Abstract
Purpose
To analyze the impact of axial length (AL) on the visual outcome and rate of perioperative complications in phacoemulsification surgery.
Design
Retrospective clinical database study.
Methods
Cataract surgery data of 217,556 eyes was extracted from the electronic medical records of 8 ophthalmic centers in the United Kingdom from July 2003 to March 2015. A total of 88,774 eyes without ocular co-pathologies were grouped eyes according to AL (mm): short AL (< 22), average AL (22–26; reference group), and long AL (> 26).
Main outcomes and measures
We analyzed visual acuity (VA) outcomes at 4 weeks, 4–12 weeks, and 12–24 weeks postoperatively, as well as the incidence of posterior capsular rupture (PCR), torn iris (TI), cystoid macular edema (CME), and retinal detachment (RD).
Results
Mean pre-operative VA (logMAR) was the worst in eyes with long AL compared to average and short AL eyes (VA 0.59 vs. 0.58 and 0.56; p < 0.001). However, post-operative VA at 4–12 weeks was slightly better in the long AL group (0.14 in short and average AL; 0.12 in long AL, p < 0.001). We observed an increased odds of TI in the short AL group (OR 2.09, 95% CI 1.60–2.75). There was increased risk of RD in long AL eyes (p < 0.001). However, PCR and CME rates were not different.
Conclusion
In the absence of any coexisting ocular pathology, AL alone did not have an impact on VA improvement or the risk of encountering PCR or CME. The risk of TI was greater in the short AL group, and the risk of RD was higher in the long AL group.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Cataract is the leading cause of blindness in the world, and cataract surgery is the most commonly performed surgery in the entire medical profession [1]. Axial length (AL) is an important parameter in determining the dioptric power of the intraocular lens (IOL) implant and may potentially influence several aspects of cataract surgery. There is limited literature on the effect of AL on cataract surgery outcomes [2, 6]. Previous studies have shown that cataract surgery on eyes with short or long AL may be associated with an increased risk of perioperative complications [2, 7]. Eyes with short AL may have shallow anterior chamber with limited space for maneuvering instruments [8], while eyes with long AL can pose a challenge due to altered anterior chamber stability and fluidics, greater incidence of lens/iris diaphragm retropulsion, and hypermobility of the posterior capsule [9, 11].Few studies have shown a worse postoperative visual acuity (VA) in extreme AL eyes[12, 13], with one study finding that only 20–29% of extreme AL eyes achieved 20/25 postoperative VA [4]. In addition, there are conflicting results with regards to incidence of posterior capsular rupture (PCR) with some studies reporting higher rates of PCR in extreme AL eyes of up to 5.7–12.5% [14, 19]. Studies on the outcome of long and short AL eyes are generally limited by their smaller sample sizes, absence of comparison groups, and more importantly inclusion of eyes with other ocular co-pathologies. These factors make it difficult to accurately evaluate the impact of AL on the outcome of cataract surgery.
Large database studies analyzing the surgical outcomes in cataract surgery are important for guiding decision-making and surgical management to improve patient care. In this study, we utilized a large multicenter dataset from the United Kingdom (UK) National Health Service to evaluate the impact of AL on visual outcomes and incidence of intraoperative complications, cystoid macular edema (CME), and retinal detachment (RD) in eyes undergoing phacoemulsification.
Methods
Data extraction
We pooled phacoemulsification data sets from ophthalmology departments at 8 different UK National Health Service sites to a centralized database for analysis. All centers used the same electronic medical record (EMR) system (Medisoft Ophthalmology; Medisoft Limited). A previous study by Chu et al. utilizing the same data set described the standard of care at these various sites [20]. A period of about 12 years (July 2003 to March 2015) was selected and postoperative care included at least one visit 4–6 weeks following surgery conducted by a specialty nurse, optometrist, or ophthalmologist.
Extracted data fields included: age, gender, AL, pre- and postoperative VA, presence of postoperative CME, intraoperative complications including PCR, PCR with vitreous loss, torn iris (TI), and RD. Fields extracted from the operative record for cataract surgery included: pupil size, grade of operating surgeon, and type of surgery performed. Recording of intraoperative complications was a compulsory field. Surgeons were mandated to record intraoperative complications in the EMR by selecting from a prespecified list of cataract surgery complications prior to finalizing the operative record or select “other” and record the complication using free text. If no complication occurred, the surgeon then selects “none”.
This study was conducted in accordance with the Declaration of Helsinki. Approval for data extraction was provided by the lead clinician at each center and the Caldicott Guardian, who oversees the data protection [20]. The extracted patient information was de-identified and therefore was not classified as human-subject research, and informed consent from patients was not required.
Exclusion criteria and data categorization
We identified an initial database of a total of 217,556 eyes that underwent phacoemulsification with some undergoing combined surgery between July 2003 to March 2015. We further grouped these eyes based on their AL. The short AL group (< 22 mm) included 23,095 eyes, the average AL group (22–26 mm) included 184,054 eyes, and the long AL group (> 26 mm) included 10,427 eyes. Figure 1 details the distribution of eyes and filtering criteria. Of this cohort, we then excluded eyes that underwent combined surgery or multiples surgeries by selecting only eyes that had phacoemulsification with or without IOL implantation and with or without anterior vitrectomy. Combined surgeries include any additional surgical procedures done in conjunction with phacoemulsification during the same operative session. We excluded eyes of patients younger than 18 years of age and of patients with unknown diabetic status (26,574 eyes). In addition, we excluded eyes with any other prior ocular co-pathologies (i.e., age-related macular degeneration, glaucoma, diabetic retinopathy, etc). High myopia was not considered a co-pathology since our study examines outcomes in relation to AL. For this analysis, we excluded all second-operated eyes in patients undergoing bilateral cataract surgery to remove possible intrasample correlation between both eyes of the same patient.
Follow-up and study outcomes
The main outcome variables for this study were postoperative VA, and incidence of TI, PCR (with or without vitreous loss), postoperative CME, and RD. All VA values were automatically converted at the time of data extraction to the logarithm of the minimum angle of resolution (logMAR). We designated VA as the best value of unaided or best-corrected distance VA available at each visit. We defined preoperative VA as the recorded VA at the closest visit to the surgery date, no more than 3 months prior. The visual gain was defined as a postoperative visual improvement of ≥ 0.3 logMAR units (~ 3 Snellen lines). We defined postoperative CME as the recorded occurrence of CME within 90 days of cataract surgery. The study centers over the study period performed imaging studies, including optical coherence tomography (OCT) or fluorescein angiography (FA) at the clinicians’ discretion, typically in patients with unexpected VA outcomes following cataract surgery. Therefore, CME in our study reflects visually significant CME rather than subclinical disease. To eliminate the confounding effects of diabetic macular edema, any eyes with diabetic retinopathy were already excluded under the exclusion of eyes with other ocular co-pathologies. We divided the follow-up time into 3 time periods: 0–4 weeks, 4–12 weeks, and 12–24 weeks postoperatively.
Statistical analysis
We compared demographic, clinical characteristics, and intraoperative/postoperative complications of patients at varying AL categories with χ2 tests for categorical variables and ANOVA for continuous variables. We followed up significant endpoints from χ2 with simple and multivariate logistic regression models. We then assessed potential confounders in these models based on prior knowledge and included age, gender, surgeon grade, diabetic status, preoperative VA, and pupil size. We generated odds ratios (ORs) from final analyses for significant endpoints using logistic regression models. Each model used eyes with average AL as the reference group. We chose the AL categorizations in this study based on existing literature. However, as the cut-off AL values have slightly varied in prior studies and may be argued, we also conducted a sensitivity analysis of complications rate with AL as a continuous variable, using receiver operating characteristic (ROC) curves. We performed the primary analyses using Stata Release 14.2 (StataCorp LP) and GraphPad Prism version 8 (La Jolla, CA, USA, www.graphpadprism.com). We considered tests with 2-sided P values < 0.05 as statistically significant.
Results
Demographics of study eyes
There was a total of 9,412 eyes in the short AL group, 74,165 eyes in the average AL group, and 5,197 eyes in the long AL group. Table 1 summarizes the demographic and intraoperative characteristics of the study eyes. Patients in the short AL group were older (75.8 years, p < 0.001) than patients in the average and long AL groups (74.7 and 70.3 years, respectively). The proportion of patients with diabetes mellitus was greater in the average AL group (17.9%, p = 0.001). The number of eyes left aphakic and required secondary IOL implantation was 149 (17 in short AL, 108 in average AL, and 24 in long AL eyes). The number of attending-level (consultant) surgeons performing primary surgeries was 48,718 (54.88%), while 40,056 (45.12%) were non-attendings (residents, fellows, middle-grade surgeons). The number of attending surgeons by AL included 5,136 (54.6%) in the short AL, 40,795 (56%) in the average AL, and 2,787 (53.6%) in the long AL group. There was no difference in probability distributions of surgeon grade by AL group (p = 0.126).
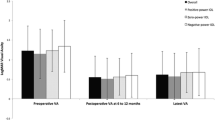
Visual acuity assessment
Figure 2 and Table 2 summarize the preoperative and postoperative LogMAR VA outcomes in the study groups. Mean preoperative VA (logMAR) was only slightly worst in eyes with long AL compared to average and short AL eyes (VA 0.59 vs. 0.58 and 0.56; p < 0.001). However, postoperative VA at 4–12 weeks was slightly better in the long AL group (0.14 in short and average AL; 0.12 in long AL, p < 0.001). We observed similar findings at 12–24 weeks.
In examining visual improvement using a threshold of 0.30 logMAR or more (≥ 3 Snellen lines), we found that the proportion of eyes in the long AL group (65.6% at 12–24 weeks) that achieved this threshold was slightly greater than those in the short (61.9%) or average AL (62.0%) groups (p = 0.033).
Torn iris
We found that the short AL group had a significantly higher incidence of TI at 0.76%, compared to 0.38% for average AL group and 0.08% for long AL group (p < 0.001). Odds ratios from adjusted logistic regression model revealed that TI was 2 times more likely to develop in eyes with short AL than in eyes with average AL (p < 0.001), while the odds ratio of TI for eyes with long AL was 0.21 (p < 0.01) (Table 3 and Fig. 3).
Posterior capsular rupture
The proportion of eyes that developed PCR with or without vitreous loss was not greater in long (73 eyes,1.40%) or short (142 eyes,1.51%) AL groups compared to the average (1,397 eyes,1.88%) AL group (p = 0.003). Odds ratios from the adjusted logistic regression model showed that the odds of the development of PCR were 0.76 for the short AL group (p < 0.01) and 0.71 for the long AL group (p < 0.05) (Table 3). There was no difference in the rate of zonular dialysis between the 3 study groups.
Cystoid macular edema
Cystoid macular edema developed in 106 (1.13%) eyes in the short AL group, in 692 (0.93%) eyes in the average AL group, and in 43 (0.83%) eyes in long AL group (p = 0.124) (Table 3). To control for unmatched differences in preoperative characteristics, we conducted a logistic regression analysis to determine the OR of CME according to AL. The model was adjusted for age, gender, surgeon grade, diabetic status, preoperative VA, pupil size, and TI. We used TI as a covariate in this analysis because we hypothesized that iris manipulations may influence CME development. We found no difference in the adjusted OR for development of CME in different AL groups (OR: 1.19 times greater than average in short AL group, p = 0.097) (Table 3).
Retinal detachment and choroidal hemorrhage
We found that there was a significantly greater proportion of eyes developing RD in the long AL group (1.08%) compared to the short AL (0.05%) and average AL (0.17%), (p < 0.001). On logistic regression analysis, this corresponded to 6.27 higher odds of RD as compared to average AL group (Confidence interval: 4.54–8.66) (Table 3 and Fig. 3). Choroidal hemorrhage was slightly higher in eyes with short AL (0.08%) but the differences were not significant from average and short eyes (0.04% each).
Sensitivity analysis for complications
Figure 4 depicts the predictive performance of AL as a continuous variable for development of CME, TI, zonular dialysis, PCR, choroidal hemorrhage, and RD following phacoemulsification. Using area under the curve (AUC) analysis, AL was most accurate for predicting RD (AUC 0.78), choroidal hemorrhage (AUC 0.62), and TI (AUC 0.61) but not for zonular dialysis, PCR, and CME. Overall, these results were in line with our logistic regression analyses of complications using AL as a non-categorized criterion.
Discussion
In this multicenter database study, we evaluated the relationship of the AL and the visual outcome and the incidence of intra- and post-operative complications in eyes undergoing phacoemulsification. To be able to precisely determine the impact of AL, we eliminated the impact of other ocular co-pathologies on the selected outcome measures. We found that long eyes achieved slightly better VA outcomes than short and average AL eyes, but the difference in VA between any of the three AL groups at any time point was ≤ 0.04 logMAR which is equivalent to ≤ 2 Snellen letters and therefore clinically insignificant. Our analysis showed that there were 2 times greater odds of TI in the short AL group and 6 times greater odds of RD in the long AL group as compared to the average AL eyes. In addition, the proportion of eyes that experienced a PCR or CME were not greater in the short or long AL group compared to the average AL group.
We found that the final postoperative VA in the long AL group was not inferior to average and shorter AL groups at postoperative time periods: at 4–12 weeks, VA was 0.14 logMAR in the short AL group, 0.14 logMAR in the average AL group, and 0.12 logMAR in the long AL group. The proportion of eyes that achieved visual improvement threshold (> 0.3 logMAR) after surgery at 12–24 weeks was slightly greater in the long AL group, which may be due to that the long AL group started off with worse preoperative VA. It is of note that our results contradict those from other studies that found that non-average AL eyes have slightly worse postoperative VA [4, 12]. Mohammadi et al. conducted a retrospective review of 353 patients (405 eyes) that underwent cataract surgery, found that the mean postoperative VA was lower in long (AL > 24.5 mm; 0.59 LogMAR) and short (AL < 22 mm, 0.34 LogMAR) eyes compared to average AL (AL 22–24.5, 0.17 LogMAR) and that the former groups have a 3.24 greater odds of poorer visual outcomes than average AL eyes [4]. The authors suggested that high AL may correlate with degenerative myopia and short AL eyes have higher frequency of amblyopia. Another retrospective review of 171 consecutive cataract surgery cases found better outcomes in average AL (22–25 mm) eyes than in short AL (< 22 mm) eyes [12]. However, those studies excluded cases with intra- and post-operative complications and data regarding associated co-pathologies is unknown. By contrast, in our study, we excluded amblyopic eyes and other ocular co-pathologies which allowed us to precisely determine the impact of AL on VA.
The typically reported incidence of CME after uncomplicated cataract surgery in contemporary studies is cited as 1–2% [21, 22]. In our cohort, the overall incidence of postoperative CME was comparable at 0.95%. We found no differences in the CME rate between the average, and short and long AL groups. Other studies also found no difference in the rate of CME with AL changes. One such study examined 1,659 patients and did not find a significant difference in AL between eyes that did or did not develop CME [23]. Their analysis grouped patients according to CME development and compared AL averages among the groups; conversely, our study grouped patients according to AL and analyzed CME rates among the groups.
We also found a significantly increased odds of TI in the short AL group and a decreased odds in the long AL when compared to the average AL group. We hypothesize that this may be because short AL eyes may have a shallow anterior chamber which increases the risk of iris prolapse and damage during surgery. To our knowledge, the increased likelihood of TI in shorter AL eyes has not previously been reported in the literature. However, our interpretation is limited because the preoperative anterior chamber depth measurements were not available in the current data extract and a large proportion of eyes with short AL may have a normal anterior chamber depth [8].
Examining studies of PCR rates according to AL has shown differing results. Most studies report higher rates of PCR in eyes with long AL [14, 18]. Fesharaki et al. reported PCR rate of 5.7% in eyes with AL of > 26 mm [14]. A recent study by Yao et al. reported the incidence of PCR in high myopia to range from 1.8% to 15.6% compared to 1.8–1.92% in the general population [2]. In short eyes, studies have reported PCR rates of 11.7% in AL < 20.5 mm; however, the study included only 17 nanophthalmic eyes [24]. On the other hand, Day et al. evaluated 103 microphthalmic and nanophthalmic eyes with mean AL of 20.5 mm and reported no cases of PCR [7]. In a database study of 180,114 eyes with co-pathologies, PCR rates (mean 1.95%) showed minimal change with AL measured as a continuous variable [3]. Similarly, in our study, we did not find an increased risk of PCR in the extreme AL groups as compared to average AL, and we found lower rates of PCR overall. Compared to the study by Day et al., we maintained cut-off AL < 22 mm for our short AL group and excluded patients with ocular co-pathologies [3]. These exclusions may account for our slightly lower PCR rates of 1.82%. Long AL has long been established in the literature as a risk factor for RD after cataract surgery [25, 29]. A large retrospective series out of Taiwan found an adjusted risk ratio of 4.19 in patients with AL > 26 mm compared to AL of < 23 mm [30]. In another study from France examining over 2.5 million eyes, the 4-year probability of RD after cataract surgery was 9.21% with high myopia (collected using ICD 10 codes), and the hazard ratio was 6.12 [31]. Our study had similar findings to these previous studies with 6.27 times greater odds of RD in patients with AL > 26 mm.
Our study must be interpreted with the following limitations in mind. First, AL categorizations were chosen based on existing literature, however, the cut-off AL values have slightly varied in prior studies and may be argued; to account for this, we conducted a non-categorized analysis with AL as a continuous variable, but the results were not different. Secondly, in the short AL group, we did not differentiate between microphthalmos and nanophthalmos and did not analyze the anterior chamber depth which may have influenced the rate of complications. Third are the limitations inherent to a non-randomized retrospective study including the presence of unaccounted differences in preoperative characteristics between the three AL groups influencing the results of the outcomes measured. To lessen this bias, we included strict filtering criteria in our analysis including both crude and adjusted statistics and logistic regression analyses to account for potential confounding factors. Additionally, as with other database studies, not all patients had follow-up data for all postoperative time points leading to some missing information in the dataset. Some cases of CME and RD may not have been captured in our study as patients were followed up to 24 weeks. Future studies with longer follow-up periods may provide additional insights into the long-term risk of these complications. Furthermore, there is limited knowledge of the uniformity of variables evaluated, for example, the methods of CME detection may have varied. While this may be a source of bias, we expect this to be non-differential, across the three AL groups, and not to change the significance of our findings. Data on the type of anesthesia used was not available in the original dataset, thus we could not explore the impact of anesthesia type on outcomes. Finally, data on potentially important postoperative complications, such as pseudophakic bullous keratopathy and ocular hypertension, were also not included in our analysis due to the lack of available data in the dataset.
The study has several strengths. To our knowledge, this is the largest scale study to use AL to stratify pre-, intra-, and post-operative outcomes undergoing cataract surgery. While a prospective randomized controlled study represents the gold-standard level of evidence, conducting such a study design to assess the impact of AL on cataract surgery outcomes on a large scale and with the exclusion of ocular co-morbidities is not feasible. A distinct strength of this study is its pragmatic nature with the automatic extraction of highly structured data collected from multiple centers. This makes the results more accurate than those from surgical registry studies, where surgeons manually enter the details of their cases. The large sample size allowed precise analysis of the impact of AL on VA and perioperative complications by eliminating the influence of ocular co-pathologies, which previous studies were not able to do. In addition, we did not include the second operated eye in our analysis, thereby minimizing the influence of intrasample correlation. A further strength of the study is the completeness and accuracy of intraoperative complications recording. Recording of intraoperative complications in the EMR was a mandatory field and thus physicians could not forget or omit to record a complication unless they make a false declaration. Given that AL is a universally obtained value prior to cataract surgery, knowing its associations with certain perioperative complications, and expected VA outcomes is valuable. Our study may help surgeons tailor discussions of expected visual outcomes and perioperative cataract surgery complications with patients according to their AL ranges.
In conclusion, in the absence of coexisting ocular pathologies, longer or shorter AL in and of itself did not negatively impact VA improvement or increased the risk for developing PCR or CME. We also found that shorter AL was associated with increased incidence of TI and decreased incidence of RD, while longer AL was associated with increased incidence of RD.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Pascolini D, Mariotti SP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96:614–618. https://doi.org/10.1136/bjophthalmol-2011-300539
Yao Y, Lu Q, Wei L, Cheng K, Lu Y, Zhu X (2021) Efficacy and complications of cataract surgery in high myopia. J Cataract Refract Surg 47:1473–1480. https://doi.org/10.1097/j.jcrs.0000000000000664
Day AC, Donachie PH, Sparrow JM, Johnston RL, Database RCoONO (2015) The Royal College of Ophthalmologists’ National Ophthalmology Database Study of cataract surgery: report 2, relationships of axial length with ocular copathology, preoperative visual acuity, and posterior capsule rupture. Eye (Lond) 29:1528–1537. https://doi.org/10.1038/eye.2015.198
Mohammadi SF, Hashemi H, Mazouri A, Rahman-A N, Ashrafi E, Mehrjardi HZ, Roohipour R, Fotouhi A (2015) Outcomes of cataract surgery at a referral center. J Ophthalmic Vis Res 10:250–256. https://doi.org/10.4103/2008-322X.170358
Elhusseiny AM, Salim S (2023) Cataract surgery in myopic eyes. Curr Opin Ophthalmol 34:64–70. https://doi.org/10.1097/ICU.0000000000000914
Elhusseiny AM, Sallam AB (2023) Cataract surgery in adult eyes with short axial length. Curr Opin Ophthalmol 34:84–93. https://doi.org/10.1097/ICU.0000000000000913
Day AC, MacLaren RE, Bunce C, Stevens JD, Foster PJ (2013) Outcomes of phacoemulsification and intraocular lens implantation in microphthalmos and nanophthalmos. J Cataract Refract Surg 39:87–96. https://doi.org/10.1016/j.jcrs.2012.08.057
Hoffman RS, Vasavada AR, Allen QB, Snyder ME, Devgan U, Braga-Mele R (2015) Cataract surgery in the small eye. J Cataract Refract Surg 41:2565–2575. https://doi.org/10.1016/j.jcrs.2015.10.008
Chong EW, Mehta JS (2016) High myopia and cataract surgery. Curr Opin Ophthalmol 27:45–50. https://doi.org/10.1097/ICU.0000000000000217
Li X, Li Q, Bano S, Li S (2021) Phacoemulsification in vitrectomized eyes: Maintaining the stability of the anterior chamber via a new technique. Eur J Ophthalmol 31(3):1492–1496. https://doi.org/10.1177/1120672120940192
Fernández-Buenaga R, Alio JL, Pérez-Ardoy AL, Larrosa-Quesada A, Pinilla-Cortés L, Barraquer R, Muñoz-Negrete FJ (2013) Late in-the-bag intraocular lens dislocation requiring explantation: risk factors and outcomes. Eye (Lond) 27:795–801; quiz 802. https://doi.org/10.1038/eye.2013.95
de Juan V, Martín R, Pérez I, Herreras JM (2010) Influence of axial length in refractive outcome after cataract surgery. Arch Soc Esp Oftalmol 85:144–148
Zheng T, Chen Z, Xu J, Tang Y, Fan Q, Lu Y (2017) Outcomes and prognostic factors of cataract surgery in adult extreme microphthalmos with axial length <18 mm or corneal diameter <8 mm. Am J Ophthalmol 184:84–96. https://doi.org/10.1016/j.ajo.2017.09.028
Fesharaki H, Peyman A, Rowshandel M, Peyman M, Alizadeh P, Akhlaghi M, Ashtari A (2012) A comparative study of complications of cataract surgery with phacoemulsification in eyes with high and normal axial length. Adv Biomed Res 1:67. https://doi.org/10.4103/2277-9175.102971
Lyle WA, Jin GJ (1996) Phacoemulsification with intraocular lens implantation in high myopia. J Cataract Refract Surg 22:238–242. https://doi.org/10.1016/s0886-3350(96)80225-2
Fan DS, Lam DS, Li KK (1999) Retinal complications after cataract extraction in patients with high myopia. Ophthalmology 106: 688-691; discussion 691-682. https://doi.org/10.1016/S0161-6420(99)90152-5
Alio JL, Ruiz-Moreno JM, Shabayek MH, Lugo FL, Abd El Rahman AM (2007) The risk of retinal detachment in high myopia after small incision coaxial phacoemulsification. Am J Ophthalmol 144:93–98. https://doi.org/10.1016/j.ajo.2007.03.043
Zuberbuhler B, Seyedian M, Tuft S (2009) Phacoemulsification in eyes with extreme axial myopia. J Cataract Refract Surg 35:335–340. https://doi.org/10.1016/j.jcrs.2008.10.044
Yuzbasioglu E, Artunay O, Agachan A, Bilen H (2009) Phacoemulsification in patients with nanophthalmos. Can J Ophthalmol 44:534–539. https://doi.org/10.3129/i09-142
Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC, Group UKPMES (2016) Risk factors and incidence of macular edema after cataract surgery: a database study of 81984 eyes. Ophthalmology 123:316–323. https://doi.org/10.1016/j.ophtha.2015.10.001
Yonekawa Y, Kim IK (2012) Pseudophakic cystoid macular edema. Curr Opin Ophthalmol 23:26–32. https://doi.org/10.1097/ICU.0b013e32834cd5f8
Lobo C (2012) Pseudophakic cystoid macular edema. Ophthalmologica 227:61–67. https://doi.org/10.1159/000331277
Henderson BA, Kim JY, Ament CS, Ferrufino-Ponce ZK, Grabowska A, Cremers SL (2007) Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg 33:1550–1558. https://doi.org/10.1016/j.jcrs.2007.05.013
Jung KI, Yang JW, Lee YC, Kim SY (2012) Cataract surgery in eyes with nanophthalmos and relative anterior microphthalmos. Am J Ophthalmol 153:1161-1168.e1161. https://doi.org/10.1016/j.ajo.2011.12.006
Haug SJ, Bhisitkul RB (2012) Risk factors for retinal detachment following cataract surgery. Curr Opin Ophthalmol 23:7–11. https://doi.org/10.1097/ICU.0b013e32834cd653
Erie JC, Raecker ME, Baratz KH, Schleck CD, Robertson DM (2006) Risk of retinal detachment after cataract extraction, 1980–2004: a population-based study. Trans Am Ophthalmol Soc 104:167–175
Boberg-Ans G, Villumsen J, Henning V (2003) Retinal detachment after phacoemulsification cataract extraction. J Cataract Refract Surg 29:1333–1338. https://doi.org/10.1016/s0886-3350(03)00057-9
Russell M, Gaskin B, Russell D, Polkinghorne PJ (2006) Pseudophakic retinal detachment after phacoemulsification cataract surgery: Ten-year retrospective review. J Cataract Refract Surg 32:442–445. https://doi.org/10.1016/j.jcrs.2005.12.095
Neuhann IM, Neuhann TF, Heimann H, Schmickler S, Gerl RH, Foerster MH (2008) Retinal detachment after phacoemulsification in high myopia: analysis of 2356 cases. J Cataract Refract Surg 34:1644–1657. https://doi.org/10.1016/j.jcrs.2008.06.022
Sheu SJ, Ger LP, Chen JF (2006) Axial myopia is an extremely significant risk factor for young-aged pseudophakic retinal detachment in taiwan. Retina 26:322–327. https://doi.org/10.1097/00006982-200603000-00011
Daien V, Le Pape A, Heve D, Carriere I, Villain M (2015) Incidence, risk factors, and impact of age on retinal detachment after cataract surgery in France: a national population study. Ophthalmology 122:2179–2185. https://doi.org/10.1016/j.ophtha.2015.07.014
Acknowledgements
None
Funding
We have no financial disclosures, and this project did not receive any funding.
Author information
Authors and Affiliations
Contributions
Conception and design: Yang, Soliman, Elhusseiny, and Sallam.
Analysis and interpretation: Chauhan, Elhusseiny, Soliman, and Sallam.
Data collection: Yang and Sallam.
Writing the manuscript: Ahmad, Soliman, and Elhusseiny.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval for data extraction was provided by the lead clinician at each center and the Caldicott Guardian, who oversees data protection. The extracted patient information was de-identified and therefore was not classified as human subject research and informed consent from patients was not required.
The study was conducted under Health Insurance Portability and Accountability Act (HIPAA) compliance and adhered to the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahmad, K.T., Chauhan, M.Z., Soliman, M.K. et al. Impact of axial length on visual outcomes and complications in phacoemulsification surgery: a multicenter database study. Graefes Arch Clin Exp Ophthalmol 261, 3511–3520 (2023). https://doi.org/10.1007/s00417-023-06120-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06120-2