Abstract
Purpose
To compare long-term visual function after implantation of diffractive extended depth-of-focus (EDF) intraocular lenses (IOLs) using echelett optics and monofocal IOLs with the same platform.
Methods
In this prospective comparative case series, diffractive EDF or monofocal IOLs were implanted binocularly and followed up for 2 years. At the last visit, distance-corrected binocular visual acuities were measured at distances of 0.3, 0.5, 0.7, 1, 2, 3, and 5 m. Photopic and mesopic contrast sensitivity was also examined. Dynamic visual function was evaluated in terms of functional visual acuity (FVA), standard deviation of visual acuity (SDVA), visual maintenance ratio (VMR), mean response time, and number of blinks. The outcomes were compared between the two IOLs, and the influence of posterior capsule opacification (PCO) on contrast sensitivity and FVA was examined.
Results
Binocular visual acuity of eyes with EDF IOLs was better at distances of 0.5 and 0.7 m than that of eyes with monofocal IOL (P < 0.026). There were no differences in binocular visual acuity at other distances, contrast sensitivities, or dynamic visual functions. The influence of PCO on the visual functions was not found in eyes with EDF IOLs.
Conclusion
Up to 2 years postoperatively, eyes with diffractive EDF IOLs sustained superior intermediate visual acuity together with visual function comparable to that of eyes with monofocal IOLs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.

Introduction
Currently, various presbyopia-correcting intraocular lenses (IOLs) are available, and diffractive extended depth-of-focus (EDF) IOLs are chosen to obtain postoperative vision between far and intermediate distances with the least photic phenomena [1]. The Symfony® IOL (Johnson & Johnson Surgical Vision, Santa Ana, CA, USA) is designed for providing an EDF function by utilizing echelle optics and could provide 20/20 or better visual acuities at distances of 0.7 m or greater [1, 2]. Owing to the compensation of chromatic aberrations [3], postoperative contrast sensitivities of EDF IOLs are comparable with those of monofocal IOLs [4, 5].
Presbyopia correction in eyes with bifocal IOLs is sensitive to optical distortions such as mild posterior capsule opacification (PCO) [6, 7]. Thus, it is difficult to sustain the postoperative quality of vision over time, and neodymium:YAG (Nd:YAG) laser capsulotomy is more frequently performed in eyes with bifocal IOLs than in eyes with monofocal IOLs [8]. As the through-focus property of EDF IOLs is comparable to that of monofocal IOLs [9], it was anticipated that there would be no difference in long-term visual function between eyes with EDF and monofocal IOLs. This comparative prospective study aimed to compare the long-term visual function after implantation of two types of IOLs. Functional visual acuity (FVA) testing was performed to detect slight impairment of visual function, such as that seen in eyes with mild PCO [10] and subnanometer vacuoles in the IOL surface layer [11].
Methods
Participants
Patients with bilateral cataracts were recruited for this study. Inclusion criteria were age between 61 and 80 years, no postoperative complication, and residual astigmatism of 1.25 diopter (D) or less. Exclusion criteria were previous ocular surgery and diseases influencing visual function except for cataract, such as chronic or recurrent uveitis, acute ocular disease, or external/internal infection, diabetes with retinal changes, glaucoma, exfoliation syndrome, pathological miosis, keratoconus, corneal endothelial dystrophy, and abnormalities in the capsule, zonule, or pupil. For comparing visual functions promptly, eyes with postoperative corrected distance visual acuities (CDVAs) below 20/30 were also excluded. This study was approved by the ethics committee of Miyata Eye Hospital (identifier: CS-295) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment.
EDF IOLs (ZXR00V, Johnson & Johnson Surgical Vision) or monofocal IOLs (ZCB00V, Johnson & Johnson Surgical Vision) were binocularly implanted. As the postoperative outcomes and surgical costs of each IOL were quite different, implanted IOL types were determined based on the patient’s preferences regarding postoperative vision. When patients preferred vision between far and intermediate distances with less use of spectacles, EDF IOL was recommended. Otherwise, monofocal IOL was recommended. Consequently, no randomization was performed.
The minimum sample size for enrolled patients was determined to be 17 for detecting differences in the FVA values of 0.15 logarithm of the minimum angle of resolution (logMAR), which corresponded to approximately 1.5 steps, with a significance level of 0.05 and a detection power of 0.90 when the standard deviation (SD) of FVA was 0.13 logMAR [11]. This calculation was assumed the use of unpaired t-test.
Intraocular lenses
The implanted diffractive EDF IOLs were ZXR00V, which were one-piece, violet-light blocking, and hydrophobic acrylic IOLs. The optics had a 6.0-mm diameter, an aspheric design on the anterior surface, continuous sharp optic edges on the posterior surface, and anteriorly shifted haptics. The EDF function was produced with echelett optics; the 1st-order diffraction formed the distance focus and the 2nd-order diffraction added + 1.75 D power for extending the focus range [3], providing vision from far to 0.7 m. The materials and platforms of monofocal IOL ZCB00V were identical to those of ZXR00V, except for no echelle optics.
Using biometry data obtained with a swept-source biometer OA-2000 (Tomey, Nagoya, Japan), IOL powers were calculated to determine postoperative emmetropia. Cataract surgery was performed by experienced surgeons in the same procedure. Through a superior corneoscleral incision of a width of 2.2 or 2.4 mm, cataract was removed using the phacoemulsification and aspiration technique (Centurion® Vision System, Alcon), and IOLs were implanted completely within the capsules using the inserter system.
Postoperative examinations
Two years after surgery, CDVA, manifest refraction spherical equivalent (MRSE), binocular all-distance visual acuity, and contrast sensitivity were measured without masking. Particular experienced examiners measured CDVA using Landolt ring charts at distance of 5 m. As there is a significant difference between subjective and objective refraction in eyes with EDF IOLs [12], spherical refraction was determined by increasing the spherical powers until the corrected visual acuity decreased from the best-corrected values, and the power before the decrease was recorded [2].
Binocular visual acuities at distances of 0.3, 0.5, 0.7, 1, 2, 3, and 5 m were examined under distance correction to avoid the influence of refractive errors, using an all-distance vision tester (AS-15; Kowa, Nagoya, Japan) [13, 14]. At each distance, a Landolt ring was randomly displayed, and the best visual acuity was measured. All visual acuity data were converted to the logMAR for analysis.
Contrast sensitivity at 1.5, 3, 6, 12, and 18 cycles per degree (cpd) was measured using the Optec6500 (Stereo Optical, Chicago, IL, USA) under photopic and mesopic illumination (85 and 3.0 cd/m2, respectively). The area under the logarithmic contrast sensitivity function (AULCSF) [15] was also calculated from the measured data.
Postoperative FVA was measured monocularly using the AS-28 (Kowa, Nagoya, Japan) as described previously [10, 11, 16]. Under distance-corrected conditions, static visual acuity was initially measured using the Landolt ring chart, which was automatically indicated on the screen (Start visual acuity). The participants delineated the orientation of the ring by handling the joystick. The optotype size was changed in single steps, depending on the subject’s responses: The optotype was enlarged when the patient’s response was incorrect or reduced for the correct response. When there was no response within 2 s, an error was recorded and the optotype was enlarged. After testing for 60 s, the FVA value that was the mean of visual acuity over the testing period, standard deviation of visual acuities (SDVA), visual maintenance ratio (VMR), mean response time, and number of blinks were obtained. VMR is the ratio of the FVA value with respect to start visual acuity. Response time was the mean of the time from changes in optotype size until correct responses were recorded.
To explore the influence of PCO on the visual functions of eyes with EDF IOLs, PCO was quantitatively evaluated at 2 years postoperatively. After dilation, Scheimpflug images in four directions (0°, 45°, 90°, and 135°) were captured using an anterior segment analyzer (EAS-1000, Nidek, Gamagori, Japan) under 7-mm-long slit illumination from a 200-W flash lamp. Densitometry values (CCT) at the IOL posterior center were analyzed for each direction and averaged [17].
Statistical analysis
As contrast sensitivity and FVA are altered by age [18,19,20], difference in ages between the groups were verified using the t-test. Differences in CDVA, distance-corrected binocular visual acuities in the range of 0.3–5 m, and visual acuity values in FVA testing were examined using the Mann–Whitney test, since their distributions were inherently non-Gaussian. VMR, the mean response time, number of blinks, and contrast sensitivity were examined using the t-test after confirmation with the Shapiro–Wilk test; otherwise, the Mann–Whitney test was used. Associations between PCO densitometry values and FVA, SDVA, VMR, and AULCSFs were examined using regression analysis. P < 0.05 was considered significant.
Results
EDF and monofocal IOLs were implanted in 31 and 28 patients, respectively, and 18 and 14 patients completed the 2-year observations, respectively. Nd:YAG laser posterior capsulotomy was not performed in all eyes. The demographic data of the subjects are shown in Table 1. While there was no significant difference in the mean age and postoperative CDVA, the postoperative MRSE of patients with EDF IOLs was significantly lower than that of patients with monofocal IOL (P = 0.048, t-test), while the mean difference was 0.21 D.
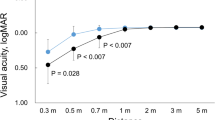
Figure 1 shows the binocular distance-corrected visual acuities at distances of 0.3, 0.5, 0.7, 1, 2, 3, and 5 m. The mean visual acuities in eyes with EDF IOLs were 20/20 or better, except for at 0.3 m, while the visual acuities at 0.7 m and lesser were worse than 20/20 in eyes with monofocal IOLs. Between the two IOLs, there was a significant difference in visual acuities at distances of 0.5 and 0.7 m (P = 0.009, Mann–Whitney test with the Holm correction). Figure 2 shows the proportion of patients who achieved 20/20 or better visual acuity at each distance. All patients obtained 20/20 or better at 1 m or longer with both the IOLs. At 0.7 m or nearer, the rate decreased in patients with monofocal IOLs. Over 70% of patients with EDF IOLs were 20/20 or better at 0.5 and 0.7 m.
Binocular distance-corrected visual acuities at distances of 0.3, 0.5, 0.7, 1, 2, 3, and 5 m of patients with EDF (white) and monofocal (black) IOLs. #: significant differences between 2 IOLs (P = 0.009, Man–Whitney test with Holm correction). EDF, extended depth of focus; IOL, intraocular lens; logMAR, logarithm of the minimum angle of resolution
Figure 3 shows photopic and mesopic contrast sensitivities of 34 eyes of 17 patients with EDF IOLs and 28 eyes of 14 patients with monofocal IOLs. Under photopic illumination, there was no difference at any spatial frequency (P > 0.086, t-test), and the mean AULCSFs were 1.76 and 1.81 with SDs of 0.19 and 0.18, respectively, with no significant difference (P = 0.24, t-test). Under mesopic illumination, no difference was found in the contrast sensitivity (P > 0.21) and AULCSF (P = 0.31) values.
Table 2 compares the FVA parameters between the EDF and monofocal IOLs. No significant differences were observed (P > 0.095).
PCO analysis was performed in 20 eyes of 10 patients, as EAS-1000 was available for a limited period. The mean densitometry values were 23.4 (SD: 4.2) CCT, ranging from 17.8 to 31.3 CCT. Table 3 shows the associations with the mean FVA, SDVA, VMR, and AULCSFs. No significant association was observed (P > 0.13, regression analysis).
Discussions
Two years postoperatively, there was no difference in binocular all-distance visual acuity, contrast sensitivity, and FVA between the EDF and monofocal IOLs, except for binocular visual acuity at distances of 0.5 and 0.7 m. In previous studies, there were no differences in the CDVAs and contrast sensitivities of eyes with EDF and monofocal IOLs until 6 months postoperatively [4, 5, 21]. To the best of our knowledge, there has been no long-term comparison between EDF and monofocal IOLs. The previous findings and current results demonstrate that the superior visual function in eyes with EDF IOLs was maintained for up to 2 years.
Dynamic visual acuity was examined using the FVA test, which has been used to evaluate slight differences in visual functions [11, 22]. A previous comparison between the same EDF and monofocal IOLs 3 months after implantation [21] also showed the comparability of these two types of IOLs. Thus, we concluded that EDF IOLs could sustain visual function for 2 years.
In the use of presbyopia-correcting IOLs, deterioration of visual function due to slight opacification [22] has been a concern. With slight to mild PCO, contrast sensitivity and FVA can be degraded using EDF IOLs. In this study, there was no difference in the long-term contrast sensitivity and FVA between EDF and monofocal IOLs. Additionally, densitometry of the posterior IOL surface did not influence FVA with the use of the EDF IOL; however, the sample size was limited. From an optical perspective, the disturbance inherent to echelett optics would be small [4, 9]. We speculated that the influence of opacification on an EDF IOL would be the same as that on a monofocal IOL. Further investigation is necessary to verify this hypothesis.
This study had some limitations. First, the sample size was small. It was not easy to follow up patients for 2 years; therefore, 39–50% of patients were not followed up. Despite the sample size being limited, the sustainability of EDF function and comparability with monofocal IOL could be identified. However, the number of PCO examinations performed was lower. The malfunction of the obsolete EAS-1000 limits the examination results; thus, it is important to use an alternative instrument with a Scheimpflug camera [23]. Wavefront aberrations were not examined. Higher-order aberrations can affect FVA [24]; however, as there was no difference in the current results, the influence of higher-order aberrations would be the smallest. A more detailed evaluation is necessary to confirm the comparability of visual function between the two types of IOLs.
In conclusion, 2-year observations of eyes with diffractive EDF IOLs demonstrated the sustainability of superior intermediate visual acuity and visual function, which was comparable to that of monofocal IOLs.
References
Cochener B, Concerto Study Group (2016) Clinical outcomes of a new extended range of vision intraocular lens: International Multicenter Concerto Study. J Cataract Refract Surg. 42:1268–1275. https://doi.org/10.1016/j.jcrs.2016.06.033
Ota Y, Bissen-Miyajima H, Nakamura K, Hirasawa M, Minami K (2020) Binocular visual function after staged implantation of extended depth-of-focus intraocular lens targeting emmetropia and -0.5 diopter: a prospective comparison. PloS One 15:e0238135. https://doi.org/10.1371/journal.pone.0238135
Millán MS, Vega F (2017) Extended depth of focus intraocular lens: chromatic performance. Biomed Opt Express 8:4294–4309. https://doi.org/10.1364/BOE.8.004294
Pedrotti E, Bruni E, Bonacci E, Badalamenti R, Mastropasqua R, Marchini G (2016) Comparative analysis of the clinical outcomes with a monofocal and an extended range of vision intraocular lens. J Refract Surg 32:436–442. https://doi.org/10.3928/1081597X-20160428-06
Pedrotti E, Carones F, Aiello F, Mastropasqua R, Bruni E, Bonacci E et al (2018) Comparative analysis of visual outcomes with 4 intraocular lenses: monofocal, multifocal, and extended range of vision. J Cataract Refract Surg 44:156–167. https://doi.org/10.1016/j.jcrs.2017.11.011
Alio JL, Plaza-Puche AB, Férnandez-Buenaga R, Pikkel J, Maldonado M (2017) Multifocal intraocular lenses: an overview. Surv Ophthalmol 62:611–634. https://doi.org/10.1016/j.survophthal.2017.03.005
de Vries NE, Nuijts RM (2013) Multifocal intraocular lenses in cataract surgery: literature review of benefits and side effects. J Cataract Refract Surg 39:268–278. https://doi.org/10.1016/j.jcrs.2012.12.002
Biber JM, Sandoval HP, Trivedi RH, de Castro LE, French JW, Solomon KD (2009) Comparison of the incidence and visual significance of posterior capsule opacification between multifocal spherical, monofocal spherical, and monofocal aspheric intraocular lenses. J Cataract Refract Surg 35:1234–1238. https://doi.org/10.1016/j.jcrs.2009.03.013
Yoo YS, Whang WJ, Byun YS, Piao JJ, Kim DY, Joo CK, Yoon G (2018) Through-focus optical bench performance of extended depth-of-focus and bifocal intraocular lenses compared to a monofocal lens. J Refract Surg 34:236–243. https://doi.org/10.3928/1081597X-20180206-04
Wakamatsu TH, Yamaguchi T, Negishi K, Kaido M, Matsumoto Y, Ishida R, Kojima T, Ibrahim OM, Saiki M, Dogru M, Tsubota K (2011) Functional visual acuity after neodymium: YAG laser capsulotomy in patients with posterior capsule opacification and good visual acuity preoperatively. J Cataract Refract Surg 37:258–264. https://doi.org/10.1016/j.jcrs.2010.08.048
Hiraoka T, Miyata K, Hayashidera T, Iida M, Takada K, Minami K, Oshika T (2017) Influence of intraocular lens subsurface nanoglistenings on functional visual acuity. PLoS ONE 12:e0173574. https://doi.org/10.1371/journal.pone.0173574. (Erratum in: PLoS One. 2017 Apr 17;12 (4):e0176318)
Ota Y, Minami K, Oki S, Bissen-Miyajima H, Okamoto K, Nakashima M, Tsubota K (2021) Subjective and objective refractions in eyes with extended-depth-of-focus intraocular lenses using echelette optics: clinical and experimental study. Acta Ophthalmol 99:e837–e843. https://doi.org/10.1111/aos.14660
Hayashi K, Yoshida M, Hayashi H (2009) All-distance visual acuity and contrast visual acuity in eyes with a refractive multifocal intraocular lens with minimal added power. Ophthalmology 116:401–408. https://doi.org/10.1016/j.ophtha.2008.09.052
Ohtani S, Gekka S, Honbou M, Kataoka Y, Minami K, Miyata K, Oshika T (2009) One-year prospective intrapatient comparison of aspherical and spherical intraocular lenses in patients with bilateral cataract. Am J Ophthalmol 147(984–989):989.e1. https://doi.org/10.1016/j.ajo.2008.12.037
Applegate RA, Howland HC, Sharp RP, Cottingham AJ, Yee RW (1998) Corneal aberrations and visual performance after radial keratotomy. J Refract Surg 14:397–407. https://doi.org/10.3928/1081-597X-19980701-05
Kaido M (2018) Functional visual acuity. Invest Ophthalmol Vis Sci 59:DES29–DES35. https://doi.org/10.1167/iovs.17-23721
Hayashi H, Hayashi K, Nakao F, Hayashi F (1998) Quantitative comparison of posterior capsule opacification after polymethylmethacrylate, silicone, and soft acrylic intraocular lens implantation. Arch Ophthalmol 116:1579–1582. https://doi.org/10.1001/archopht.116.12.1579
Kaido M, Toda I, Ishida R, Konagai M, Dogru M, Tsubota K (2011) Age-related changes in functional visual acuity in healthy individuals. Jpn J Ophthalmol 55:183–189. https://doi.org/10.1007/s10384-011-0026-2
Derefeldt G, Lennerstrand G, Lundh B (1979) Age variations in normal human contrast sensitivity. Acta Ophthalmol (Copenh) 57:679–690. https://doi.org/10.1111/j.1755-3768.1979.tb00517.x
Yoshino M, Bissen-Miyajima H, Minami K (2013) Assessment of whether visual outcomes with diffractive multifocal intraocular lenses vary with patient age. J Cataract Refract Surg 39:1502–1506. https://doi.org/10.1016/j.jcrs.2013.04.031
Sakisaka T, Minami K, Takada K, Mori Y, Miyata K (2021) Functional visual acuity after implantation of diffractive extended depth-of-focus intraocular lenses using an echelett optics. BMC Ophthalmol 21:418. https://doi.org/10.1186/s12886-021-02189-7
Gatinel D, Loicq J (2016) Clinically relevant optical properties of bifocal, trifocal, and extended depth of focus intraocular lenses. J Refract Surg 32:273–280. https://doi.org/10.3928/1081597X-20160121-07
Minami K, Honbo M, Mori Y, Kataoka Y, Miyata K (2015) Area densitometry using rotating Scheimpflug photography for posterior capsule opacification and surface light scattering analyses. J Cataract Refract Surg 41:2444–2449. https://doi.org/10.1016/j.jcrs.2015.05.038
Hiraoka T, Yamamoto T, Okamoto F, Oshika T (2013) Changes in functional visual acuity and ocular wavefront aberration after administration of eye ointment. J Ocul Pharmacol Ther 29:770–775. https://doi.org/10.1089/jop.2013.0024
Funding
This investigator-initiated study was funded by AMO Japan (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the ethics committee of Miyata Eye Hospital (identifier: CS-295) and adhered to the tenets of the Declaration of Helsinki.
Informed consent
Written informed consent was obtained from all the participants prior to enrollment.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hisai, T., Takada, K., Tokuda, S. et al. Visual function in eyes with diffractive extended depth-of-focus and monofocal intraocular lenses: 2-year comparison. Graefes Arch Clin Exp Ophthalmol 261, 2567–2573 (2023). https://doi.org/10.1007/s00417-023-06051-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06051-y