Abstract
Oxidative stress has been established as the key mechanism of the cochlear damage underlying noise-induced hearing loss, however, emerging evidence suggests that cochlear inflammation may also be a major contributor. This study aimed to improve our understanding of the cochlear inflammatory response associated with acute and chronic noise exposure. C57BL/6 mice were exposed to acute traumatic noise (100 dBSPL, 8–16 kHz for 24 h) and their cochleae collected at various intervals thereafter, up to 7 days. Using quantitative RT-PCR and immunohistochemistry, changes in expression levels of proinflammatory cytokines (TNF-α, IL-1β), chemokines (CCL2) and cell adhesion molecules (ICAM-1) were studied. All gene transcripts displayed similar dynamics of expression, with an early upregulation at 6 h post-exposure, followed by a second peak at 7 days. ICAM-1 immunoexpression increased significantly in the inferior region of the spiral ligament, peaking 24 h post-exposure. The early expression of proinflammatory mediators likely mediates the recruitment and extravasation of inflammatory cells into the noise-exposed cochlea. The occurrence of the latter expression peak is not clear, but it may be associated with reparative processes initiated in response to cochlear damage. Chronic exposure to moderate noise (90 dBSPL, 8–16 kHz, 2 h/day, up to 4 weeks) also elicited an inflammatory response, reaching a maximum after 2 weeks, suggesting that cochlear damage and hearing loss associated with chronic environmental noise exposure may be linked to inflammatory processes in the cochlea. This study thus provides further insight into the dynamics of the cochlear inflammatory response induced by exposure to acute and chronic noise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hearing loss is the most common sensory disability and has considerable social and economic implications. According to the World Health Organisation (WHO 2015), 360 million people worldwide suffer from moderate to profound hearing loss (over 5 % of the world’s population). Exposure to excessive noise, in either occupational or recreational settings, is one of the major causes of permanent sensorineural hearing loss, secondary only to age-related hearing loss (presbyacusis). Acoustic overstimulation inflicts injury on the cochlea, affecting almost all cell types, but particularly the sensory hair cells. Since sensory cells in the mammalian cochlea are incapable of regeneration, this damage is irreversible, leading to cochlear dysfunction and permanent hearing loss. A significant proportion (16 %) of the disabling hearing loss in the adult population worldwide is attributed to occupational noise exposure, ranging from 7 % in the most developed countries to 21 % in developing regions (Nelson et al. 2005). Hence, substantial efforts have been made over the years to understand the pathophysiological mechanisms underlying noise-induced cochlear injury in order to develop pharmacological interventions to reduce or prevent noise-induced hearing loss (NIHL).
Oxidative stress in the cochlea has been identified as a key mechanism of NIHL. This involves the excessive production of reactive oxygen species (ROS) and free radicals in cochlear tissues, which can lead to substantial sensory hair cell loss via both apoptotic and necrotic cell death pathways. However, there is emerging evidence that cochlear inflammation may also be a major contributor to noise-induced cochlear injury [see review by Tan et al. (2013)]. Several studies have demonstrated an inflammatory response in the cochlea following exposure to traumatic noise that involves an upregulation of proinflammatory mediators (cytokines, chemokines and cell adhesion molecules), followed by a rapid recruitment and infiltration of inflammatory cells from the systemic circulation (Discolo et al. 2004; Fujioka et al. 2006; Hirose et al. 2005; Tan et al. 2008; Tornabene et al. 2006; Wakabayashi et al. 2010). Cochlear inflammation has also been implicated as a major pathogenic factor in other conditions that cause sensorineural hearing loss, including otitis media, meningitis, autoimmune inner ear disease, and ototoxicity (Cayé-Thomasen et al. 2009; Gloddek et al. 1999; Hirose et al. 2005; Kawauchi et al. 1988; So et al. 2007, 2008; Tornabene et al. 2006; Trinidad et al. 2005). Inflammation has also been shown to potentiate drug-induced ototoxicity (Koo et al. 2015; Oh et al. 2011). Furthermore, cochlear surgery and the insertion of cochlear implants can evoke cochlear inflammation (Backhouse et al. 2008; Okano et al. 2008).
It is recognised that noise-induced cochlear inflammation involves complex signalling pathways. To date, various inflammation-related genes and proteins have been implicated in the cochlear inflammatory response (Fujioka et al. 2006; Hirose and Keasler 2004; Jo et al. 2010b; Kirkegaard et al. 2006; Nakamoto et al. 2012; Shi and Nuttall 2007; Tornabene et al. 2006; Wakabayashi et al. 2010; Yamamoto et al. 2009), yet the precise molecular mechanisms, the time course, and the role of the response in the development of cochlear injury remain to be elucidated. Hearing loss in the workplace is normally caused by repeated exposure to moderate sound levels over an extended period of time. There is a possibility that chronic environmental noise exposure, such as that in the workplace or with personal listening devices, could be linked to the development of a chronic inflammatory response, which could contribute to cochlear injury and hearing loss, but this has not been studied. Thus the purpose of the present study was to investigate the dynamics of the inflammatory response in the mammalian cochlea following both acute and chronic noise exposure. Here, we evaluated the expression of several key inflammatory markers in the cochlea including the proinflammatory cytokines tumour necrosis factor alpha (TNF-α) and interleukin-1beta (IL-1β), the chemokine chemokine (C–C motif) Ligand 2 (CCL2), and the cell adhesion molecule intercellular adhesion molecule-1 (ICAM-1), which all have important roles in the recruitment and infiltration of inflammatory cells into tissues. Our study provides comparative analysis of the inflammatory markers in the cochlea after exposure to acute and chronic noise and first evidence for the existence of cochlear inflammation at moderate sound pressure levels.
Materials and methods
Animals
All experiments were carried out on male inbred C57BL/6 mice (6–8 weeks). The animals were supplied by the animal facility at The University of Auckland, and were housed in standard wire restraint cages, and maintained on a 12-h light/dark cycle under controlled temperatures and relative humidity. Mice had free access to standard rodent chow and filtered water at all times. All animal experimental procedures were approved by The University of Auckland Animal Ethics Committee, and the animals were cared for according to the recommendations in the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health). Animals were euthanised using sodium pentobarbitone (90 mg/kg, i.p.) for tissue collection. From each animal, one cochlea was collected for mRNA extraction and the other for immunohistochemistry.
Noise exposure
Mice were exposed to acute open-field octave band (8–16 kHz) noise for 24 h at 100 dB SPL, whereas those in the chronic noise exposure groups were subjected to an octave band (8–16 kHz) noise for 2 h per day at 90 dB SPL for 1 to 4 weeks. Control animals were exposed to ambient noise levels in the animal facility (55–65 dB SPL). Noise exposures were carried out in a small custom-built sound exposure booth (Shelburg Acoustics Pty Ltd, Croydon North, VIC, Australia), equipped with an internal light source and ventilation system. The sound stimulus was produced by a noise generator, filtered, amplified, and delivered via two internal speakers suspended from the ceiling of the booth. The controls for sound generation and frequency selection were externally located. Animals were placed in their cages positioned in the centre of the sound chamber directly underneath the suspended speakers. The sound exposure levels within the chamber at the level of the animal cages were measured using a handheld calibrated sound level meter (Brüel & Kjær Precision Sound Level Meter Type 2235, Nærum, Denmark). Mice were conscious, were unrestrained and had free access to food and water throughout the duration of the noise exposure.
Cochlear tissue preparation
At selected time points after noise exposure, mice were deeply anaesthetised with sodium pentobarbitone (90 mg/kg, i.p.) and perfused intracardially with flush solution (saline containing 10 % NaNO2 and heparin). Both cochleae were then dissected out, and one was immersed in 4 % paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; fixative solution; pH 7.4) and used for immunohistochemical staining, while the contralateral cochlea was placed in lysis buffer and used for quantitative real-time RT-PCR. The cochleae collected for immunohistochemistry were fixed overnight in 4 % PFA at 4 °C, and decalcified in 5 % ethylenediaminetetraacetate (EDTA) in 0.1 M PB for 6–7 days at 4 °C. The cochleae were then cryo-protected in 30 % sucrose in 0.1 M PB for 24 h, rinsed twice with 0.1 M phosphate-buffered saline (PBS; pH 7.4), embedded in Tissue-Tek® O.C.T. (Optimal Cutting Temperature) Compound (Sakura Finetek USA, Inc., Torrance, CA, U.S.A.), snap-frozen and stored at −80 °C.
Quantitative real-time RT-PCR
To identify the changes in expression levels of proinflammatory cytokines and cell adhesion molecules associated with noise-induced cochlear inflammation (TNF-α, IL-1β, CCL2, ICAM-1), a two-step quantitative real-time RT-PCR analysis was carried out. For each group, cochlear mRNA samples (n = 8 mice; one cochlea per mouse) were used for gene expression analysis. Isolation of mRNA from cochlear tissues was firstly carried out using the Dynabeads® mRNA DIRECT™ Kit (Ambion®, Life Technologies, Oslo, Norway), followed by first-strand cDNA synthesis (reverse transcription) using the SuperScript® III First-Strand Synthesis SuperMix Kit (Invitrogen™, Life Technologies, Carlsbad, CA, U.S.A.). For each mRNA sample, a negative reverse transcriptase control (−RT) was also included. The synthesised first-strand cDNA templates were then amplified by real-time PCR using TaqMan® Universal PCR Master Mix (Applied Biosystems®, Life Technologies, Branchburg, NJ, U.S.A.) and predesigned TaqMan® Gene Expression Assays (Table 1). These assays contain unlabelled mouse-specific PCR primers and a TaqMan® MGB probe proprietary to Applied Biosystems®. The housekeeping gene β-actin served as an internal control to normalise gene expression levels of proinflammatory mediators. For each TaqMan® Gene Expression Assay (TNF-α, IL-1β, CCL2, ICAM-1, and β-actin), a separate PCR master reaction mix was prepared and aliquoted in a MicroAmp® Optical 384-Well Reaction Plate (Applied Biosystems®, Life Technologies, Foster City, CA, U.S.A.). The template (1 µL of sample cDNA) was added to a total reaction volume of 12.5 µL. Each sample was run in triplicates (two +RT reactions and a single −RT reaction). Two no-template controls (NTC; first-strand cDNA omitted) were also included in each TaqMan® Gene Expression Assay. Real-time PCR was performed using the 7900HT Fast Real-Time PCR System (Applied Biosystems®). The thermal cycling protocol included 2 min at 50 °C, 10 min at 95 °C and 40 cycles of 15 s at 95 °C and 1 min at 60 °C.
The PCR data were analysed using the Sequence Detection System (SDS) Software v2.3 (Applied Biosystems®). Relative quantification of gene expression for each inflammatory marker was determined using the comparative CT method (also referred to as the 2(−ΔΔC )T method) (Livak and Schmittgen 2001), and the results were expressed as a fold change relative to the control cochlea exposed to ambient noise. Measured C T values were used to calculate delta C T values (ΔC T = C T target − C T β-actin). ΔΔC T values per sample were then determined by subtracting the average ΔC T of the control group from the ΔC T of each sample of the experimental group. This was followed by averaging the ΔΔC T values in each group. Finally, the fold change in the target gene expression relative to the control group was calculated from the formula: 2(−ΔΔC )T if ΔΔC T ≤ 0 or (−1)/2(−ΔΔC )T if ΔΔC T > 0.
Immunohistochemistry
As leucocyte adhesion to endothelial cells and transmigration through the vascular wall are mediated by cell adhesion molecules, we investigated the distribution of ICAM-1 in the cochlea and changes in ICAM-1 immunoexpression after noise exposure using immunoperoxidase. The antibodies used, including their dilutions, are listed in Table 2. Frozen cochlear tissues were cryosectioned at 20 µm using a cryostat (Leica CM3050 S Cryostat, Leica Microsystems, Wetzlar, Germany). The fixed cochlear cryosections were then transferred into a BD Falcon™ 24-well plate (BD Biosciences, San Jose, CA, U.S.A.) prefilled with 0.1 M PBS.
After two washes with 0.1 M PBS, the free floating cochlear sections were incubated in 0.6 % H2O2/methanol for 20 min at room temperature (RT) to quench endogenous peroxidase activity. Cochlear sections were then incubated for 1 h at RT in blocking/permeabilisation solution containing 10 % normal goat serum (NGS; Invitrogen™, Life Technologies, Frederick, MD, U.S.A.) and Triton X-100 (1 %) in 0.1 M PBS. Cryosections were then incubated overnight at 4 °C with the primary ICAM-1 antibody (BD Biosciences) diluted in 0.1 M PBS containing 10 % NGS and 0.1 % Triton X-100. In the control reactions, the ICAM-1 primary antibody was either omitted or replaced with the corresponding IgG isotype control, hamster IgG1 κ chain (BD Biosciences). The ICAM-1 antibody (BD Biosciences) was used previously to characterise ICAM-1 immunolabelling in the mammalian cochlea (Miyao et al. 2008; Tornabene et al. 2006). After three washes with 0.1 M PBS, sections were incubated for 40 min at RT with biotinylated goat anti-hamster IgG conjugate (Vector Laboratories, Inc., Burlingame, CA, U.S.A.) in 0.1 M PBS containing 5 % NGS. After washes in 0.1 M PBS, sections were incubated in avidin-biotinylated horseradish peroxidase complex (Vectastain® Elite ABC Kit, Vector Laboratories, Inc.) for 40 min at RT, washed and soaked in 0.1 M PBS overnight at 4 °C. Finally, sections were developed by the addition of the chromogenic peroxidase substrate 3, 3′-diaminobenzidine (DAB Peroxidase Substrate Kit; Vector Laboratories, Inc.). Development times were consistent in all experimental groups. Sections from the control cochleae were stained for the same duration as the corresponding sections from noise-exposed cochleae. Sections were mounted in glycerol/PBS (1:1) and stored at 4 °C until imaging. Cochlear sections were viewed using brightfield microscopy (Nikon Eclipse 80i Microscope, Nikon Instruments Inc., Melville, NY, USA.), and images were obtained with a digital microscope camera (Nikon DS-5Mc) attached to the microscope. Image acquisition was controlled by NIS-Elements Basic Research imaging software (version 2.30; Nikon), and images were captured using identical acquisition parameters.
Quantitative image analysis of ICAM-1 immunostaining
In addition to assessing ICAM-1 immunostaining qualitatively, image analysis based on pixel intensity and area of staining was carried out using ImageJ (version 1.46r; NIH) to provide semi-quantitative assessment of the protein expression levels. The inferior region of the spiral ligament in the upper basal cochlear turn was selected as the region of interest (ROI) as an area with strong ICAM-1 immunolabelling. Using the freehand selection tool, the DAB-stained ROI was selected, and pixel intensity and area were calculated. For staining intensity measurements, the mean grey value was determined by converting the RGB pixels in the image to greyscale/brightness values. The mean grey value represents the sum of the grey values of all the pixels in the selection divided by the total number of pixels. The lower the pixel value, the higher the intensity (pixel values range from 0 to 255, with 0 representing black and 255 representing white). To present area measurements in calibrated units (µm2), the scale of the images was firstly set. In each noise exposure group, one cochlea per mouse (n = 5) was analysed using three to five mid-modiolar sections. The mean grey values and areas of the ROI were averaged for each group and presented relative to the non-exposed control group.
Statistical analysis
A one-way analysis of variance (ANOVA) followed by Tukey’s post hoc multiple comparisons test (IBM SPSS Statistics v20) was used to compare gene expression levels (ΔΔC T values) and ICAM-1 immunostaining intensity/area at different time points after noise exposure. A p value of less than 0.05 (p < 0.05) was considered statistically significant. All data were presented as the mean ± standard error of the mean (SEM).
Results
Acute noise exposure: gene expression levels of proinflammatory mediators
Selected gene transcripts displayed similar dynamics of expression, with an early upregulation at 6 h after noise exposure followed by a subsequent peak at 7 days (Fig. 1). At 6 h post-exposure, TNF-α, CCL2, ICAM-1 and IL-1β increased by 4.6-, 22.9-, 3.8- and 1.6-fold, respectively, relative to non-exposed controls (p < 0.001 for TNF-α and CCL2, p < 0.05 for ICAM-1, p > 0.05 for IL-1β). At 7 days post-exposure, the respective fold changes for TNF-α, CCL2, ICAM-1 and IL-1β were 5.7, 4.7, 5.1 and 1.6 (p < 0.001 for TNF-α and ICAM-1, p < 0.01 for CCL2, p > 0.05 for IL-1β). No amplification was detected in the no-template controls or in the −RT controls in all real-time PCR runs. Of the four inflammatory mediators examined, CCL2 showed the greatest increase in mRNA expression at 6 h post-exposure relative to controls (Fig. 1b), about five times higher than that for TNF-α. Furthermore, the gene expression level of CCL2 at 7 days post-exposure was significantly lower than that of the initial peak at 6 h post-exposure (p < 0.01). However, for TNF-α, ICAM-1 and IL-1β, gene expression levels at these two time points were comparable (p > 0.05).
Quantitative real-time RT-PCR analysis of TNF-α, CCL2, ICAM-1 and IL-1β gene expression in the C57BL/6 mouse cochlea following acute noise exposure. The graphs illustrate the fold change in gene expression of TNF-α (a), CCL2 (b), ICAM-1 (c) and IL-1β (d) at 6 h, 1, 3 and 7 days following acute exposure to traumatic noise (100 dB SPL, 8–16 kHz for 24 h) relative to controls exposed to ambient noise (55–65 dB SPL). Data presented as mean ± SEM (n = 8 per time point). *p < 0.05; **p < 0.01; ***p < 0.001; relative to the control group (one-way ANOVA followed by Tukey’s post hoc test)
Acute noise exposure: ICAM-1 immunoexpression
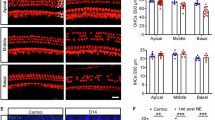
ICAM-1 immunoreactivity was localised primarily in the inferior region of the spiral ligament, which includes the type IV fibrocytes and vascular endothelial cells (Fig. 2a). Blood vessels elsewhere in the spiral ligament, and those in the stria vascularis, spiral limbus and spiral ganglion also showed ICAM-1 immunolabelling, but it was qualitatively much weaker. In addition, the endothelium of collecting venules below the lateral wall were also immunolabelled (Fig. 2b). Cochlear sections were completely devoid of staining in the absence of the ICAM-1 antibody or when the primary antibody was replaced with hamster IgG1 κ chain (Fig. 2c). Following acute noise exposure, ICAM-1 immunoexpression increased markedly, reaching maximum 1 day after acoustic exposure (Fig. 3a). Qualitatively, the ICAM-1 immunopositive area in the spiral ligament became more intense and expanded to cover a considerably greater area of the spiral ligament. ICAM-1 immunoexpression therefore corroborates the gene expression data (Fig. 1c), with maximum protein expression occurring shortly following peak levels of gene expression.
Distribution of ICAM-1 in the non-noise-exposed C57BL/6 mouse cochlea. a ICAM-1 in the cochlea exposed to ambient noise was localised predominantly in the inferior region of the spiral ligament among type IV fibrocytes and vascular endothelial cells (arrow). ICAM-1 immunolabelling was also observed in blood vessels elsewhere in the spiral ligament as well as in the spiral limbus and spiral ganglion (arrows). b In addition, the endothelium of collecting venules (arrowhead) located below the spiral ligament was also immunolabelled. c No immunostaining was detected when the primary ICAM-1 antibody was replaced with the corresponding IgG isotype control (hamster IgG1 κ chain). The small dark coloured spots in the stria vascularis are melanin pigment granules located in the intermediate cells. OC organ of Corti, SG spiral ganglion, SL spiral ligament, SLm spiral limbus, SM scala media, ST scala tympani, SV stria vascularis. Scale bars 50 µm
ICAM-1 immunolabelling in the spiral ligament of the C57BL/6 mouse cochlea following acute noise exposure. a Time course of ICAM-1 immunoexpression in the inferior region of the basal turn spiral ligament following acute exposure to traumatic noise (100 dB SPL, 8–16 kHz for 24 h). b Semi-quantitative analysis of ICAM-1 immunostaining. The graphs illustrate the ratio of the intensity and area of the immunostaining at 6 h, 1, 3 and 7 days following acute noise exposure relative to controls exposed to ambient noise (55–65 dB SPL). SL spiral ligament, SM scala media, ST scala tympani. Scale bar 50 µm. Data presented as mean ± SEM (n = 5 per time point). ***p < 0.001, relative to the control group (one-way ANOVA followed by Tukey’s post hoc test)
Image analysis confirmed that the intensity of the ICAM-1 immunolabelling with noise exposure increased to a maximum 1 day after noise exposure and thereafter returned to the level seen in the non-exposed cochlea (Fig. 3b). Quantitatively, the average staining intensity 1 day post-exposure was 15 % (82.4 ± 1.9 versus 94.8 ± 1.2) higher than in the non-exposed cochlea (p < 0.001). The average area of the ICAM-1 immunolabelling also increased to a maximum 1 day post-exposure (Fig. 3b), with the area (11,599 ± 704 µm2) double that of the non-exposed cochlea (5800 ± 289 µm2; p < 0.001). Thereafter, the immunolabelled area declined in size, but still remained significantly larger than in the non-exposed cochlea (p < 0.001). The area of immunolabelling was similar at 6 h, 1 and 3 days after exposure (p > 0.05). The noise-induced change in ICAM-1 immunostaining intensity and area in the cochlear middle turn followed a similar pattern (data not shown).
Chronic noise exposure: gene expression levels of proinflammatory mediators
Markedly elevated transcript levels of TNF-α, CCL2, ICAM-1 and IL-1β were also detected in the cochleae of mice exposed chronically to moderate noise. All four genes displayed a similar pattern of expression following chronic noise exposure (Fig. 4). Increased mRNA expression of these inflammatory mediators was first observed after 1 week of noise exposure and reached a maximum after 2 weeks. The respective fold changes in gene expression of TNF-α, CCL2, ICAM-1 and IL-1β after exposure for 2 weeks were 2.2, 3.6, 2.1 and 1.9 (p < 0.001 for TNF-α, CCL2 and ICAM-1, p < 0.05 for IL-1β). CCL2 again showed the largest upregulation relative to controls (Fig. 4b). No statistically significant differences were observed between the 1-week- and 2-week-exposure groups (p > 0.05). Transcript levels of the four genes returned to basal pre-noise levels after the second week post-exposure.
Quantitative real-time RT-PCR analysis of TNF-α, CCL2, ICAM-1 and IL-1β gene expression in the C57BL/6 mouse cochlea following chronic noise exposure. The graphs illustrate the fold change in gene expression of TNF-α (a), CCL2 (b), ICAM-1 (c) and IL-1β (d) following 1, 2, 3 and 4 weeks of exposure to moderate noise (90 dB SPL, 8–16 kHz, 2 h/day) relative to controls exposed to ambient noise (55–65 dB SPL). Data presented as mean ± SEM (n = 8 per time point). *p < 0.05; **p < 0.01; ***p < 0.001; relative to the control group (one-way ANOVA followed by Tukey’s post hoc test)
Chronic noise exposure: ICAM-1 immunoexpression
As with acute noise exposure, prominent ICAM-1 immunolabelling was observed in the inferior region of the spiral ligament following chronic noise exposure (Fig. 5a). Maximum intensity of ICAM-1 immunostaining occurred after 1 week of exposure to moderate noise (Fig. 5a,b). The average mean grey value decreased significantly (p < 0.001) from 94.8 ± 1.2 in non-exposed controls to 79.4 ± 1.7 after 1 week of noise exposure, representing a 20 % increase in staining intensity, slightly higher than the peak intensity seen following acute noise exposure (15 % increase). Thenceforth, ICAM-1 immunostaining intensity decreased to levels comparable with the non-exposed control cochlea. The area of ICAM-1 immunostaining doubled in size after noise exposure for 1 week (Fig. 5b), from 5800 ± 289 to 11,574 ± 558 µm2 (p < 0.001), almost the same size observed 24 h after acute noise exposure (11,599 µm2). The ROI remained a similar size after 2 (11,964 ± 843 µm2) and 3 weeks (10,238 ± 421 µm2) (p > 0.05), however, after 4 weeks, the ROI area returned to the pre-noise size. Comparable findings were observed in the middle turn of the cochlea (data not shown).
ICAM-1 immunolabelling in the spiral ligament of the C57BL/6 mouse cochlea following chronic noise exposure. a Time course of ICAM-1 immunoexpression in the inferior region of the basal turn spiral ligament following chronic exposure to moderate noise (90 dB SPL, 8–16 kHz, 2 h/day for 1–4 weeks). b Semi-quantitative analysis of ICAM-1 immunostaining. The graphs illustrate the ratio of the intensity and area of the immunostaining after 1, 2, 3 and 4 weeks of noise exposure relative to controls exposed to ambient noise (55–65 dB SPL). SL spiral ligament, SM scala media, ST scala tympani. Scale bar 50 µm. Data presented as mean ± SEM (n = 5 per time point). ***p < 0.001, relative to the control group (one-way ANOVA followed by Tukey’s post hoc test)
It is possible that early increase in ICAM-1 immunoexpression is partly due to changes in gene expression, and partly due to ICAM-1 trafficking from intracellular stores to the cell surface upon cytokine stimulation (e.g. with IL-1, TNF-α). ICAM-1 is known to be continuously redistributed between the cell surface and an intracellular pool, at least in some immune cells (Jo et al. 2010a).
Discussion
The present study demonstrates the time course of the inflammatory response in the cochlea following acute exposure to traumatic noise and provides first evidence of cochlear inflammation induced by chronic exposure to moderate noise levels. We observed significant upregulation in expression of proinflammatory cytokines and cell adhesion molecules in both types of noise exposure. The presence of a biphasic inflammatory response in the cochlea exposed to acute noise, demonstrated by the increased expression of TNF-α, CCL2, ICAM-1 and IL-1β at 6 h and 7 days post-exposure, was unexpected, as previous studies reported the upregulation of inflammatory mediators only in the early phase of noise-induced cochlear trauma (Fujioka et al. 2006; Hirose and Keasler 2004; Jo et al. 2010b; Nakamoto et al. 2012; Tornabene et al. 2006; Wakabayashi et al. 2010). We speculate that the initial rise in expression levels of these cytokines, chemokines and cell adhesion molecules would mediate the recruitment and extravasation of inflammatory cells. The occurrence of the latter peak in gene expression is not clear, but we postulate that it may be associated with reparative processes. This view, however, will need further investigation.
Expression of TNF-α, considered one of the primary mediators of inflammation, was substantially increased following acoustic overstimulation. This cytokine is sufficient to induce the recruitment of inflammatory cells into the cochlea even in the absence of antigens or pathogens (Keithley et al. 2008). Furthermore, blocking TNF-α using Etanercept, a soluble TNF-α-receptor-Fc fusion protein, significantly attenuates the cochlear inflammatory response in animal models of labyrinthitis (Satoh et al. 2002; Wang et al. 2003). Taken together, these findings strongly suggest a pivotal role of TNF-α in the development of cochlear inflammation.
It is likely that these proinflammatory mediators are expressed and secreted by various resident cells in the cochlea, particularly the fibrocytes in the spiral ligament. In vitro studies using cultured murine spiral ligament fibrocytes have shown that upon stimulation with proinflammatory cytokines (TNF-α, IL-1β), fibrocytes secrete various inflammatory mediators such as TNF-α, IL-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), keratinocyte-derived chemokine (KC), soluble intercellular adhesion molecule-1 (sICAM-1) and vascular endothelial growth factor (VEGF) (Ichimiya et al. 2000; Maeda et al. 2005; Moon et al. 2006; Yoshida et al. 1999). The exact mechanisms of how proinflammatory mediators are generated by fibrocytes following noise exposure are unknown. It is possible that an early release of TNF-α and IL-1β from intracellular stores following noise exposure can activate corresponding receptors on fibrocytes and the NF-κB signalling pathway which will lead to the expression of other proinflammatory mediators such as cytokines, chemokines and cell adhesion molecules (Lawrence 2009). In addition, ROS produced during oxidative stress can also induce the generation of proinflammatory mediators such as TNF-α, iNOS and COX-2 due to the activation of the transcription factor STAT1 (Kaur et al. 2011). The JNK and p38 signalling pathways are activated by proinflammatory cytokines such as TNF-α and IL-1β, suggesting that proinflammatory cytokines may be directly involved in the apoptotic death of hair cells (Tabuchi and Hara 2012).
This study demonstrated expression and distribution of ICAM-1 in the normal and noise-exposed mouse cochlea largely in the area of type IV fibrocytes and vascular endothelial cells in the lowermost region of spiral ligament. The significant increase in ICAM-1 protein 24 h after noise exposure is consistent with the early upregulation (6 h) in the gene expression of ICAM-1, suggesting that ICAM-1 protein is newly produced in the first 24 h after noise exposure. These findings are consistent with previous studies (Miyao et al. 2008; Tornabene et al. 2006; Yamamoto et al. 2009), showing that ICAM-1 likely plays a crucial role in the extravasation of inflammatory cells in the cochlea during the inflammatory response. A previous study (Seidman et al. 2009) demonstrated that treatment of rats with anti-ICAM-1 antibody significantly attenuated noise-induced temporary threshold shifts, suggesting the pivotal role of ICAM-1 in inflammation-induced cochlear damage. The observed immunoexpression pattern of ICAM-1 in the noise-exposed cochlea resembles the pattern observed in immune-mediated labyrinthitis (Pawankar et al. 1998; Suzuki and Harris 1995) and in response to cochlear electrode implantation (Kel et al. 2013). Increased ICAM-1 expression can be attributed to stimulation by proinflammatory cytokines such as TNF-α (Ichimiya et al. 2003) or ROS (Linas et al. 1995; Lo et al. 1993). ICAM-1 expression by spiral ligament fibrocytes suggests that they may serve a role in regulating and directing the infiltration and migration of inflammatory cells following noise exposure.
Previous studies have reported a large influx of inflammatory cells, peaking between 3 and 7 days after acute traumatic noise exposure (Discolo et al. 2004; Hirose et al. 2005; Sato et al. 2008; Tan et al. 2008; Tornabene et al. 2006; Wakabayashi et al. 2010). The recruitment of inflammatory cells from the systemic circulation to the noise-damaged cochlea is presumably mediated by chemoattractant molecules (cytokines and chemokines), while their transendothelial migration is most likely mediated by cell adhesion molecules such as ICAM-1. The vast majority of the inflammatory cell population in the cochlea have been found to be derived from the monocyte/macrophage lineage (Hirose et al. 2005; Okano et al. 2008; Tan et al. 2008; Tornabene et al. 2006; Yang et al. 2015).
The exact role inflammatory cells play in the noise-damaged cochlea remains unclear. These cells are probably recruited to serve predominantly as phagocytes to remove cellular debris caused by acoustic trauma. This hypothesis is based on the fact that infiltrating cells are largely observed in the region of the spiral ligament (inferior region) where noise-induced fibrocyte loss is most evident (Hirose et al. 2005; Ohlemiller 2008; Sato et al. 2008; Tornabene et al. 2006; Wang et al. 2002). Macrophages may also contribute to the repair process by altering the local environment via the secretion of cytokines and growth factors, such as TNF-α, IL-6, IL-1, transforming growth factor-α/β, platelet-derived growth factor and insulin-like growth factor-1 that regulate angiogenesis, fibroplasia, and matrix synthesis, effectively representing a wound healing response (Park and Barbul 2004). It is also possible that the damage to the fibrocytes in the spiral ligament and spiral limbus is caused directly by macrophages as a result of bystander injury. Macrophages can cause cell death by releasing cytotoxic substances such as quinolinate, ROS and nitric oxide (Ladrech et al. 2007; Park and Barbul 2004). Infiltration of inflammatory cells may therefore be a source of secondary damage in the cochlea. Proinflammatory cytokines may also be directly involved in the apoptotic death of hair cells. The JNK and p38 signalling pathways, which are often associated with apoptosis, are activated by proinflammatory cytokines such as TNF-α and IL-1β (Tabuchi and Hara 2012).
In support of the detrimental role of inflammation in the cochlea, glucocorticoids such as dexamethasone and methylprednisolone exhibit protective effect against noise-induced cochlear injury and hearing loss (Hirose et al. 2007; Sendowski et al. 2006; Tabuchi et al. 2006; Takemura et al. 2004). In addition, post-exposure inhibition of IL-6 attenuates the cochlear inflammatory response and significantly improves hearing (Wakabayashi et al. 2010). It was recently demonstrated that TNF-α inhibition improves cochlear blood flow and prevents permanent threshold shift after noise overexposure (Arpornchayanon et al. 2013). TNF-α inhibition also suppresses cellular infiltration and reduces hearing loss in an experimental model of labyrinthitis (Satoh et al. 2002; Wang et al. 2003).
Based on our findings, we postulate the following scenario of the noise-induced cochlear inflammatory response. Damage to sensory and non-sensory cells of the cochlea by noise overexposure causes an early release of proinflammatory mediators and cell adhesion molecules that recruit inflammatory cells from the circulation to phagocytose the cellular debris. However, as the macrophages migrate among the cells to clear debris, they cause significant bystander tissue injury, exacerbating the noise-induced cochlear damage. In direct response to this, a wound healing response is initiated via the secretion of various cytokines and growth factors. According to our study, this recovery phase of cochlear inflammation likely occurs by the end of the first week post-exposure, presumably with the contribution of resident cochlear cells (fibrocytes and resident macrophages). However, such a speculative view needs to be studied further.
This study also demonstrated that an inflammatory response was induced in the cochlea following repeated exposure to moderate noise levels. To our knowledge, this is the first study demonstrating the presence of cochlear inflammation in animals exposed to chronic moderate level noise, the type of environmental noise exposure that can be found in the workplace. Further work will need to be done to examine the extent of structural and functional injury to the cochlea associated with this type of low-level chronic noise exposure.
Conclusion
Noise-induced cochlear inflammation is a complex pathophysiological process characterised by the coordinated activation of various signalling pathways that regulate the expression of various proinflammatory mediators, which in turn orchestrate the directed migration of inflammatory cells to the noise-damaged cochlea. However, the exact mechanisms by which noise elicits this response still remain unclear. With improved understanding of the cochlear inflammatory response, we can explore and develop novel therapeutic interventions to protect, and perhaps rescue, cochlear tissues from inflammation-induced injury and thus mitigate NIHL.
Abbreviations
- CCL2:
-
Chemokine (C–C motif) ligand 2
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL-1β:
-
Interleukin-1beta
- IL-6:
-
Interleukin-6
- NIHL:
-
Noise-induced hearing loss
- TNF-α:
-
Tumour necrosis factor alpha
References
Arpornchayanon W, Canis M, Ihler F, Settevendemie C, Strieth S (2013) TNF-α inhibition using etanercept prevents noise-induced hearing loss by improvement of cochlear blood flow in vivo. Int J Audiol 52:545–552. doi:10.3109/14992027.2013.790564
Backhouse S, Coleman B, Shepherd R (2008) Surgical access to the mammalian cochlea for cell-based therapies. Exp Neurol 214:193–200. doi:10.1016/j.expneurol.2008.08.002
Cayé-Thomasen P, Worsøe L, Brandt CT, Miyazaki H, Østergaard C, Frimodt-Møller N, Thomsen J (2009) Routes, dynamics, and correlates of cochlear inflammation in terminal and recovering experimental meningitis. Laryngoscope 119:1560–1570
Discolo CM, Keasler JR, Hirose K (2004) Inflammatory cells in the mouse cochlea after acoustic trauma. In: 27th Association for research in otolaryngology (ARO) MidWinter Meeting, Daytona Beach, Florida, USA, Noise injury: mechanisms. 23 Feb 2004
Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H (2006) Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res 83:575–583
Gloddek B, Lassmann S, Gloddek J, Arnold W (1999) Role of S-100β as potential autoantigen in an autoimmune disease of the inner ear. J Neuroimmunol 101:39–46
Hirose K, Keasler JR (2004) Proinflammatory cytokine and chemokine expression in the noise-exposed murine cochlea. In: 27th Association for research in otolaryngology (ARO) MidWinter Meeting, Daytona Beach, Florida, USA, Noise injury: mechanisms. 23 Feb 2004
Hirose K, Discolo CM, Keasler JR, Ransohoff R (2005) Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J Comp Neurol 489:180–194
Hirose Y, Tabuchi K, Oikawa K, Murashita H, Sakai S, Hara A (2007) The effects of the glucocorticoid receptor antagonist RU486 and phospholipase A2 inhibitor quinacrine on acoustic injury of the mouse cochlea. Neurosci Lett 413:63–67. doi:10.1016/j.neulet.2006.11.029
Ichimiya I, Yoshida K, Hirano T, Suzuki M, Mogi G (2000) Significance of spiral ligament fibrocytes with cochlear inflammation. Int J Pediatr Otorhinolaryngol 56:45–51
Ichimiya I, Yoshida K, Suzuki M, Mogi G (2003) Expression of adhesion molecules by cultured spiral ligament fibrocytes stimulated with proinflammatory cytokines. Ann Otol Rhinol Laryngol 112:722–728
Jo J-H, Kwon M-S, Choi H-O, Oh H-M, Kim H-J, Jun C-D (2010a) Recycling and LFA-1-dependent trafficking of ICAM-1 to the immunological synapse. J Cell Biochem 111:1125–1137. doi:10.1002/jcb.22798
Jo MH, Kim CJ, Koh SH, Nam GS, Jeong HM, Lee JH, Lee SH (2010b) Expression and distribution of tumor necrosis factor-alpha in mice cochlea exposed to noise. Korean J Otorhinolaryngol-Head Neck Surg 53:527–533. doi:10.3342/kjorl-hns.2010.53.9.527
Kaur T, Mukherjea D, Sheehan K, Jajoo S, Rybak LP, Ramkumar V (2011) Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death Dis 2:e180. doi:10.1038/cddis.2011.63
Kawauchi H, Demaria TF, Lim DJ (1988) Endotoxin permeability through the round window. Acta Otolaryngol 105:100–115. doi:10.3109/00016488809138892
Keithley EM, Wang X, Barkdull GC (2008) Tumor necrosis factor α can induce recruitment of inflammatory cells to the cochlea. Otol Neurotol 29:854–859
Kel GE, Tan J, Eastwood HT, Wongprasartsuk S, O’Leary SJ (2013) Early cochlear response and ICAM-1 expression to cochlear implantation. Otol Neurotol 7:1595–1602. doi:10.1097/MAO.0b013e31828f4929
Kirkegaard M, Murai N, Risling M, Suneson A, Jarlebark L, Ulfendahl M (2006) Differential gene expression in the rat cochlea after exposure to impulse noise. Neuroscience 142:425–435
Koo J-W et al (2015) Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci Transl Med 7:298ra118. doi:10.1126/scitranslmed.aac5546
Ladrech S, Wang J, Simonneau L, Puel J-L, Lenoir M (2007) Macrophage contribution to the response of the rat organ of Corti to amikacin. J Neurosci Res 85:1970–1979
Lawrence T (2009) The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1:1–10. doi:10.1101/cshperspect.a001651
Linas SL, Whittenburg D, Parsons PE, Repine JE (1995) Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM-1. Kidney Int 48:1584–1591
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Lo SK, Janakidevi K, Lai L, Malik AB (1993) Hydrogen peroxide-induced increase in endothelial adhesiveness is dependent on ICAM-1 activation. Am J Physiol Lung Cell Mol Physiol 264:L406–L412
Maeda K, Yoshida K, Ichimiya I, Suzuki M (2005) Dexamethasone inhibits tumor necrosis factor-α-induced cytokine secretion from spiral ligament fibrocytes. Hear Res 202:154–160. doi:10.1016/j.heares.2004.08.022
Miyao M, Firestein GS, Keithley EM (2008) Acoustic trauma augments the cochlear immune response to antigen. Laryngoscope 118:1801–1808
Moon S-K, Park R, Lee H-Y, Nam G-J, Cha K, Andalibi A, Lim DJ (2006) Spiral ligament fibrocytes release chemokines in response to otitis media pathogens. Acta Otolaryngol 126:564–569
Nakamoto T et al (2012) Geranylgeranylacetone suppresses noise-induced expression of proinflammatory cytokines in the cochlea. Auris Nasus Larynx 39:270–274. doi:10.1016/j.anl.2011.06.001
Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M (2005) The global burden of occupational noise-induced hearing loss. Am J Ind Med 48:446–458. doi:10.1002/ajim.20223
Oh G-S et al (2011) Activation of lipopolysaccharide–TLR4 signaling accelerates the ototoxic potential of cisplatin in mice. J Immunol 186:1140–1150. doi:10.4049/jimmunol.1002183
Ohlemiller KK (2008) Recent findings and emerging questions in cochlear noise injury. Hear Res 245:5–17
Okano T, Nakagawa T, Kita T, Kada S, Yoshimoto M, Nakahata T, Ito J (2008) Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res 86:1758–1767
Park JE, Barbul A (2004) Understanding the role of immune regulation in wound healing. Am J Surg 187:S11–S16. doi:10.1016/S0002-9610(03)00296-4
Pawankar R, Tomiyama S, Jinnouchi K, Ikezono T, Nonaka M, Yagi T (1998) Intercellular adhesion molecule-1 expression in the inner ear of rats following secondary immune reaction in the endolymphatic sac. Acta Otolaryngol (Stockh) 118:5–14
Sato E, Shick HE, Ransohoff RM, Hirose K (2008) Repopulation of cochlear macrophages in murine hematopoietic progenitor cell chimeras: the role of CX3CR1. J Comp Neurol 506:930–942
Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM (2002) Tumor necrosis factor-α, an initiator, and etanercept, an inhibitor of cochlear inflammation. Laryngoscope 112:1627–1634
Seidman MD, Tang W, Shirwany N, Bai U, Rubin CJ, Henig JP, Quirk WS (2009) Anti-intercellular adhesion molecule-1 antibody’s effect on noise damage. Laryngoscope 119:707–712. doi:10.1002/lary.20109
Sendowski I, Abaamrane L, Raffin F, Cros A, Clarençon D (2006) Therapeutic efficacy of intra-cochlear administration of methylprednisolone after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res 221:119–127. doi:10.1016/j.heares.2006.08.010
Shi X, Nuttall AL (2007) Expression of adhesion molecular proteins in the cochlear lateral wall of normal and PARP-1 mutant mice. Hear Res 224:1–14. doi:10.1016/j.heares.2006.10.011
So H et al (2007) Cisplatin cytotoxicity of auditory cells requires secretions of proinflammatory cytokines via activation of ERK and NF-κB. J Assoc Res Otolaryngol 8:338–355
So H et al (2008) Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res Otolaryngol 9:290–306. doi:10.1007/s10162-008-0126-y
Suzuki M, Harris JP (1995) Expression of intercellular adhesion molecule-1 during inner ear inflammation. Ann Otol Rhinol Laryngol 104:69–75
Tabuchi K, Hara A (2012) Implications of cytokines in cochlear pathophysiology. In: Kamkin A, Kiseleva I (eds) Mechanosensitivity in cells and tissues, vol 5. Springer, The Netherlands, pp 189–199. doi:10.1007/978-94-007-2004-6_8
Tabuchi K, Murashita H, Sakai S, Hoshino T, Uemaetomari I, Hara A (2006) Therapeutic time window of methylprednisolone in acoustic injury. Otol Neurotol 27:1176–1179. doi:10.1097/01.mao.0000226313.82069.3f
Takemura K et al (2004) Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res 196:58–68. doi:10.1016/j.heares.2004.06.003
Tan BTG, Lee MMG, Ruan R (2008) Bone marrow-derived cells that home to acoustic deafened cochlea preserved their hematopoietic identity. J Comp Neurol 509:167–179
Tan WJ, Thorne PR, Vlajkovic SM (2013) Noise-induced cochlear inflammation. World J Otorhinolaryngol 3:89–99. doi:10.5319/wjo.v3.i3.89
Tornabene SV, Sato K, Pham L, Billings P, Keithley EM (2006) Immune cell recruitment following acoustic trauma. Hear Res 222:115–124. doi:10.1016/j.heares.2006.09.004
Trinidad A, Ramírez-Camacho R, García-Berrocal JR, Verdaguer JM, Daza R (2005) Labyrinthitis secondary to experimental otitis media. Am J Otolaryngol 26:226–229
Wakabayashi K et al (2010) Blockade of interleukin-6 signaling suppressed cochlear inflammatory response and improved hearing impairment in noise-damaged mice cochlea. Neurosci Res 66:345–352
Wang Y, Hirose K, Liberman MC (2002) Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol 3:248–268. doi:10.1007/s101620020028
Wang X, Truong T, Billings PB, Harris JP, Keithley EM (2003) Blockage of immune-mediated inner ear damage by etanercept. Otol Neurotol 24:52–57
WHO (2015) Deafness and hearing loss (fact sheet No. 300). World Health Organisation (WHO) Media Centre. http://www.who.int/mediacentre/factsheets/fs300/en/. Accessed 19 April 2016
Yamamoto H, Omelchenko I, Shi X, Nuttall AL (2009) The influence of NF-κB signal-transduction pathways on the murine inner ear by acoustic overstimulation. J Neurosci Res 87:1832–1840. doi:10.1002/jnr.22018
Yang W, Vethanayagam RR, Dong Y, Cai Q, Hu BH (2015) Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience 303:1–15. doi:10.1016/j.neuroscience.2015.05.081
Yoshida K, Ichimiya I, Suzuki M, Mogi G (1999) Effect of proinflammatory cytokines on cultured spiral ligament fibrocytes. Hear Res 137:155–159. doi:10.1016/s0378-5955(99)00134-3
Acknowledgments
The authors wish to acknowledge the financial support from The University of Auckland and the Auckland Medical Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tan, W.J.T., Thorne, P.R. & Vlajkovic, S.M. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem Cell Biol 146, 219–230 (2016). https://doi.org/10.1007/s00418-016-1436-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-016-1436-5