and hair cell death in neomycin treated cultures of the neonatal rat cochlea. Hear Res 128(1):16–26
Marcotti W, Van Netten SM, Kros CJ (2005) The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol 567(2):505–521
Marcotti W, Corns LF, Desmonds T, Kirkwood NK, Richardson GP, Kros CJ (2014) Transduction without tip links in cochlear hair cells is mediated by ion channels with permeation properties distinct from those of the mechano-electrical transducer channel. J Neurosci 34(16):5505–5514
Mazurek B, Amarjargal N, Haupt H, Gross J (2006) High potassium concentrations protect inner and outer hair cells in the newborn rat culture from ischemia-induced damage. Hear Res 215:31–38
Momiyama J, Hashimoto T, Matsubara A, Futai K, Namba A, Shinkawa H (2006) Leupeptin, a calpain inhibitor, protects inner ear hair cells from aminoglycoside ototoxicity. Tohoku J Exp Med 209(2):89–97
Noben-Trauth K, Zheng QY, Johnson KR (2003) Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet 35(1):21–23
Osborne M, Comis S, Pickles J (1988) Further observations on the fine structure of tip links between stereocilia of the guinea pig cochlea. Hear Res 35(1):99–108
Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R (2008) Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27(50):6407–6418
Poirrier A, Pincemail J, Van Den Ackerveken P, Lefebvre P, Malgrange B (2010) Oxidative stress in the cochlea: an update. Curr Med Chem 17(30):3591–3604
Richardson GP, Russell IJ (1991) Cochlear cultures as a model system for studying aminoglycoside induced ototoxicity. Hear Res 53(2):293–311
Rybak L, Ramkumar V (2007) Ototoxicity Kidney Int 72(8):931–935
Sha S-H, Taylor R, Forge A, Schacht J (2001) Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res 155(1):1–8
Stepanyan RS, Indzhykulian AA, Vélez-Ortega AC, Boger ET, Steyger PS, Friedman TB, Frolenkov GI (2011) TRPA1-mediated accumulation of aminoglycosides in mouse cochlear outer hair cells. J Assoc Res Otolaryngol 12(6):729–740
Steyger P, Peters S, Rehling J, Hordichok A, Dai C (2003) Uptake of gentamicin by bullfrog saccular hair cells in vitro. J Assoc Res Otolaryngol 4:565–578
Vu AA, Nadaraja GS, Huth ME, Luk L, Kim J, Chai R, Ricci AJ, Cheng AG (2013) Integrity and regeneration of mechanotransduction machinery regulate aminoglycoside entry and sensory cell death. PloS One 8(1):e54794
Waguespack J, Salles FT, Kachar B, Ricci AJ (2007) Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27(50):13890–13902
Wang J, Van De Water T, Bonny C, De Ribaupierre F, Puel J, Zine A (2003) A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci 23(24):8596–8607
Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J (2001) Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague–Dawley rat. Hear Res 158(1–2):165–178
Xie J, Talaska AE, Schacht J (2011) New developments in aminoglycoside therapy and ototoxicity. Hear Res 281(1–2):28–37
Zhao Y-D, Yamoah EN, Gillespie PG (1996) Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci USA 93(26):15469–15474
Acknowledgements
This study was supported by the Auckland Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this article have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Lin, S.C.Y., Thorne, P.R., Housley, G.D. et al. Resistance to neomycin ototoxicity in the extreme basal (hook) region of the mouse cochlea. Histochem Cell Biol 150, 281–289 (2018). https://doi.org/10.1007/s00418-018-1683-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-018-1683-8