Abstract
Purpose
Sector retinitis pigmentosa (RP) is a rare form of rod-cone degeneration typically associated with mutations in the RHO gene. We describe six unrelated patients presenting with this atypical phenotype in association with biallelic mutations in EYS gene.
Methods
Multinational, multicentre cross-sectional case series. Patients with biallelic disease-causing variants in EYS and a clinical diagnosis of sector RP were recruited from specialized centres in Portugal and Brazil. All patients underwent a comprehensive ophthalmologic examination complemented by deep phenotyping. Peripheral blood samples were collected from all probands and available relatives for genetic analysis. Genetic counselling was provided to all subjects.
Results
Seven disease-causing variants (4 pathogenic; 3 likely pathogenic) were identified in 6 unrelated female patients. Best-corrected visual acuity ranged from 75 to 85 ETDRS letters. All eyes showed bilateral and symmetrical areas of outer retinal atrophy distributed along the inferior vascular arcades and extending temporally and/or nasally in a crescent-shaped pattern. On fundus autofluorescence (AF), a foveal-sparing curvilinear band of hyperAF encroaching the optic nerve head and extending temporally was seen in 4 patients. The remaining 2 presented bilateral and symmetrical patches of hypoAF inside crescent-shaped areas of hyperAF along the inferior temporal vascular arcade. Visual field testing revealed superior visual field defects of varying extents, always in close association with the fundus AF findings.
Conclusions
Even though EYS has only recently been listed as a cause of the sector RP phenotype, we believe that this presentation is not infrequent and should be considered an important differential for sector RP.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sector retinitis pigmentosa (RP) is a rare, atypical, and milder form of rod-cone degeneration in which only one or two quadrants of the retina are involved [1, 2]. The disorder is characterized by bilateral and symmetrical regionalized areas of retinal pigment epithelium (RPE) atrophy and bone spicule hyperpigmentation, usually in the inferior or inferonasal quadrants, corresponding to superior defects on visual field testing [3, 4]. This peculiar pattern of degeneration is most noticeable on fundus autofluorescence (FAF) imaging with hypoautofluorescent (hypoAF) regions corresponding to the areas of RPE atrophy and bone spicule hyperpigmentation, and a thick, crescent-shaped band of hyperautofluorescence (hyperAF) separating these areas from the unaffected, iso-autofluorescent retina [4,5,6]. Nevertheless, unilateral or asymmetrical involvement, as well as predominantly nasal, superotemporal, or superior quadrants degeneration have been reported [7]. Affected individuals may be asymptomatic or present with nyctalopia, mild visual loss and/or visual field defects of varying extent, depending on the affected regions of the retina [4, 5]. Although historically considered a stationary to slowly progressive RP phenotype, sector RP may ultimately lead to a more severe, diffuse rod-cone degeneration [8, 9].
Most cases of sector RP are inherited in an autosomal dominant pattern and are caused by missense mutations in the rhodopsin gene (RHO, 3q22.1, MIM *180380) [4, 5, 8, 10]. Nevertheless, mutations in the usherin (USH1C, 11p15.1, MIM *605242) [10, 11], cadherin 23 (CDH23, 10q22.1, MIM *605516) [10, 12], retinol dehydrogenase 5 (RDH5, 12q13.2, MIM *601617) [13], arrestin (SAG, 2q37.1, MIM *181031) [14, 15], and in the RP GTPase Regulator (RPGR, Xp11.4, MIM *312610) [4, 10] genes have also been reported in association with this unique phenotype. Recently, Georgiou et al. [10] further expanded the mutational spectrum of sector RP by identifying causative variants in 5 genes that were not previously implicated (PRPS1, MYO7A, EYS, IMPDH1, and RP1). Biallelic mutations in one of these genes, the eyes shut homolog gene (EYS, 6q12, MIM *612424) are among the most commonly found disease-causing variants in autosomal-recessive RP in Asian and European populations [16,17,18,19]. With 44 exons, spanning 2.0 Mb of genomic DNA, EYS is the largest-known retina-specific gene and encodes a product 3165 amino acids in length [20, 21]. High phenotypic and genetic heterogeneity exists in EYS-related retinal degeneration [17, 19, 22,23,24]. However, a clear association with the sector RP phenotype was only recently established [10].
We describe the genotypes and phenotypes of six unrelated patients with EYS-related sector RP and provide a review of previously reported mutations in EYS associated with phenotypic descriptions that fall into the spectrum of this clinical entity.
Methods
Study design and diagnostic criteria
Multinational, multicentre cross-sectional case series. Patients with biallelic disease-causing variants in EYS gene and a clinical diagnosis of sector RP were recruited from the IRD-PT registry [25] in Portugal and from the INRET Clínica e Centro de Pesquisa in Brazil. The clinical diagnosis of sector RP was based on the presence of regionalized areas of RPE atrophy (± bone spicule hyperpigmentation), with corresponding FAF abnormalities and visual field defects, and the exclusion of any known reasons, such as trauma, infection or inflammation, for the RP-like appearance of the fundus.
The study was conducted at the Retinal Dystrophies Clinic and Medical Genetics Unit of Centro Hospitalar e Universitário de Coimbra (CHUC), Coimbra, Portugal (patients 1, 2 and 3), and INRET Clínica e Centro de Pesquisa, Belo Horizonte, Brazil (patients 4, 5 and 6). Informed consent was obtained for every included subject. The study was approved by the local Ethics Committee and followed the tenets of the Declaration of Helsinki for biomedical research.
Ophthalmic examination and imaging
All patients underwent a comprehensive ophthalmologic examination including best-corrected visual acuity (BCVA, ETDRS letters), dilated slit-lamp anterior segment and fundus biomicroscopy, seven standard 45°-field color fundus photographs (CFP) taken with a Nikon Digital SLR Camera D7000 (Nikon Corporation, Japan) mounted on either a TRC-NW7SF or TRC-NW8 Mark II Retinal Camera (Topcon Corporation, Japan), spectral-domain optical coherence tomography (SD-OCT) (Spectralis, Heidelberg Engineering, Heidelberg, Germany or Avanti RTVue-XR 100, Optovue Inc, Fremont, CA, USA), FAF (HRAII, Heidelberg Engineering, Heidelberg, Germany), and Humphrey visual field testing (Zeiss 750i, Carl Zeiss, Germany). Four probands (Patients 2, 4, 5, and 6) underwent full-field electroretinogram (ffERG) using the RETIscan system (Roland Consult, Germany) or UTAS Sunburst (LKC Technologies, USA), with DTL-Plus electrodes, according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standards in photopic and scotopic states [26].
Genetic testing
Peripheral blood samples were collected from all probands and available relatives for genetic analysis. The genomic DNA was extracted using a genomic DNA extraction and purification kit based on the manufacturer’s protocol. Variants were classified in accordance with the American College of Medical Genetics and Genomics (ACMG). The genetic study of patients 1, 2, and 3 was coordinated by a medical geneticist (ALC) from the Medical Genetics Unit of CHUC. In these patients, genetic studies were carried out at different times. Therefore, different tests were performed according to their availability. In patient 1, a NGS panel for RP (187 genes) was used. Patient 2 initially underwent Sanger sequencing for the RHO gene. Since no clinically significant variants were found, a NGS panel for retinal dystrophies (309 genes) was then used. In patient 3 targeted mutation analysis (Sanger sequencing and MLPA) was used to confirm the pathogenic variants previously identified in a family member (a sister in whom EYS-related pathogenic variants causing a typical RP phenotype had previously been identified using the 187 genes NGS panel for RP). Patients 4, 5, and 6 were genotyped using a NGS panel of 224 genes known to cause retinal disease. Captured DNA was sequenced on the Illumina HiSeq platform and NGS data was processed by an in-house bioinformatics pipeline leading to annotated variant calls [27]. All clinically significant variants were further confirmed by Sanger sequencing. A published cDNA sequence for EYS (GenBank NM_001142800.1) was compared with the sequencing results. Sanger sequencing and next-generation sequencing (NGS) were performed at diagnostic labs, with good quality control at the raw data stage, the alignment and the variant calling. Regarding raw data of Sanger sequencing, there were not any artifacts, peaks were well-resolved and with acceptable heights, data start points were not deviated from others, length of the read was the expected, and baseline noise was very little or not present. Genetic counselling provided by a medical geneticist was granted to all subjects.
Results
Clinical phenotypes
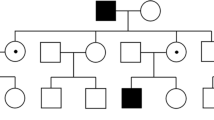
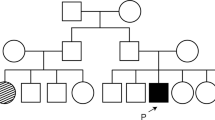
Demographic and genetic information is presented in Table 1. The average age at diagnosis was 45 years (range 29–58 years), and all patients were female. Parental consanguinity (r = 1/8) was reported in patient 2, while a positive family history of RP was identified both in patient 2 and in patient 3. Patient 2 has 1 affected brother and had 1 sister (now deceased) with a clinical diagnosis of RP. Unfortunately, they were never observed at our centre. Patient 3 has an affected sister presenting with a typical RP phenotype (thus not included in this cohort).
Three patients (1, 4, and 5) had obvious symptoms at presentation. Patient 1 complained of nyctalopia from age 30; patient 4 presented loss of central vision and difficulties while reading; and patient 6 described an upper visual field restriction. The other 3 patients (patients 2, 3, and 6) were asymptomatic and the abnormal appearance of the fundus was noted upon routine ophthalmic examination.
All patients had a relatively good BCVA, ranging from 20/32 to 20/20 (Table 1). Posterior subcapsular lens opacification was observed in 3 patients (patients 1, 2, and 6). On CFP, all eyes showed bilateral and symmetrical areas of outer retinal atrophy distributed along the inferior vascular arcades and extending temporally and/or nasally in a crescent-shaped pattern (Fig. 1). Peripapillary atrophy was also present in all eyes. Intraretinal pigment migration in the form of bone-spicule hyperpigmentation was observed in both eyes of patients 1, 2, and 6. On FAF, patients 1, 2, 4, and 5 showed bilateral and symmetrical hypoAF regions corresponding to the regionalized retinal degeneration seen on CFP, along with a foveal-sparing curvilinear band of hyperAF encroaching the optic nerve head and extending temporally (Figs. 2A, B, 4A and B). Patients 3 and 6 presented bilateral and symmetrical patches of hypoAF inside crescent-shaped areas of hyperAF along the inferior temporal vascular arcade, even though the FAF changes did not encroach the optic nerve head (Fig. 2C). Visual field testing revealed superior visual field defects of varying extents, always in close association with the observed FAF findings (Fig. 2). The horizontal cross-scan of the SD-OCT showed a normal foveal architecture in all but patient 4 who presented bilateral, centre-involving cystoid macular edema (Fig. 4C and D). Outer retinal atrophy and RPE thinning were noted in scans over the affected areas of retinal degeneration (Figs. 3C, D, 4C and D). The ffERG of patients 2 (Fig. 1), 4, 5, and 6 (Supplemental Fig. 1) revealed marginally recordable, albeit significantly decreased, scotopic and photopic responses.
Montage color fundus photography (CFP) and full field electroretinography (ffERG) from Patient 2 (right and left eye, respectively). On CFP, bilateral and symmetrical areas of outer retinal atrophy distributed along the inferior vascular arcades and extending temporally and nasally in a crescent-shaped pattern can be observed. Vascular attenuation and intraretinal pigment migration in the form of bone-spicule hyperpigmentation are also seen. Mild peripapillary atrophy is present in both eyes. The following ffERG waves are shown from top to bottom (for the right and left eye, respectively): dark-adapted (DA) 0.01 cd.s.m−2; DA 3.0 cd.s.m−2; DA oscillatory potentials; light-adapted (LA) 3.0 cd.s.m−2; and LA 30 Hz flicker ERG. The ffERG was non-recordable for the DA 0.01 and DA oscillatory potentials waveforms, while very residual electrical activity (low amplitude and poorly defined waves) was observed for the DA 3.0, LA 3.0 and LA 30 Hz flicker ERG waveforms
Fundus autofluorescence (FAF) and corresponding Humphrey 24–2 grayscale visual field maps. In patients 1 (A) and 2 (B), hypoautofluorescent regions corresponding to the areas of regionalized retinal degeneration are seen along with a thick curvilinear band of hyperautofluorescence separating the unaffected, iso-autofluorescent retina from the affected regions. In both cases, the affected area encroaches the optic nerve head and extends temporally, although sparing the fovea. In patient 3 (C), bilateral and symmetrical patches of hypoautofluorescence are observed inside crescent-shaped areas of hyperaurofluorescence along the inferior temporal vascular arcade. In all cases, the anatomo-functional correlation can be appreciated in the 24–2 Humphrey visual fields
Color fundus photography (CFP), near-infrared (NIR) imaging and spectral-domain optical coherence tomography (SD-OCT) of the right eye of patient 1. (A) On CFP, outer retinal atrophy distributed along the inferior temporal vascular arcade in a crescent-shaped pattern is seen along with intraretinal pigment migration in the form of bone-spicule hyperpigmentation. Mild peripapillary atrophy is also observed. (B) The demarcation area between normal and abnormal retina is better appreciated on NIR imaging. (C) Horizontal SD-OCT scan shows normal foveal anatomy and preservation of the subfoveal inner and outer retinal layers and retinal pigment epithelium (RPE)/Bruch membrane complex. Loss of the ellipsoid zone, external limiting membrane and outer nuclear layer is observed temporally (yellow arrowhead). In the same area, thinning of the RPE/Bruch membrane complex is also observed. (D) Vertical SD-OCT scan shows similar findings but with loss of the outer retinal layers and thinning of the RPE/Bruch membrane complex in the inferior macula (yellow arrowhead)
Fundus autofluorescence (FAF) and vertical spectral-domain optical coherence tomography (SD-OCT) scans of patient 4. Bilateral areas of hypoautofluorescence along the inferior temporal vascular arcade can be observed in OD (A) and OS (B). A subtle hyperautofluorecent band is also present. Bilateral, centre-involving cystoid macular oedema is seen on the SD-OCT scans of OD (C) and OS (D). Loss of integrity of the outer retinal layers and the retinal pigment epithelium/Bruch membrane complex is present over the regionalized atrophy seen inferiorly on FAF
Mutational spectrum
Seven different disease-causing variants were identified across 12 alleles of 6 unrelated patients (Table 1). Four novel EYS variants are herein reported for the first time: 1 pathogenic, 1 likely pathogenic and 2 variants of uncertain significance (VUS) according to the ACMG classification. In the case of the latter 2 variants, family studies allowed reclassification of the variants as likely pathogenic. Detailed information of all variants is shown on Table 2. Except for the above reported EYS variants, no additional clinically significant variants (ACMG classes IV or V) were found in genes associated with inherited retinal dystrophies.
Discussion
The mutational spectrum of sector RP is evolving, with recent additions [4, 10] to the list of associated genes. One of these genes is EYS, a frequent cause of autosomal-recessive retinal degeneration in Asian and European populations [16,17,18,19]. Phenotypic heterogeneity exists in EYS-related disease [17, 19, 22,23,24] but despite previous clinical descriptions compatible with a sectoral phenotype, the gene has only recently been listed in a publication reviewing the genomic landscape of sector RP [10]. In 2010, Audo et al. [17] reported a case where distinct fundus abnormalities with predominance of pigmentary changes in the inferior retina were found in an Egyptian-descent patient with a homozygous deletion of exon 12 (p.Cys590TyrfsX4). Interestingly, ERG responses were not detectable for this patient, consistent with severe generalized rod-cone dysfunction, which is unusual for RHO-related sector RP. A similar phenotype was described by Muciollo et al. [24] in an Italian patient with compound heterozygosity for the c.8133_8137del (p.Phe2712Cysfs*33) and c.9383_9387del (p.Lys3128Argfs*7) variants. Here, recordable scotopic rod-specific B-waves were present in both eyes, which is more consistent to what has been described for RHO-related sector RP [28]. Additionally, Bandah-Rozenfeld et al. [29] described a patient homozygous for the p.His2740TyrfsX27 variant in EYS that initially presented with sector RP at the age of 25 (based on funduscopic and visual field findings) and later progressed to widespread, generalized retinal involvement. Recently, Cundy et al. [30] reported one asymptomatic patient with a phenotype consistent with inferior sector RP. The patient was homozygous for the c.5834delA variant. In a Japanese cohort of EYS-related retinal degeneration [19], FAF imaging of a patient harbouring homozygous c.2528 G > A, p.(Gly843Glu) variants is also consistent with sector RP, even though this was not acknowledged by the authors. Two recent studies [22, 23] highlighted the presence of crescent-shaped hyperAF changes in patients with EYS-related RP, advancing most from the temporal and inferior quadrants and whose edge encroached on the central macula. As expected, these patients had larger visual fields, longer ellipsoid zones on macular SD-OCT and, therefore, milder disease. It seems from these descriptions that sector RP is not an infrequent finding in association with disease-causing variants in EYS. However, the individual cases previously reported were either diluted in large EYS cohorts [17, 24, 29], or given a different name [22, 23], thus losing visibility as a distinctive molecular cause for the sector RP phenotype. We are deeply convinced that EYS is a frequent cause of sector RP, thus meriting scientific awareness. We identified seven different disease-causing variants in 6 unrelated female patients. The female predominance in our cohort is unexpected but probably just fortuitous. Intriguingly, the case reported by Georgiou et al. [10] was also of a female patient. Further studies are needed to clarify if there is a correlation between EYS-related sector RP and female sex.
Despite the small cohort, two variants were identified in 33% (4/12) and 25% (3/12) of the alleles: the c.5982-2A > G p.? and the c.2225del p.(Cys742Leufs*36) variants, respectively. According to the literature and patient registries of the two centres, none of these variants is particularly frequent in Portuguese or Brazilian populations. The c.5982-2A > G p.? variant has been reported in association with retinitis pigmentosa across different populations [31, 32], including a Spanish cohort [33]. The c.2225del p.(Cys742Leufs*36) variant is a frameshift variant leading to a premature stop codon and is not present in population databases (dbSNP and gnomAD). This variant has not been reported in the literature, but has been identified in Portuguese patients with EYS-related retinitis pigmentosa (CHUC cohort; unpublished data). The fact that all variants reported in our cohort have been identified in association with a typical RP phenotype highlight the interfamilial phenotypic heterogeneity associated with disease-causing variants in EYS. Furthermore, the fact that patient 3 has an affected sibling with a typical EYS-related RP phenotype, demonstrates the intrafamilial heterogeneity.
Our study shows that the phenotypic resemblances to RHO-related sector RP are evident, and physicians should keep in mind EYS as an important differential for this atypical presentation. Understanding the characteristics of mutant proteins and establishing genotype–phenotype correlations is not always an easy task. Sengillo et al. [23] was able to correlate the presence of typical parafoveal hyperAF rings and atypical crescent-shaped hyperAF rings with variants in the distal portion, or C-terminal one third of the EYS protein. The authors concluded that the position of the variant within the gene could explain disease severity among patients. This was not the case in our cohort, where only 3 out of 7 variants were located in the distal portion of the EYS protein.
Although the exact mechanisms involved in sector RP remain unidentified, the fact that intra- and interfamily phenotypic variability exists in the presence of the same mutations, suggests the influence of external factors [9]. Some evidence indicates that the direct action of incident light on rhodopsin (Rho) may trigger retinal degeneration, thus contributing to the observed sector phenotype [9]. In fact, a disease-exacerbating role for incident light has been proposed, based on a theoretical model showing that light exposure is greatest in the inferior retina [34]. The EYS protein is important for photoreceptor morphology but its precise function in photoreceptor biology is still unknown. It has been hypothesized that it could be involved in maintaining the stability of the ciliary axoneme in both rods and cones. Nevertheless, the variability of its isoform structure suggests that other roles are also possible and yet to be established [35]. Even though no accurate explanations exist, it can be speculated that EYS interacts or influences Rho expression as a genetic modifier, thus triggering the sectoral phenotype [17].
There are limitations to this study. First, electrophysiology testing was only performed in patients 2, 4, 5, and 6 and was limited to the ffERG strategy. Given the pattern of retinal involvement associated with sector RP, it would be interesting to have multifocal ERG data, as recently described by Giambene et al. [28]. Second, the small number of subjects and the study design preclude the establishment of genotype–phenotype correlations and evaluation of disease progression. Further genetic analyses of larger cohorts are needed to better understand the pathophysiology of EYS-related sector RP and longitudinal natural history studies with functional testing will be useful to understand if progression does exist in EYS-related sector RP. Nevertheless, by using multimodal imaging and functional testing, and providing a review of the current literature, this study provides robust evidence to establish EYS as a cause for the sector RP phenotype.
In conclusion, we have described here a phenotype of sector RP in 6 unrelated patients harbouring disease-causing mutations in EYS. Even though EYS has only recently been listed as a cause of the sector RP phenotype, we believe that this presentation is not infrequent and it should be considered an important differential for this distinctive phenotype.
References
Massof Rw, Finkelstein D (1979) Vision threshold profiles in sector retinitis pigmentosa. Arch Ophthalmol 97(10):1899–1904
Van Woerkom C, Ferrucci S (2005) Sector retinitis pigmentosa. Optometry 76(5):309–317
Napier Ml, Durga D, Wolsley Cj et al (2015) Mutational analysis of the rhodopsin gene in sector retinitis pigmentosa. Ophthalmic Genet 36(3):239–243
Coussa Rg, Basali D, Maeda A et al (2019) Sector retinitis pigmentosa: report of ten cases and a review of the literature. Mol Vis 25:869–889
Xiao T, Xu K, Zhang X et al (2019) Sector retinitis pigmentosa caused by mutations of the Rho gene. Eye (Lond) 33(4):592–599
Oliveira Ma, Neves E, Marques Jp (2020) Genetic, anatomical, and functional correlation of sector retinitis pigmentosa. Jama Ophthalmol 138(6):E193133
Bisantis C (1971) [G.B. Bietti's sectorial pigmentary retinopathy. contribution to the knowledge of its various clinical aspects]. Ann Ocul (Paris) 204(9):907–54
Xt Nguyen, Talib M, Van Cauwenbergh C et al (2020) Clinical characteristics and natural history of Rho-associated retinitis pigmentosa: a long-term follow-up study. Retina 41(1):213–223
Ramon E, Cordomi A, Aguila M et al (2014) Differential light-induced responses in sectorial inherited retinal degeneration. J Biol Chem 289(52):35918–35928
Georgiou M, Ps Grewal, Narayan A et al (2020) Sector Retinitis pigmentosa: extending the molecular genetics basis and elucidating the natural history. Am J Ophthalmol 221:299–310
Saihan Z, StabejPle Q, Robson Ag et al (2011) Mutations in the Ush1c gene associated with sector retinitis pigmentosa and hearing loss. Retina 31(8):1708–1716
Branson Sv, Mcclintic Ji, Stamper Th et al (2016) Sector retinitis pigmentosa associated with novel compound heterozygous mutations of Cdh23. Ophthalmic Surg Lasers Imaging Retina 47(2):183–186
Sato M, Oshika T, Kaji Y et al (2004) A novel homozygous Gly107arg mutation in the Rdh5 gene in a japanese patient with fundus albipunctatus with sectorial retinitis pigmentosa. Ophthalmic Res 36(1):43–50
Nakamachi Y, Nakamura M, Fujii S et al (1998) Oguchi disease with sectoral retinitis pigmentosa harboring adenine deletion at position 1147 in the arrestin gene. Am J Ophthalmol 125(2):249–251
Pappalardo J, Heath Jeffery RC, Thompson JA et al (2021) Progressive sector retinitis pigmentosa due to C.440g>T mutation in SAG in an Australian family. Ophthalmic Genet 42(1):62–70
Arai Y, Maeda A, Hirami Y et al (2015) Retinitis pigmentosa with eys mutations is the most prevalent inherited retinal dystrophy in Japanese populations. J Ophthalmol 2015:819760
Audo I, Ja S, Mohand-Said S et al (2010) Eys is a major gene for rod-cone dystrophies in France. Hum Mutat 31(5):E1406–E1435
Barragan I, Borrego S, Ji P et al (2010) Mutation spectrum of eys in spanish patients with autosomal recessive retinitis pigmentosa. Hum Mutat 31(11):E1772–E1800
Yang L, Fujinami K, Ueno S et al (2020) Genetic spectrum of eys-associated retinal disease in a large Japanese cohort: identification of disease-associated variants with relatively high allele frequency. Sci Rep 10(1):5497
Abd El-Aziz Mm, Barragan I, O’driscoll Ca et al (2008) Encoding an ortholog of drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet 40(11):1285–1287
Collin Rw, Littink Kw, Klevering Bj et al (2008) Identification of A 2 Mb human ortholog of drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet 83(5):594–603
Pierrache Lhm, Messchaert M, Thiadens A et al (2019) Extending the spectrum of eys-associated retinal disease to macular dystrophy. Invest Ophthalmol Vis Sci 60(6):2049–2063
Sengillo Jd, Lee W, Nagasaki T et al (2018) A distinct phenotype of eyes shut homolog (eys)-retinitis pigmentosa is associated with variants near the c-terminus. Am J Ophthalmol 190:99–112
Mucciolo Dp, Sodi A, Passerini I et al (2018) Fundus phenotype in retinitis pigmentosa associated with eys mutations. Ophthalmic Genet 39(5):589–602
Marques Jp, Carvalho Al, Henriques J et al (2020) Design, development and deployment of a web-based interoperable registry for inherited retinal dystrophies in Portugal: the ird-pt. Orphanet J Rare Dis 15(1):304
Mcculloch Dl, Marmor Mf, Brigell Mg et al (2015) Iscev standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Fbo Porto, Em Jones, Branch J et al (2017) Molecular screening of 43 Brazilian families diagnosed with leber congenital amaurosis or early-onset severe retinal dystrophy. Genes (Basel) 8(12):355
Giambene B, Verdina T, Pennino M et al (2020) Multifocal electroretinographic responses in sector retinitis pigmentosa. Int Ophthalmol 40(3):703–708
Bandah-Rozenfeld D, Littink Kw, Ben-Yosef T et al (2010) Novel null mutations in the eys gene are a frequent cause of autosomal recessive retinitis pigmentosa in the Israeli population. Invest Ophthalmol Vis Sci 51(9):4387–4394
Cundy O, Broadgate S, Halford S et al (2020) Genetic and clinical findings in an ethnically diverse retinitis pigmentosa cohort associated with pathogenic variants in eys. Eye (Lond) 35(5):1440–1449
Db Mcguigan, Heon E, Av Cideciyan et al (2017) Eys mutations causing autosomal recessive retinitis pigmentosa: changes of retinal structure and function with disease progression. Genes (Basel) 8(7):178
Habibi I, Chebil A, Falfoul Y et al (2016) Identifying mutations in tunisian families with retinal dystrophy. Sci Rep 6(1):37455
Gonzalez-Del Pozo M, Borrego S, Barragan I et al (2011) Mutation screening of multiple genes in Spanish patients with autosomal recessive retinitis pigmentosa by targeted resequencing. PLoS ONE 6(12):E27894
Schwartz L, Boelle Py, D’hermies F et al (2003) Blue light dose distribution and retinitis pigmentosa visual field defects: an hypothesis. Med Hypotheses 60(5):644–649
Alfano G, Kruczek Pm, Shah Az et al (2016) Eys is a protein associated with the ciliary axoneme in rods and cones. PLoS ONE 11(11):E0166397
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the HREC of Centro Hospitalar e Universitário de Coimbra (CHUC) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Full-field electroretinogram (ffERG) of Patients 4, 5 and 6. On patients 4 and 5, bilateral marginally recordable scotopic and photopic responses were recorded. Patient 6 presented a subnormal scotopic and photopic ffERG. (PNG 1308 kb)
Rights and permissions
About this article
Cite this article
Marques, J.P., Porto, F.B.O., Carvalho, A.L. et al. EYS-Associated Sector Retinitis Pigmentosa. Graefes Arch Clin Exp Ophthalmol 260, 1405–1413 (2022). https://doi.org/10.1007/s00417-021-05411-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05411-w