Abstract
Purpose
To assess the feasibility and reliability of biometric measurements taken with the Eyestar 900 device in keratoconus eyes in comparison with those taken with the Pentacam HR and IOLMaster 700.
Methods
Seventy-five eyes of 75 patients with keratoconus were included. The central corneal thickness (CCT), thinnest point of corneal thickness (TCT), axial length (AL), flat (K1) and steep (K2) anterior and posterior (Kp1, Kp2) keratometry, maximal keratometry (KMax) and anterior chamber depth (ACD) were compared between the Eyestar 900, Pentacam HR and IOLMaster 700. Reliability parameters such as the coefficient of variation (CoV) and intraclass correlation coefficient (ICC) were calculated. Pearson’s r was determined to assess the correlation between devices.
Results
A high repeatability (CoV < 1%) and intraclass correlation (ICC > 0.9) was found for all devices, led by AL, TCT, K1 and K2 (CoV 0.01–0.36%; ICC 0.994–1.00). The largest correlation between devices was found for AL (Eyestar vs. IOLMaster, r = 1.0), K1 (Eyestar vs. IOLMaster, r = 0.997) and ACD (Eyestar vs. IOLMaster, r = 0.995; Pentacam vs. IOLMaster, r = 0.987; Eyestar vs. Pentacam, r = 0.983), but there were significant differences in measured values between devices (p < 0.001), whereas the correlation was only slightly lower (r = 0.947 to 0.994) for KMax, CCT, TCT, K2, Kp1 and Kp2.
Conclusion
Keratometric and axial length measurements with the Eyestar 900 were feasible and revealed a high repeatability and a good correlation to the other devices in eyes with keratoconus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Keratoconus is a progressive ectatic disorder of the cornea, characterized by thinning, protrusion and irregularity of the curvature. It is mainly a bilateral disease but may manifest asymmetrically [1]. The prevalence varies across geographic regions [2]. In Europe, a prevalence of about 55–86 per 100, 000 with a male predominance has been reported, whereas in Saudi Arabia, it is higher at 4.79% [3,4,5].
For imaging, different measurement techniques are used in the clinical routine. Scheimpflug-based systems produce slit-light images with maximal resolution where the objective, focus and image planes meet at a common intersection point. The resulting images allow three dimensional topo- and tomographic maps of the anterior and posterior corneal surface and the anterior segment to be calculated with high accuracy [6].
Scheimpflug systems as well as devices based on optical coherence tomography (OCT) allow corneal tomographies, including anterior and posterior corneal surface identification, to be performed [7].
Optical coherence tomography has been an established method for non-invasive retinal imaging for more than two decades. In the last years, different OCT devices for anterior segment evaluation, including that of the cornea, have been made available. Due to the high resolution and short acquisition time, as well as the use of an infrared laser beam, OCT measurements are presumably less prone to imaging artifacts and able to depict the geometrical shape of the cornea better than the Scheimpflug technique, which is based on the analysis of video-recorded slit-beam images and is therefore more susceptible to errors (e.g. poor surface conditions, corneal haze or scars) [8, 9].
OCT-based devices can master these obstacles, via infrared wavelength laser-light and a high processing speed, passing clear to slightly opaque media and thereby producing high- resolution images of the anterior and posterior corneal surfaces [10].
Topographic measurements, at present often performed with Scheimpflug-based devices, are essential for monitoring keratoconus progression, deciding on treatment indications and assessing the post-surgical course and outcome. Therefore, accurate imaging with a high reliability and repeatability is crucial yet difficult to achieve in irregular corneas, especially those with keratoconus [11].
The identification and reliable measurement of an irregular cornea is also crucial in biometric measurements and the pre-requisite for an optimal intraocular lens (IOL) power calculation before cataract surgery [12, 13]. In eyes with keratoconus, obtaining accurate keratometric and pachymetric measurements for tracking possible disease progression and obtaining reliable axial length (AL) measurements for IOL calculation is challenging. While ultrasonic pachymetry was the gold standard for measuring central corneal thickness (CCT), the Pentacam HR (Oculus, Wetzlar, Germany) has been shown to measure pachymetry (e.g. CCT) and keratometry with a high repeatability in eyes with advanced keratoconus, and the IOLMaster 700 (Carl Zeiss, Oberkochen, Germany) performs additionally precise AL measurements in such patients [14,15,16]. Determination of accurate keratometry values in keratoconus patients is feasible in mild to moderate ectasias but is prone to lower reproducibility in advanced stages [15, 17].
The Eyestar 900 (Haag-Streit AG, Koeniz, Switzerland) is a new biometer based on swept-source OCT (ss-OCT) technology. To the best of our knowledge, only limited data on the feasibility and repeatability of corneal measurements taken with the Eyestar and its agreement with other biometric or topographic devices is available for keratoconus eyes. The aim of this study is to evaluate the feasibility and repeatability of corneal measurements in keratoconus eyes with the Eyestar 900 and compare it to the IOLMaster 700, also a ss-OCT based biometry device, and to the Pentacam HR, a Scheimpflug device.
Methods
Patients
A total of 75 patients’ right eyes with keratoconus were enrolled in the Department of Ophthalmology, Inselspital, University of Bern, Bern, Switzerland for this study. All stages of keratoconus were accepted (sublinical, early to advanced); eyes with hydrops, corneal scarring or any history of corneal surgeries (except corneal crosslinking performed > 1 year prior) were excluded. The keratoconus diagnosis was based on slit-lamp findings and corneal topography with a Placido (Tomey TMS) and a Scheimpflug device (Oculus Pentacam HR).
Each cornea was measured three times with each of the devices named below, in a randomized order and a dark environment, according to the manufacturer’s guidelines. Patients were instructed to blink repeatedly before each measurement. The mean values were determined and included in the analyses. Measurements of low quality or with relevant imaging artefacts were discarded and repeated.
Devices
The Pentacam HR (software version 1-22r05), a Scheimpflug device, produces a single slit-lamp frame series in one 180° rotation, using a 475 nm blue LED, calculating a detailed topography of the anterior and posterior corneal surfaces and also measuring the anterior chamber depth.
The IOLMaster 700 (software version 1.90.12.05), biometer is based on ss-OCT technology, operating with a 1055 nm wavelength laser, allowing a full scan of the visual system. Apart from keratometry, measuring from the anterior corneal apex to the fovea allows AL measurements as well as verification of fixation quality and the central (4 mm) topography of the anterior surface and total corneal power.
The Eyestar 900 (software version 1.2.3), which is also an ss-OCT device, uses a wavelength of 1060 nm with a scan speed of 30 kHz. This biometer enables measurement and topographical assessment of the anterior and posterior corneal surface, measurement and illustration of the anterior chamber and lens and measurement of the AL (cornea-to-retina biometry).
Parameters
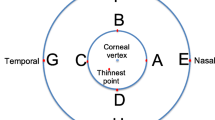
The following parameters were measured: CCT (all three devices) and thinnest point of corneal thickness (TCT; Pentacam and Eyestar), AL (IOLMaster and Eyestar), flat (K1) and steep (K2) anterior keratometry and flat posterior (Kp1) and steep posterior (Kp2) keratometry (all three devices), maximal keratometry (KMax; Pentacam and Eyestar) within the measured zone and anterior chamber depth (ACD, all three devices).
Statistical analysis
Statistical analysis was performed using SPSS version 28 (IBM, Armonk, New York, United States). The Shapiro–Wilk test was used to determine the normal distribution. For intrasubject repeatability and accuracy, the within-subject standard deviation (Sw; SD) was used to calculate precision (1.96xSw) and the repeatability index (RI = 2.77 × Sw for test–retest variability). The within-subject coefficient of variation (CoV) was determined by dividing Sw by the means, expressing high repeatability in lower values (in %). A two-way mixed model was used to assess the intraclass correlation coefficient (ICC) for reliability or variance between measurements within a device (0 = no agreement; < 0.75 = low agreement; 0.75–0.90 = moderate agreement; > 0.75 = good agreement; 1 = perfect agreement) [18].
Pearson’s r was determined to assess the correlation between devices (0–0.3 = small; 0.3–0.5 = medium; > 0.5 = large correlation; independent of the algebraic sign) [19]. Bland–Altman plots were used to evaluate the agreement between devices with 95% limits of agreement (LoA) [20, 21]. To compare means, the repeated-measures analysis of variance (ANOVA) for parametric data and the Friedman test for non-parametric data were used. A p-value of < 0.05 was considered statistically significant. However, in view of the number of comparisons (three), the significance level α was adjusted to p < 0.0167 to avoid α-error accumulation according to the principle of Bonferroni correction [22].
Ethical approval
The study was approved by the cantonal Ethics Committee of Bern (Swissethics ID #2020–0119). A written informed consent was obtained from all participants. The study was performed in accordance with the Declaration of Helsinki.
Results
In this study, a total of 75 eyes of 75 patients with keratoconus were enrolled. The mean patient age was 29.2 ± 10.8 years, and 65% of the participants were male (Table 1). All patients were Caucasians, except for one Asian and one African patient. The mean KMax value obtained with the Pentacam was 53.0 ± 6.12 D, with 34.7% of the eyes revealing KMax values below 50 D and as many above 55 D (Table 1).
Table 2 shows the repeatability of biometric measurements in keratoconus eyes for each device. Leading with an overall high repeatability value were the measurements of AL by the Eyestar (RI = 0.008, CoV = 0.01%, ICC = 1.00) and the IOLMaster (RI = 0.01, CoV = 0.02%, ICC = 1.00). A slightly lower but not significantly different (p > 0.05) repeatability between repeated measurements within each device was found for measurements of the CCT (Eyestar: RI = 5.84, CoV = 0.44%, ICC = 0.997; Pentacam: RI = 3.94, CoV = 0.30%, ICC = 0.999; IOLMaster RI = 5.41, CoV = 0.40%, ICC = 0.999), TCT (Eyestar: RI = 2.76, CoV = 0.21%, ICC = 1.0; Pentacam: RI = 4.60, CoV = 0.36%, ICC = 0.999;) and KMax (Eyestar: RI = 0.90, CoV = 0.60% ICC = 0.994; Pentacam: RI = 0.52, CoV = 0.34%, ICC = 0.999). The ICC generally showed an overall good correlation, highest for TCT measurements with the Pentacam, for AL with the Eyestar and IOLMaster and for K2 with the Eyestar (ICC = 1.0, each). Overall, there were no significant differences between the repeated measurements of each device. The differences in repeated CCT measurements with the IOLMaster were not significant. The only exception was the measurement of K1 with the IOLMaster, where the differences between the three repeated measurements was significant (p = 0.012). However, when excluding all eyes with KMax > 55 D, no significant differences were found (p = 0.82). The lowest ICC was found for K2 measured with the IOLMaster, for KMax with the Pentacam (both, ICC = 0.994), for Kp1 with the Eyestar (ICC = 0.995) and the Pentacam (ICC = 0.996) and for Kp2 with the Eyestar (ICC = 0.996; Table 2).
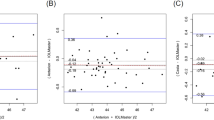
The means and SD of the differences in the device comparisons and their LoAs are shown in Table 3, whereas the corresponding Bland Altman plots are depicted in Figs. 1, 2 and 3. Overall, the measurements of all parameters with the three devices, correlated largely with each other, with r values > 0.947 for KMax, and r values > 0.96 for K1 (Eyestar vs. Pentacam) and Kp2 (Eyestar vs. IOLMaster). The largest correlation was found for AL between Eyestar and IOLMaster, for K1 between Eyestar and IOLMaster, and for ACD between Eyestar and IOLMaster (r = 1.0 for each).
Bland–Altman plots with limits of agreement for the different biometric parameters measured with the Eyestar, Pentacam and IOLMaster. Central corneal thickness (Eyestar, Pentacam, IOLMaster); KMax, Maximal Keratometry (Eyestar vs. Pentacam); Thickness at the thinnest corneal point (Eyestar vs. Pentacam); Axial length (Eyestar vs. IOLMaster)
Bland–Altman plots with limits of agreement for the different biometric parameters measured with the Eyestar, Pentacam and IOLMaster. Kp1, Posterior flat keratometry (Eyestar, Pentacam, IOLMaster); Kp2, Posterior steep keratometry (Eyestar, Pentacam, IOLMaster); Anterior chamber depth (Eyestar, Pentacam, IOLMaster)
Despite the large correlation, significant differences were found for all parameters between the three devices (p < 0.001, each), except for K1 Eyestar vs. Pentacam (p = 0.31), for K2 Pentacam vs. IOLMaster and for Kp1 Eyestar vs. Pentacam (both p = 0.54). No significant difference was found for Kp2 between Eyestar and Pentacam. The best agreement was found for CCT, TCT, Kp1 and Kp2 between Eyestar and Pentacam, for ACD between Eyestar, Pentacam and IOLMaster and for AL, K1, K2 and ACD between Eyestar and IOLMaster (Figs. 1, 2 and 3). The lowest agreement was found for KMax and CCT between Pentacam and IOLMaster, for K2 between Eyestar and Pentacam and for Kp1 and Kp2 between Pentacam and IOLMaster. The values outside the LoAs were mainly from corneas with KMax > 55 D, which contributed the most to the dispersion (Figs. 1, 2 and 3, coloured markings).
Discussion
To the best of our knowledge and up until now, there is only very limited data comparing biometric measurements with the Eyestar 900 to other devices in keratoconus eyes. In our prospective study we therefore compared measurements taken with the Pentacam, Eyestar 900 and IOLMaster 700 devices in keratoconus eyes and analysed the feasibility and repeatability of such measurements with the Eyestar 900. All measurements showed a very good repeatability for each device (Table 2) as well as correlation between the three devices (Table 3). This is consistent with the current literature, reporting such biometric measurements in patients with and without keratoconus [15, 17, 23,24,25].
Our results are in line with those of Galzignato et al., who also reported a very high repeatability of AL measurements with the Eyestar and IOLMaster 700 and a rather lower one for CCT [15, 23]. Our study found a lower repeatability of CCT measurements than that of Galzignato et al. This is most likely because they investigated biometric values in a healthy cohort and excluded keratoconus patients. The accuracy of corneal thickness measurements is known to be reduced in keratoconus patients and to depend on the extent of ectasia [15].
A study by Herber et al. showed that ss-OCT devices measured corneal thickness (CCT and TCT) in keratoconus patients with a higher accuracy and reproducibility than Scheimpflug devices, but the SD was higher than that in healthy eyes [26]. According to our data, the same also applies to TCT measurements. The ICC of the CCT values measured with the Pentacam in our results (ICC = 0.999) are quite similar to the results reported by Kumar et al. (ICC = 0.998) [27]. In our Pentacam measurements the ICC for KMax was consistent with the findings of Hashemi et al. (ICC = 0.970–0.996, depending on the keratoconus grade) [15].
To our best knowledge, there is only limited data analysing KMax measurements taken with the Eyestar. Our study shows a large correlation between the two ss-OCT devices, the Eyestar and the IOLMaster. Accurate measurement of the AL is crucial for IOL calculations and is best ensured by optimal identification of the cornea and retina with the ss-OCT device [28].
Indeed, AL measurements taken with the Eyestar and the IOLMaster revealed a high reproducibility and correlation between the two devices. However, the absolute measurement values differed significantly. Despite high accuracy, they are therefore not interchangeable. It is also well known that the AL can be prone to error in patients with a fixation problem, which might be a relevant problem in patients with advanced keratoconus [29, 30].
Anterior keratometry measurements generally showed consistent results, especially measurements of K2 with the Eyestar. This same observation has already been reported for healthy corneas [23]. In our study, those values were slightly superior to the measurements with taken with the IOLMaster and Pentacam regarding ICC and CoV (Table 2). The superiority of the Eyestar in comparison with the IOLMaster regarding K1 (Eyestar CoV = 0.16%; IOLMaster CoV = 0.218%) and K2 (Eyestar CoV = 0.19%; IOLMaster CoV = 0.226%) was also reported by Galzignato et al. but only for healthy eyes [23].
The posterior keratometry measurements with the Eyestar in our study performed rather worse, with a higher SD for repeated measurements (Kp1 = -6.56 D ± 0.68 SD; Kp2 = -7.19 D ± 0.89SD) compared to the findings by Sorkin et al. (Kp1 = 6.1 D ± 0.3 SD, Kp2 = 6.3 D ± 0.3 SD) [25].
However, their publication included only a healthy cohort and no keratoconus patients [25].
Such higher SDs are to be expected, as the posterior corneal float is also subject to pathological changes in keratoconus patients [31]. We observed no significant differences within the repeated keratometry measurement values of K1 with each device in mild to moderate keratoconus stages. However, in the whole cohort (all keratoconus stages), a significant difference between repeated measurement values of K1 was found with the IOLMaster. This again underlines that more progressive stages contribute to measurement fluctuations and lower repeatability of measurements, as has been observed by Seiler et al. for the MS39, a spectral domain OCT device, and also with the Pentacam HR [17].
The best agreement between Eyestar and IOLMaster was found for ACD, AL, K1, K2, comparable to the findings of Lender et al., whose measurements however showed slight deviations in the ACD values between the IOLMaster and the Eyestar [24]. Interestingly, the correlation between Eyestar, IOLMaster and Pentacam reported by Lender et al. were lower than in our study for certain parameters (rAL between Eyestar and IOLMaster, rK1 between IOLMaster and Eyestar, rK1 between IOLMaster and Pentacam, rK1 between Eyestar and Pentacam), although they have excluded eyes with keratoconus. On the other hand, the correlation coefficient r of K2 was similar to ours (Table 3) [24].
In our case, unlike in the study of Lender et al., three measurements were performed with each device, and the calculated means from these measurements were analysed which reduced the influence of possible outliers. Our study confirms that ss-OCT devices reveal a good agreement of biometric parameters not only in healthy eyes but even in ectatic corneas [16, 32]. Interestingly, Sorkin et al., comparing Anterion (ss-OCT) with Eyestar in a healthy cohort, found a better agreement when Eyestar reflective anterior keratometric measurements were used instead of Eyestar ss-OCT anterior keratometric measurements for IOL calculation [25].
Low agreement and correlation were found for posterior keratometry values between the Eyestar and IOLMaster in particular. Fluctuating measurements of the corneal back surface have already been observed with different biometers in the previous literature [25, 33, 34]. The significant differences in almost all devices (Table 3) indicates that measurements, though largely correlated, are not interchangeable. Furthermore, follow-up measurements and progression monitoring should be performed with the same device.
Considering the high reproducibility of measurements, changes during follow-up should likely be considered as possible signs of progression. However, measurement difficulties and fluctuations in advanced stages of keratoconus may still make interpretation difficult, and defining cut-off values for relevant changes is still challenging [15]. It should be discussed whether reproducibility and correlation are useful parameters that are sensitive enough to evaluate devices for this type of disease. The same issue has already been addressed in a recent publication by Seiler et al. [17].
The significant differences between the devices underscores the importance of monitoring a keratoconus patient ideally on the same device during follow-up. This ensures that changes in parameters are related to disease progression and not to a device change. If a clinic replaces a device, at least the first measurement(s) should ideally be taken with the old and the new device to better allow interpretation of the first set of measurements with the new device that then can be used as a new baseline. Other parameters such as refraction, visual acuity and clinical findings should additionally be included in the assessment of potential progression.
Perhaps the CoV as a percentage parameter, describing the ratio of the mean and the within-person standard deviation, allows for a better assessment of the presence of a relevant fluctuation. As shown in Table 2, even measurements with a high ICC (for example K1, all ICC = 0.999) show discrete differences in the CoV (0.21% and 0.23%). Further investigation is needed to pursue this idea in more detail.
Considering the measurement of corneal parameters in keratoconus patients, the Eyestar device may be useful because of its high repeatability, especially regarding measurements of the TCT. Unfortunately, the KMax (as an important progression and severity parameter) cannot be exported automatically at present from the Eyestar's database but must be exported manually [35]. Nevertheless, with a software update integrating such parameters, the device might have the potential for systematic assessments of ectasia severity as well as for progression analyses in keratoconus patients.
The strengths of this study are the prospective design and the even distribution of different keratoconus stages in the cohort (Table 1). However, this study also has two main limitations: The analyses were conducted without separation by severity of ectasia. It would be interesting for future studies to compare different severities. The KMax value of the Eyestar cannot be automatically exported by the software currently but must be manually extracted from the tangential corneal maps.
In summary, this study reveals good reproducibility of the biometric parameters in keratoconus corneas for the Eyestar and a large correlation to the IOLMaster and the Pentacam. In corneas with advanced stages of keratoconus, measurements appear to be less reliable.
Change history
29 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00417-023-06258-z
References
Rabinowitz YS (1998) Keratoconus. Surv Ophthalmol 42(4):297–319
Gokhale NS (2013) Epidemiology of keratoconus. Indian J Ophthalmol 61(8):382–383
Kennedy RH, Bourne WM, Dyer JA (1986) A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol 101(3):267–273
Nielsen K, Hjortdal J, Aagaard Nohr E et al (2007) Incidence and prevalence of keratoconus in Denmark. Acta Ophthalmol Scand 85(8):890–892
Torres Netto EA, Al-Otaibi WM, Hafezi NL et al (2018) Prevalence of keratoconus in paediatric patients in Riyadh. Saudi Arabia Br J Ophthalmol 102(10):1436–1441
Scheimpflug T (1904) Improved method and apparatus for the systematic alteration or distortion of plane pictures and images by means of lenses and mirrors for photography and for other purposes. GB patent no. 1196
Shi Y (2016) Strategies for improving the early diagnosis of keratoconus. Clin Optom (Auckl) 8:13–21
Chan TCY, Biswas S, Yu M et al (2017) Comparison of corneal measurements in keratoconus using swept-source optical coherence tomography and combined Placido-Scheimpflug imaging. Acta Ophthalmol 95(6):e486–e494
Karnowski K, Kaluzny BJ, Szkulmowski M et al (2011) Corneal topography with high-speed swept source OCT in clinical examination. Biomed Opt Express 2(9):2709–2720
Yip H, Chan E (2019) Optical coherence tomography imaging in keratoconus. Clin Exp Optom 102(3):218–223
Gustafsson I, Bergstrom A, Myers AC et al (2020) Association between keratoconus disease severity and repeatability in measurements of parameters for the assessment of progressive disease. PLoS One 15(2):e0228992
Sheard R (2014) Optimising biometry for best outcomes in cataract surgery. Eye (Lond) 28(2):118–125
Cua IY, Qazi MA, Lee SF et al (2003) Intraocular lens calculations in patients with corneal scarring and irregular astigmatism. J Cataract Refract Surg 29(7):1352–1357
Sadoughi MM, Einollahi B, Einollahi N et al (2015) Measurement of Central Corneal Thickness Using Ultrasound Pachymetry and Orbscan II in Normal Eyes. J Ophthalmic Vis Res 10(1):4–9
Hashemi H, Yekta A, Khabazkhoob M (2015) Effect of keratoconus grades on repeatability of keratometry readings: Comparison of 5 devices. J Cataract Refract Surg 41(5):1065–1072
Oh R, Oh JY, Choi HJ et al (2021) Comparison of ocular biometric measurements in patients with cataract using three swept-source optical coherence tomography devices. BMC Ophthalmol 21(1):62
Seiler TG, Mueller M, Mendes Baiao T (2022) Repeatability and Comparison of Corneal Tomography in Mild to Severe Keratoconus Between the Anterior Segment OCT MS-39 and Pentacam HR. J Refract Surg 38(4):250–255
Bland JM, Altman DG (1999) Measuring agreement in method comparison studies. Stat Methods Med Res 8(2):135–160
Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale, NJ
Bland JM, Altman DG (1996) Measurement error. BMJ 313(7059):744
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310
Bonferroni CE (1936) Teoria statistica delle classi e calcolo delle probabilità. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze 8:3–62
Galzignato A, Lupardi E, Hoffer KJ et al (2023) Repeatability of new optical biometer and agreement with 2 validated optical biometers, all based on SS-OCT. J Cataract Refract Surg 49(1):5–10
Lender R, Mirsky D, Greenberger R et al (2022) Evaluation of three biometric devices: ocular parameters and calculated intraocular lens power. Sci Rep 12(1):19478
Sorkin N, Achiron A, Abumanhal M et al (2022) Comparison of two new integrated SS-OCT tomography and biometry devices. J Cataract Refract Surg 48(11):1277–1284
Herber R, Lenk J, Pillunat LE et al (2022) Comparison of corneal tomography using a novel swept-source optical coherence tomographer and rotating Scheimpflug system in normal and keratoconus eyes: repeatability and agreement analysis. Eye Vis (Lond) 9(1):19
Kumar M, Shetty R, Jayadev C et al (2015) Comparability and repeatability of pachymetry in keratoconus using four noncontact techniques. Indian J Ophthalmol 63(9):722–727
Tana-Rivero P, Tana-Sanz S, Pastor-Pascual F et al (2022) Axial length measurement failure rates using optical biometry based on swept-source OCT in cataractous eyes. Expert Rev Med Devices 19(8):633–640
Zhu X, He W, Sun X et al (2016) Fixation Stability and Refractive Error After Cataract Surgery in Highly Myopic Eyes. Am J Ophthalmol 169:89–94
Bozorg S, Pineda R (2014) Cataract and keratoconus: minimizing complications in intraocular lens calculations. Semin Ophthalmol 29(5–6):376–379
Tomidokoro A, Oshika T, Amano S et al (2000) Changes in anterior and posterior corneal curvatures in keratoconus. Ophthalmology 107(7):1328–1332
Fisus AD, Hirnschall ND, Findl O (2021) Comparison of 2 swept-source optical coherence tomography-based biometry devices. J Cataract Refract Surg 47(1):87–92
Kose B (2022) Agreement between swept-source optical biometry and Scheimpflug-based topography measurements of posterior corneal curvature. J Cataract Refract Surg 48(2):185–189
Mi H, Tan N, Ang M (2015) Comparison of anterior and posterior topographic analysis between 3 imaging systems. J Cataract Refract Surg 41(11):2533–2545
Epstein RL, Chiu YL, Epstein GL (2012) Pentacam HR criteria for curvature change in keratoconus and postoperative LASIK ectasia. J Refract Surg 28(12):890–894
Funding
The project was funded by an unrestricted grant from the Haag Streit Foundation, which is a Swiss non-profit foundation with the aim of promoting applied ophthalmology and related basic research in the field of medical technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the cantonal Ethics Committee of Bern (Swissethics ID #2020–0119).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure of potential conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the cantonal Ethics Committee of Bern and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Financial disclosure
The authors declare to have no financial interests.
Conflicts of interests
The authors decline any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. The entries in Table 3, row “Kp2 (D)2”, column “95% LoA” are now corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bograd, A., Himmel, I., Pfister, I.B. et al. Comparison of corneal measurements in keratoconus eyes with two swept-source-optical coherence tomography devices and a Scheimpflug device. Graefes Arch Clin Exp Ophthalmol 262, 891–901 (2024). https://doi.org/10.1007/s00417-023-06219-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06219-6