Abstract
Purpose
To determine the relationship between fluoroquinolone susceptibility of gram-positive cocci (GPC) isolated from patients with bacterial keratitis and the age of the patients or the date of onset.
Methods
Bacterial isolates were obtained from corneal lesions of patients with infectious keratitis treated between January 2008 and December 2016. The fluoroquinolone susceptibility of GPC was assessed, and a retrospective review of microbiological records was performed. Fluoroquinolone susceptibility was measured through broth microdilution in accordance with protocols of the Clinical and Laboratory Standards Institute. Statistical analysis was performed using a generalized estimating equation and cubic spline to determine the association between fluoroquinolone susceptibility of GPC isolated from corneal lesions and patient age.
Results
Of the 1200 bacterial isolates, 471 GPC were identified. They included Staphylococcus epidermidis (45.6%), other coagulase-negative Staphylococcus sp. (17.8%), and Staphylococcus aureus (18.3%). Levofloxacin susceptibility of GPC exhibited a negative relationship with age and had an odds ratio of 0.893 (95% confidence interval, 0.825–0.967) for every 10 years of age. A non-adjusted cubic spline curve was well correlated with year-adjusted data in a generalized additive model, and the levofloxacin susceptibility of GPC was initially stable but gradually declined after 40 years of age, before re-stabilizing again after 70 years of age.
Conclusion
The fluoroquinolone susceptibility of GPC isolated from corneal lesions of infectious keratitis is high in children under 15 years of age and declines with an increase in age of patients using a generalized estimating equation and cubic spline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Bacterial keratitis is a community-acquired infection and a potentially vision-threatening disease that requires urgent treatment. Broad-spectrum antibiotics are currently the first-line treatment for patients with bacterial keratitis. However, the treatment should be updated to include appropriate antibiotics based on the sensitivity of specimens isolated from the cornea [1]. Several studies have shown that fluoroquinolones have excellent efficacy as a primary monotherapy in the treatment of bacterial keratitis [2, 3]. However, fluoroquinolone-resistant species have been isolated from bacterial keratitis corneal lesions since the 1990s [4, 5].
The primary causative agents of bacterial keratitis include gram-positive cocci (GPC) such as Staphylococcus epidermidis and Staphylococcus aureus, and gram-negative bacteria, Pseudomonas aeruginosa. Variation in the prevalence of keratitis caused by P. aeruginosa is related to geographical and historical factors [6]. In contrast, the prevalence of GPC-related keratitis has remained relatively constant, possibly because GPCs are part of the normal microbiota of human skin and the ocular surface [7].
Fluoroquinolones interact with bacterial topoisomerase IV and gyrase [8], and have been marketed and widely prescribed for gram-negative and gram-positive bacterial infections since the 1980s. No limitations have been placed on prescribing topical fluoroquinolone for children; however, to mitigate the risk of side effects such as cartilage and tendon damage that affect physical growth, systemic prescription of fluoroquinolones for patients under 16 years of age has been restricted to a few diseases [9].
To reveal the influence of age or the date of the onset of infection on the fluoroquinolone susceptibility of bacteria causing keratitis, we analyzed the fluoroquinolone susceptibility of GPC isolated from bacterial keratitis in a single hospital.
Patients and methods
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki and was approved by the Institutional Research Ethics Committee of the Miyata Eye Hospital. Written informed consent was obtained from all patients after explanation of the nature and possible consequences of the study.
We included patients who underwent bacteriologic analysis for keratitis at Miyata Eye Hospital between January 2008 and December 2016. Microbiological records of the patients were retrospectively reviewed for age, clinical diagnosis, microbiological samples, bacterial species, and antibiotic susceptibility. We excluded patients whose medical information was not consistently available.
Microbiological methods and isolates
All isolates were obtained after administration of topical anesthesia on the ocular surface. Preservative-free 0.4% oxybuprocaine hydrochloride was used and corneal lesions were scraped with a surgical blade under a microscope. Bacterial isolation, identification, and susceptibility tests were performed at the Research Foundation for Microbial Diseases of Osaka University, Suita, Japan.
Bacterial isolation was performed for 24–48 h at 36.5 °C on TSA II 5% sheep blood agar (Becton Dickinson Japan Co. Ltd.) and Drygalski improved medium (Kyokuto Pharmaceutical Industrial Co., Ltd, Tokyo, Japan) or Chocolate II agar medium (Becton Dickinson Japan Co. Ltd.), in an atmosphere containing 5% CO2. In addition, cultivation under anaerobic conditions was conducted for 24–120 h at 36.5 °C in Chocolate II agar medium. Samples that could not be isolated through direct culture were cultured for 1–2 weeks at 36.5 °C in TGC medium (Nissui, Tokyo, Japan) and were separated.
Antibiotic susceptibility for levofloxacin (LVFX), moxifloxacin, and gatifloxacin was measured using the broth dilution method and was determined in accordance with protocols from the Clinical and Laboratory Standards Institute [10]. Isolates were graded as sensitive (S), intermediate (I), or resistant (R) to the tested antibiotics, with minimal inhibitory concentration (MIC) interpreted against breakpoints from the CLSI. The percentage of antibiotic susceptibility was calculated as 100 (S/S + I + R).
Statistical analysis
To estimate the susceptibility of bacterial isolates, a generalized linear mixed-effects model was used with patients designated as a random effect. The simple and adjusted smoothed curves of bacterial susceptibility with respect to age or year isolated were plotted using a generalized additive model (GAM) with 3 degrees of freedom. All analyses were performed using SAS version 9.4. A p value of less than 0.05 was considered to indicate a statistically significant difference. All values are presented as the mean and 95% confidence interval (CI) unless otherwise mentioned.
Results
Bacterial isolation
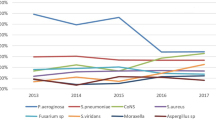
In total, 1200 bacterial isolates were obtained from corneal scrapings of 1167 eyes. The mean age of the patients was 50.4 ± 23.1 (mean ± standard deviation) years. Among them, 471 isolates (39.3%) were GPC, 500 were Cutibacterium (formerly Propionibacterium) species, and 112 were other gram-negative bacteria (GNB) (Table 1). The mean age of the group infected with the GNB was 50.5 years. Among the GPC, the majority were S. epidermidis (45.6%), other coagulase-negative Staphylococci (17.8%), and S. aureus (18.3%). Approximately 24.4% of the S. aureus isolates were methicillin-resistant. The annual number of infections caused by GPC was consistent enough to conduct statistical analysis. Among the GNB isolates, we identified Pseudomonas (n = 20), Serratia (n = 24), and Acinetobacter (n = 13) species during the observation periods (Table 1). The isolation rate was about 70% during the observation periods (Online Resource Table 1S).
Annual changes of isolates and patient age on fluoroquinolone susceptibility
The mean age of patients from whose corneal lesions GPC isolates were obtained gradually increased from 50.4 years in 2008 to 61.6 years in 2016 (p = 0.0407, Fig. 1), and the proportion of older patients gradually increased (Fig. 2). The mean age of patients from whose corneal lesions S. epidermidis isolates were obtained also gradually increased (Online Resource Table 2S). There were only a few patients under the age of 15 in the study. Thus, the number of isolates obtained from these patients was small (Online Resources Table 3S and 4S).
Aging effect on fluoroquinolone susceptibility of GPC
In models that designated both the variables of annual change and patient age as fixed effects, the odds ratio for LVFX susceptibility gradually decreased: 0.893 (0.825–0.967) for GPC and 0.787 (0.682–0.907) for S. epidermidis (p = 0.0055 and 0.0042, respectively; Table 2). In contrast, no significant association was found between LVFX susceptibility of GPC and S. epidermidis with one year of their isolation (Table 2).
The gradual decrease in the LVFX susceptibility of GPC with age in patients was also confirmed by cubic spline analysis. Non-adjusted smooth curves of the mean LVFX susceptibility of GPC and S. epidermidis against patient age (blue plots, Figs. 3 and 4) suggested that the LVFX resistance of GPC increased at around 40 years of age and plateaued around 70 years of age. The same tendency was observed by the plots of onset-date-adjusted LVFX susceptibility of GPC and S. epidermidis (red circles, Figs. 3 and 4).
Age-dependent changes in levofloxacin (LVFX) susceptibility of GPC isolated from corneal lesions. The non-adjusted smoothed curve (closed blue circle) indicates LVFX susceptibility of GPC with respect to age, using a generalized additive model with three degrees of freedom. Open red circle indicates onset-date-adjusted average LVFX susceptibility of GPC, adjusted to the year of isolation. Both plots show corresponding decrease with increase in age
Age-dependent changes in levofloxacin (LVFX) susceptibility of Staphylococcus epidermidis isolated from corneal lesions. The non-adjusted smoothed curve (closed blue circle) indicates LVFX susceptibility of GPC with respect to patient age using a generalized additive model with three degrees of freedom. Open red circle indicated onset-date-adjusted average LVFX susceptibility of GPC, adjusted to the year of isolation. Both plots show a corresponding decrease with the increase in age
Comparison of different fluoroquinolone antibiotic susceptibilities of GPC
Finally, we compared the susceptibility of S. epidermidis isolates to three different fluoroquinolones: LVFX, gatifloxacin, and moxifloxacin. No significant differences were observed between the fluoroquinolones (Table 3).
Discussion
In this study, we retrospectively analyzed the microbiological data of isolates from corneal lesions of bacterial keratitis obtained between 2008 and 2016. We hypothesized that fluoroquinolone resistance of GPC in the general population would increase over the years. However, statistical analysis revealed that fluoroquinolone resistance increases around age 40 and plateaus around age 70. Systemic administration of fluoroquinolones to infants under 16 years of age is restricted due to its toxicity to bone and cartilage. Diseases requiring systemic administration of fluoroquinolones are not generally prevalent in patients under 40 years of age. Therefore, it is possible that fluoroquinolone resistance has not developed in younger patients. A recent large cross-sectional study using longitudinal data collected in the USA revealed low fluoroquinolone susceptibility of staphylococci strains isolated from elderly patients with ocular infections [11]. Differences in susceptibility of staphylococci isolated from elderly patients may be explained by a history of previous exposure to fluoroquinolones.
The predominant isolates from corneal lesions were S. aureus, S. epidermidis, and coagulase-negative staphylococci, all of which ubiquitously colonize the ocular surface [7]. Use of topical fluoroquinolones within 3 months prior to bacteriological examination significantly increased fluoroquinolone-resistant staphylococci on the ocular surface [12]. Administration of topical fluoroquinolone after cataract surgery for one month also transiently increased fluoroquinolone-resistant staphylococci after 6–9 months [13].
Fluoroquinolone susceptibility of some bacteria, including GPC and S. aureus, decreased after extensive systemic use of fluoroquinolones [14,15,16,17]. However, we could not find any reports of ocular infection by fluoroquinolone-resistant bacteria after systemic use of fluoroquinolone. In addition, fluoroquinolone use is a risk factor for nosocomial fluoroquinolone-resistant MRSA infection [18] and colonization of the nasal mucosa by fluoroquinolone-resistant MRSA [19]. Systemic fluoroquinolone might influence the ocular surface because of the high similarity between the nasal and ocular surface flora [20].
The mean age of patients increased, and the fluoroquinolone susceptibility of GPC isolates significantly decreased during the study period. There was a significant correlation between the patient’s age and fluoroquinolone susceptibility (odds ratio 0.893, p = 0.0055) (Table 2). This study was performed at an eye hospital situated in a rural area of Japan. During the study period, according to the city hall of Miyakonojo City, Miyazaki Prefecture, the region’s population decreased from 172,405 (2008) to 167,487 (2016), the percentage of residents under the age of 15 decreased from 14.3 to 13.9%, and that of residents over the age of 64 increased from 25.4 to 29.4%. This demographic change appeared to affect the mean age of patients with bacterial keratitis. In addition, a safety campaign for contact lens users carried out in 2008 and 2009 may have led to a decrease in the incidence of contact lens–associated keratitis.
Limitations of this study include its retrospective study design, and that it was only conducted in a single hospital. When we analyzed the bacteriological profiles of the isolates and the age of patients, some information about the medical history of patients was absent, specifically regarding their history of topical and systemic use of prescription fluoroquinolones. We hope that the future studies on the prescription use of fluoroquinolones would elucidate the relationship between usage and dosage of these medications and fluoroquinolone susceptibility of various bacterial species.
In conclusion, this study revealed that the prevalence of fluoroquinolone-resistant GPC was higher among the isolates of bacterial keratitis from elderly patients than those from younger patients.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Code availability
None.
References
Daniell M (2003) Overview: Initial antimicrobial therapy for microbial keratitis. Br J Ophthalmol 87:1172–1174
Baker RS, Flowers CW Jr, Casey R et al (1996) Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Arch Ophthalmol 114:632–633
The Ofloxacin Study Group (1997) Ofloxacin monotherapy for the primary treatment of microbial keratitis: a double-masked, randomized, controlled trial with conventional dual therapy. Ophthalmology 104:1902–1909
Goldstein MH, Kowalski RP, Gordon YJ (1999) Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 106:1313–1318
Alexandrakis G, Alfonso EC, Miller D (2000) Shifting trends in bacterial keratitis in South Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497–1502
Shah A, Sachdev A, Coggon D et al (2011) Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol 95:762–767
Singer TR, Isenberg SJ, Apt L (1988) Conjunctival anaerobic and aerobic bacterial flora in paediatric versus adult subjects. Br J Ophthalmol 72:448–451
Hooper DC (2001) Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis 7:337–341
Committee on Infectious Diseases (2006) The use of systemic fluoroquinolones. Pediatrics 118:1287–1292
Clinical and Laboratory Standards Institute. M100 http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED30:2020&sbssok=CLSI%20M100%20ED30:2020%20TABLE%202C&format=HTML#CLSI%20M100%20ED30:2020%20TABLE%202C. Accessed 6 Aug 2021
Asbell PA, Sanfilippo CM, Sahm DF et al (2020) Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2019. JAMA Ophthalmology 138:439–450
Fintelmann RE, Hoskin EN, Lietman TM et al (2011) Topical fluoroquinolone use as a risk factor for in vitro fluoroquinolone resistance in ocular cultures. Arch Ophthalmol 129:399–402
Ono T, Nejima R, Iwasaki T et al (2017) Long-term effects of cataract surgery with topical levofloxacin on ocular bacterial flora. J Cataract Refract Surg 43:1129–1134
Chang SC, Hsieh WC, Luh KW (1994) Fluoroquinolone resistance among methicillin-resistant staphylococci after usage of fluoroquinolones other than ciprofloxacin in Taiwan. Diag Microbiol Infect Dis 19:143–147
Hooper DC (2002) Fluoroquinolone resistance among Gram-positive cocci. Lancet Infect Dis 2:530–538
Zervos MJ, Hershberger E, Nicolau DP et al (2003) Relationship between fluoroquinolone use and changes in susceptibility to fluoroquinolones of selected pathogens in 10 United States teaching hospitals, 1991–2000. Clin Infect Dis 37:1643–1648
Tam VH, Louie A, Fritsche TR et al (2007) Impact of drug-exposure intensity and duration of therapy on the emergence of Staphylococcus aureus resistance to a quinolone antimicrobial. J Infect Dis 195:1818–1827
Weber SG, Gold HS, Hooper DC et al (2003) Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis 9:1415–1422
Couderc C, Jolivet S, Thiebaut ACM et al (2014) Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case control study. Clin Infect Dis 59:206–215
Fahmy JA, Moller S, Weis BM (1975) Bacterial flora in relation to cataract extraction. I. Material, methods and preoperative flora. Acta Ophthalmol 53:458–475
Acknowledgements
The authors sincerely thank Toshihito Furukawa (Biostatistical Research Corporation, Tokyo, Japan) for his valuable advice and contributions to the article.
Author information
Authors and Affiliations
Contributions
The authors who contributed to the design and conduct of the study were K.U., T.I., T.O., and A.Y.; to the collection, management, analysis, and interpretation of data, K.U., T.I., J.L., R.N., Y.M., Y.N., and A.Y.; and to the preparation, review, and approval of the manuscript, T.I. and K.M.
Corresponding author
Ethics declarations
Ethics approval
This clinical study was approved by the Institutional Research Ethics Committee of the Miyata Eye Hospital.
Consent to participate
Written informed consent was obtained from all patients.
Consent for publication
Written informed consent was obtained from all patients.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ueda, K., Iwasaki, T., Ono, T. et al. Age factor in the fluoroquinolone susceptibility of gram-positive cocci isolates from bacterial keratitis cases between 2008 and 2016. Graefes Arch Clin Exp Ophthalmol 259, 3351–3357 (2021). https://doi.org/10.1007/s00417-021-05351-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05351-5