Abstract
Purpose
To assess whether early visual acuity letter score change from baseline (ΔVALS) and early spectral domain optical coherence tomography (SD-OCT) measures of center point thickness (CPT) are associated with later ΔVALS in eyes with macular edema due to central or hemiretinal vein occlusion treated with intravitreal aflibercept or bevacizumab.
Methods
Secondary analysis of a randomized clinical trial of 362 participants.
Results
Considered separately at month 3, CPT (categorized as ≤ 300 μm, > 300 μm) and ΔVALS (categorized as < 5, 5–9, ≥ 10) are predictive of ΔVALS at month 6 (aflibercept: P = 0.02 for CPT and P < 0.0001 for ΔVALS; bevacizumab: P = 0.007 for CPT and P < 0.0001 for ΔVALS) and, except for CPT in the bevacizumab arm, also predictive of ΔVALS at month 12 (aflibercept: P = 0.03 for CPT and P < 0.0001 for ΔVALS; bevacizumab: P = 0.18 for CPT and P < 0.0001 for ΔVALS). Month 3 predictors are also associated with average ΔVALS from months 4 to 12 (CPT P = 0.01 in the aflibercept arm, P = 0.02 in the bevacizumab arm; ΔVALS > 10 versus < 5; P < 0.001 for both aflibercept and bevacizumab). When month 3 measures are considered jointly, ΔVALS effect remains significant for average ΔVALS from months 4 to 12 (aflibercept: P = 0.002; bevacizumab: P < 0.0001) but not CPT (aflibercept: P = 0.18; bevacizumab: P = 0.22).
Conclusion
While both month 3 ΔVALS and CPT are predictive of ΔVALS after month 3 through month 12, early ΔVALS has a stronger relationship than CPT with later ΔVALS. SCORE2 registration number is NCT01969708.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) therapy is the standard treatment for macular edema secondary to central retinal vein occlusion (CRVO) or hemiretinal vein occlusion (HRVO). Studies have established the efficacy of anti-VEGF treatment based on monthly injections [1,2,3,4]. The Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2) demonstrated that, after 6 monthly intravitreal injections, bevacizumab was non-inferior to aflibercept in terms of mean change from baseline in visual acuity letter score (ΔVALS) [5]. Baseline central subfield thickness (CST) measured with spectral domain optical coherence tomography (SD-OCT) was associated with 6-month VALS outcomes, but only age and baseline VALS were found to predict treatment response independently in multivariate models [6]. Other studies have also examined whether early response after the first few months of anti-VEGF injections is associated with response at 6 months or later. Bhisitkul et al. [7] reported that, in the Treatment of Macular Edema following Central Retinal Vein Occlusion: Evaluation of Efficacy and Safety (CRUISE) study, an OCT-measured center point thickness (CPT) less than or equal to 250 μm at month 3 was predictive of visual acuity change from baseline to months 6 and 12. Gonzalez et al. [8] studied changes in visual acuity from baseline in patients treated with anti-VEGF therapy for diabetic macular edema and concluded that eyes with suboptimal early visual acuity response month 3 had poorer visual acuity outcomes at 3 years compared with eyes with better early visual acuity response.
The purpose of this secondary analysis from SCORE2 is to assess whether early changes from baseline in visual acuity and early SD-OCT measures of CPT are associated with later changes from baseline in VALS in eyes with macular edema secondary to CRVO or HRVO treated with aflibercept or bevacizumab. We also applied the analysis approaches used by both Bhisitkul et al. [7] and Gonzalez et al. [8] to SCORE2 data to determine if findings from SCORE2 are consistent with these studies. We then compared the strength of the associations of early VALS changes and SD-OCT CPT with later VALS changes. This assessment of early response to anti-VEGF therapy is of practical value to clinicians and patients by guiding expectations and disease management for patients with macular edema due to CRVO or HRVO.
Materials and methods
SCORE2 adhered to the tenets of the Declaration of Helsinki [9] and is registered on http://www.clinicaltrials.gov (identifier: NCT01969708). After institutional review board approval of the protocol, written informed consent was obtained from all participants. SCORE2 methods have been described in detail [10]. The current report focuses on the 180 SCORE2 participants initially randomized to aflibercept and the 182 participants initially randomized to bevacizumab. At month 0 and monthly through month 6, data were collected on best-corrected electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) VALS, CPT assessed by SD-OCT, and eye examinations. Following assessment of the primary outcome at month 6, participants originally assigned to aflibercept who met the protocol-defined criteria for a good response were re-randomized to either continuing aflibercept every 4 weeks or changing to a treat and extend (TAE) regimen; participants with a protocol-defined poor or marginal response at 6 months were to receive a dexamethasone implant. Participants originally assigned to bevacizumab who met the protocol-defined criteria for a good response were re-randomized to either continuing bevacizumab every 4 weeks or changing to a TAE regimen; participants with a protocol-defined poor or marginal response at 6 months were to receive aflibercept. SCORE2 participants’ last visit as part of the SCORE2 protocol-defined treatment schedule was at month 12.
For this visual acuity analysis to match what was reported by Gonzalez et al. [8], study eyes were categorized according to ΔVALS at month 3: < 5, 5–9, or ≥ 10. For the early CPT response at month 3, mean change from baseline in VALS was compared between study eyes with CPT ≤ 300 μm versus CPT > 300 μm to match the Bhisitkul et al. [7] analysis approach. The Bhisitkul reference analysis used a 250 μm cutoff based on central foveal thickness from Stratus OCT software (Carl Zeiss Meditec). As it is known that the newer SD-OCT systems, Cirrus (Carl Zeiss Meditec) and Spectralis (Heidelberg Engineering), have retinal thickness values that are higher than those generated by Time Domain-OCT machines [11] (median difference of 43 μm for Cirrus and 67 for Spectralis) and because SCORE2 used SD-OCT software, we chose a 300 μm CPT cutoff for the comparative analyses. The 300 μm thickness cutoff on OCT retinal measurements has relevance beyond the Bhisitkul reference as it was used in SCORE2 to define study eye eligibility (central subfield thickness [CST] > 300 μm if measured with a Carl Zeiss Meditec Cirrus OCT machine was an inclusion criterion), and < 300 μm in CST was one of the components used to define resolution of macular edema as presented in the primary SCORE2 results [5].
Statistical analysis
Analyses included calculation of simple means, standard deviations, and Pearson correlation coefficients, often graphically represented. To assess which of early ΔVALS or early CPT is a better predictor of later ΔVALS, we compared CPT-ΔVALS correlation coefficients to ΔVALS-ΔVALS correlation coefficients. We have data from months 0 to 12, so there are 91 month-pairs we could consider to be “early” versus “late,” that is, M0vM0,..., M0vM12, M1vM1,..., M1vM12, ..., M12vM12.Footnote 1 Rather than choosing a specific pair of months, we consider all of them in Fig. 3, which summarizes, for each initial treatment assignment, the distributions of the correlation coefficients. P values in Table 1 were derived from 1-way analyses of variance. Results in Table 2 were constructed by regressing average ΔVALS during months 4 to 12 using a restricted maximum likelihood model separately on month 3 CPT and ΔVALS, by treatment arm, assuming independent errors. Estimates and associated statistics are cell means derived from the model via SAS contrasts. In Table 3, where the covariates include month 3 CPT, ΔVALS, and their interaction, the estimates incorporate the interaction term, even though it is not significant. All analyses and graphics were carried out in SAS 9.4, level TS1M4.
Results
SCORE2 randomized 362 participants who had a mean (SD) age of 69 (12) years; 157 (43.4%) were women; mean (SD) VALS at baseline was 50.3 (15.2) (approximate Snellen visual acuity mean of 20/100), mean SD-OCT CST was 665.0 (223.2) microns, and mean CPT was 682. 5 (250.7) microns.
Table 1 displays the analysis for both randomized treatment arms in SCORE2, examining mean ΔVALS at months 6 and 12 as a function of whether month 3 CPT was ≤ 300 μm or month 3 ΔVALS was < 5, 5–9, or ≥ 10. CPT ≤ 300 μm at month 3 was significantly associated with improved ΔVALS at month 6 (P = 0.02) and month 12 (P = 0.03) in the aflibercept arm, with mean improvements of ΔVALS of approximately 20 compared to about 8 among those with CPT > 300 at month 3. Similar statistically significant findings and magnitude of ΔVALS differences were noted when examining the CPT ≤ 300 μm indicator at month 6 in the bevacizumab arm (P = 0.007) but not at month 12 (P = 0.18). The groupings based on ΔVALS at month 3 were also statistically significant (P < 0.0001) for both aflibercept and bevacizumab at both months 6 and 12, with mean ΔVALS at months 6 and 12 of less than 4 in both arms when month 3 ΔVALS was < 5, ranging from 8 to 14 when month 3 ΔVALS was 5 to 9, and ranging from 23 to 28 when month 3 ΔVALS was ≥ 10.
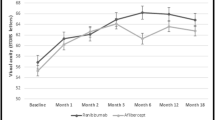
Mean ΔVALS at all follow-up visits from month 1 to month 12 showed good separation based on the 300 μm CPT indicator at month 3 in both the aflibercept and bevacizumab arms (Fig. 1). When examining the mean of ΔVALS during months 4 to 12 (Table 2), the visits that occurred after CPT groupings were defined; study eyes with CPT ≤ 300 μm at month 3 averaged ΔVALS improvements of 12.5 (P = 0.01, 95% CI: 2.8–22.2) more than those with CPT > 300 μm in the aflibercept arm between months 4 and 12 and 7.1 (P = 0.02, 95% CI:1.3–12.9) more in the bevacizumab arm, based on estimates from a regression model.
Figure 2 displays ΔVALS from month 1 to month 12 based on ΔVALS groupings at month 3 of < 5, 5–9, and ≥ 10, which showed separation for ΔVALS across all follow-up visits. Estimates from a regression model of mean ΔVALS between months 4 and 12 showed that those with ΔVALS ≥ 10 at month 3 averaged 21.8 (P < 0.0001, 95% CI: 15.7–27.8) more than those with ΔVALS < 5 in the aflibercept arm and 25.7 (P < 0.0001, 95% CI: 20.8–30.6) more in the bevacizumab arm (Table 2). Those with a ΔVALS improvement of 5–9 at month 3 in the aflibercept arm averaged 7.0 more in mean ΔVALS between months 4 and 12 than those with ΔVALS < 5 at month 3, a finding that was not statistically significant (P = 0.08, 95% CI: − 0.9 to 14.8), but the mean improvement of 9.7 comparing the ΔVALS improvement of 5–9 at month 3 to those with ΔVALS < 5 was statistically significantly in the bevacizumab arm (P = 0.004, 95% CI: 3.1–16.3).
Table 3 shows results from regressing mean continuous ΔVALS over months 4 to 12 jointly on month 3 CPT (≤ 300, > 300), month 3 ΔVALS (< 5, 5–9, ≥ 10), and their interaction. Neither interaction nor month 3 CPT is significant (interaction P = 0.50 in aflibercept, 0.35 in bevacizumab; month 3 CPT P = 0.18 in aflibercept, 0.22 in bevacizumab), but month 3 ΔVALS is significant (aflibercept P = 0.002, bevacizumab P < 0.0001). This suggests that, once month 3 ΔVALS is known, month 3 CPT adds little predictive information for subsequent mean ΔVALS. The confidence intervals of Table 3 show that, irrespective of month 3 CPT, mean ΔVALS did not differ significantly from 0 in either treatment arm when month 3 ΔVALS was < 5, but was significantly greater than 0 when month 3 ΔVALS was ≥ 10. Results were intermediate when month 3 ΔVALS was 5–9, with values significantly greater than 0 except for the aflibercept arm with month 3 CPT ≤ 300 μm. Baseline VALS was associated with ΔVALS at visits after month 3 and adjusting group- and treatment-specific ΔVALS and CPT means for baseline VALS in the analyses presented in Tables 1, 2, and 3 resulted in similar findings.
Further investigation of the association between early ΔVALS and CPT was performed by estimating correlation coefficients. Figure 3 shows the distributions of ΔVALS-ΔVALS correlation coefficients and the CPT-ΔVALS correlation coefficients across the month-pair values between baseline and month 12 (i.e., M0vM0,..., M0vM12, M1vM1,..., M1vM12, etc.). The ΔVALS-ΔVALS correlations are considerably greater in magnitude than the ΔVALS-CPT correlation coefficients. More specifically, the ΔVALS-CPT correlation coefficients have a median of − 0.09, with 90% of the values ranging between − 0.27 and 0.32, while the ΔVALS-ΔVALS correlation coefficients have a median of 0.83, with 90% of the values ranging between 0.63 and 0.92. The larger magnitude of the ΔVALS-ΔVALS correlations suggests that, to predict late ΔVALS, it is better to use early ΔVALS change than to use early CPT.
We further investigated the relationship between early month 3 visit data and later month ΔVALS outcomes. Figure 4a shows a scatter plot of ΔVALS at month 6 plotted against CPT at month 3. The correlation coefficient between month 3 CPT and month 6 ΔVALS is − 0.09 and − 0.20 in the aflibercept and bevacizumab arms, respectively. To contrast with the relationship between early and late ΔVALS, month 6 ΔVALS values are plotted against month 3 ΔVALS in Fig. 4b. The correlation between month 3 and month 6 ΔVALS is 0.81 and 0.89 in the aflibercept and bevacizumab arms, respectively. The diagonal lines of Fig. 4b describe the locus of points we would expect if the month 6 ΔVALS were identical to the month 3 ΔVALS, and the observed points fit this relationship reasonably well.
a Change from baseline in visual acuity letter score (ΔVALS) at month 6 versus center point thickness at month 3. Horizontal reference line at ΔVALS = 0 and vertical reference line at center point thickness = 300 μm. b ΔVALS at month 6 versus ΔVALS at month 3. The diagonal lines are lines from the origin with slopes of 45 degrees
Lastly, we also explored other measures of CPT at month 3 to compare to findings where absolute CPT at month 3 was examined. To address this, we fit models in which the average ΔVALS from months 4 to 12 was regressed on all possible subsets of the following set of month 3 predictors: VALS, ΔVALS, CPT, ΔCPT, and %ΔCPT. The most important predictor is ΔVALS. Models that excluded ΔVALS have R-squared values ranging from 0.01 to 0.38, while models with ΔVALS have R-squared values ranging from 0.73 to 0.75 in the aflibercept arm and 0.81 to 0.84 in the bevacizumab arm. Within the set of models containing ΔCPT, ΔCPT is the next most important (after ΔVALS), but R-squared increases by only 0.01 to 0.03 on its inclusion. This is minor compared to the effect of including ΔVALS, which improves R-squared by at least 0.52 in the aflibercept arm and 0.43 in the bevacizumab arm.
Discussion
SCORE2 analyses are consistent with previously reported findings with respect to the significance of early CPT [7] and early ΔVALS [8] in predicting later ΔVALS changes in eyes with CRVO treated with anti-VEGF therapy. Bhisitkul et al. [7] reported that, in the CRVO patients of the CRUISE study, an indicator of CPT ≤ 250 μm at month 3 was predictive of visual acuity change from baseline at months 6 and 12. The analogous SCORE2 analysis supports these results in that the month 6 ΔVALS among eyes with month 3 CPT ≤ 300 μm exceeds the month 6 ΔVALS among eyes with month 3 CPT > 300 μm. Early CPT responders in the CRUISE study had mean improvement from baseline in BCVA at 6 and 12 months of 15 to 16.5 letters [7]. In SCORE2, we observed mean improvements in ΔVALS of 19.9 to 22.4 when month 3 CPT was ≤ 300 (Table 1). Bhisitkul et al. [7] reported that the percent of early responders at month 3 was 71.2% (0.3 mg) and 78.5% (0.5 mg) in the CRUISE study; in SCORE2, the proportions were 94.7% in the aflibercept arm and 78.7% in the bevacizumab arm. The SCORE2 regression analysis of Table 2 shows that the CPT groupings differ significantly from each other, with the CPT values ≤ 300 μm predicting better VALS response. Figure 1 shows improvement in ΔVALS over months 1–12 based on CPT grouping at month 3.
Gonzalez et al. [8] studied visual acuity change from baseline in patients with diabetic macular edema divided into three groups based on their month 3 ΔVALS: < 5 letters, 5–9 letters, and ≥ 10 letters. This SCORE2 analysis presented in this report shows similar results in that the month 3 ΔVALS predicts corresponding changes in ΔVALS at later visits in CRVO and HRVO participants (Table 1; Fig. 2). The SCORE2 regression analysis of Table 2 shows that the ΔVALS groupings differ significantly from each other, with the higher values predicting better ΔVALS response. This finding suggests that early response measured by ΔVALS at month 3 predicts the month 12 outcomes in eyes with CRVO or HRVO (Fig. 2). Figure 2 also shows that early improvements of at least 10 in ΔVALS are, on average, sustained through month 12. In contrast, study eyes without early visual acuity improvements (< 5) do not improve at later time points through month 12 based on the treatment regimens specified in the SCORE2 protocol.
The SCORE2 analysis of CPT-ΔVALS correlations suggests that, the greater the CPT at baseline, before initiation of aflibercept or bevacizumab treatment, the more the participant could be expected to later improve in VALS, consistent with prior reports from SCORE2 [6]. These correlations show the beneficial effect of treatment. The mostly small negative correlations of CPT measured with subsequent ΔVALS suggest that, once treatment has begun, increased CPT portends poorer VALS improvement from baseline (Fig. 3). The ΔVALS-ΔVALS correlations (Fig. 4a) are much larger in magnitude than the correlations of CPT with later ΔVALS. While month 3 CPT and month 3 ΔVALS are predictive of month 6 ΔVALS, the ΔVALS relationship is stronger than the CPT relationship, as displayed in Fig. 4 a and b. Once month 3 ΔVALS is known, month 3 CPT does not add information about ΔVALS at months 6 or 12. We may ascertain this by regressing average ΔVALS from months 4 to 12 jointly on these two predictors. Table 3 shows that month 3 ΔVALS is strongly significant, while month 3 CPT is not. This analysis suggests that month 3 ΔVALS is a stronger predictor of month 6 and month 12 ΔVALS than month 3 CPT is, and that if month 3 ΔVALS is known, month 3 CPT does not improve the prediction of ΔVALS.
All analyses are presented separately for those randomized to aflibercept and those randomized to bevacizumab, as we reported that a higher proportion of eyes assigned to aflibercept demonstrated resolution of macular edema in the first 6 months compared to those assigned to bevacizumab [5]. This finding might suggest that the post-randomization predictors of CPT may differ between the anti-VEGF agents. The relationships between early response based on ΔVALS and CPT at month 3 and later ΔVALS are consistent between the two arms, except that CPT at month 3 was predictive of ΔVALS at month 12 in the aflibercept arm (P = 0.03) but not the bevacizumab arm (P = 0.18). The findings in each arm, confirmed in the other arm, serve as a replication and provide more confidence in the conclusions. Furthermore, patient expectations related to late ΔVALS response and treatment recommendations stemming from these findings should not differ based on whether the initial treatment plan started with aflibercept or bevacizumab.
These findings illustrate the limitations of OCT measures as a surrogate for changes in visual acuity. In multiple clinical trials for diabetic macular edema (Protocol I and T), changes in OCT were not significantly associated with changes in visual acuity [12, 13]. In clinical practice, physicians often rely on response demonstrated on OCT, as it is objective and fast to obtain while less prone to subjective aspects of visual acuity measurement, which is often not standardized, as it is in clinical trials. However, early response in visual acuity is more accurate than the anatomical outcomes observed from OCT to predict later VALS outcomes in patients with macular edema from CRVO or HRVO.
Long-term follow-up of patients with macular edema associated with CRVO reveals that treatment is often required beyond 6 months [14,15,16]. Predicting how eyes with macular edema secondary to CRVO or HRVO will respond in terms of visual acuity is of practical value to both clinicians and patients by helping guide expectations and management decisions. At baseline, CST was associated with 6-month VALS outcomes, but only age and baseline VALS were found to predict treatment response independently in multivariate models [6]. This present analysis further shows how early response on OCT and ΔVALS can predict how patients will do over a year on a SCORE2 treatment regimen. Participants who do not experience an early visual acuity score response at month 3 (< 5 gained) did not improve significantly at month 12 (Fig. 2; P = 0.17 for aflibercept arm and P = 0.48 for bevacizumab arm based on one-sample t test). Future clinical studies and/or clinical practitioners should consider looking at other treatment regimens to improve visual outcomes for eyes with a poor early visual acuity response.
Results presented in this paper have limitations, so care should be taken in their interpretation. These analyses exploring relationships between early ΔVALS and CPT and longer term ΔVALS are post hoc. Groupings of CPT and ΔVALS at month 3, and the resulting comparisons, are suggested by previous authors, but not protected by randomization. Due to the exploratory nature of the analysis, no control of type 1 error was attempted. When examining results within the aflibercept and bevacizumab arms, the monthly anti-VEGF treatment assigned at randomization continued through month 5. Treatment provided from month 6 through month 12 included the same anti-VEGF drug only in those who were deemed good responders at month 6. At random, half of these good responders received a treat and extend regimen rather than monthly injection. Study eyes with a poor or marginal response at month 6 received dexamethasone between months 6 and 12 if originally assigned to aflibercept (approximately 9% of eyes randomized to aflibercept) and switched to aflibercept treatment between months 6 and 12 if originally assigned to bevacizumab (approximately 23% of eyes randomized to bevacizumab). Another potential limitation when assessing the VALS groupings presented in this paper is that participants with month 3 ΔVALS < 5 may have little subsequent VALS change because of a “ceiling” effect, wherein they change little because their baseline VALS is already good and there is no room for improvement. However, the mean baseline VALS was 53.2 when month 3 ΔVALS was < 5, 55.8 when ΔVALS was 5–9, and 48.8 when ΔVALS was ≥ 10 (P = 0.006). These mean VALS values all leave substantial room for improvement, and the ceiling effect does not appear to be a major concern. Furthermore, adjustment of group- and treatment-specific means for baseline visual acuity in the analyses did not impact findings. Lastly, using an OCT measure of CPT ≤ 300 μm does not account for other features of macular edema that could impact vision, such as presence of subretinal or intraretinal fluid or cystoid spaces. Analyses demonstrated that another OCT-based assessment, resolution of macular edema, which is defined as CST < 300 μm, no subretinal or intraretinal fluid, and no cystoid spaces, was less strongly associated with later changes in VALS than the CPT ≤ 300 μm measure.
In conclusion, while both month 3 ΔVALS and month 3 CPT are predictive of the magnitude of ΔVALS improvement later in follow-up, early ΔVALS has a stronger relationship than CPT with later ΔVALS. Furthermore, participants without early VALS improvement continue to demonstrate a poor visual acuity response later in follow-up. These findings together are of practical value to both clinicians and patients by helping guide expectations and disease management for patients with macular edema due to CRVO or HRVO.
Data availability
Not applicable.
Notes
Some of the month-pairs add no information about correlation. For example, VALS change from month 0 is identically 0 at month 0. Also, the autocorrelation of VALS change with itself in the same month must be 1. Thus, there are 66 informative autocorrelation coefficients and 90 informative cross-correlation coefficients.
References
Campochiaro PA, Brown DM, Awh CC et al (2011) Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 118(10):2041–2049. https://doi.org/10.1016/j.ophtha.2011.02.038
Brown DM, Campochiaro PA, Bhisitkul RB et al (2011) Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 118(8):1594–1602. https://doi.org/10.1016/j.ophtha.2011.02.022
Brown DM, Heier JS, Clark WL et al (2013) Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol 155(3):429–437. https://doi.org/10.1016/j.ajo.2012.09.026
Korobelnik JF, Holz FG, Roider J et al (2014) Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology. 121(1):202–208. https://doi.org/10.1016/j.ophtha.2013.08.012
Scott IU, VanVeldhuisen PC, Ip MS, for the SCORE2 Investigator Group et al (2017) Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion. The SCORE2 randomized clinical trial. JAMA. 317(20):2072–2087. https://doi.org/10.1001/jama.2017.4568
Scott IU, Van Veldhuisen PC, Ip MS, for the SCORE2 Investigator Group et al (2017) Baseline factors associated with 6-month visual acuity and retinal thickness outcomes in patients with macular edema secondary to central retinal vein occlusion or hemiretinal vein occlusion: SCORE2 Study Report 4. JAMA Ophthalmol 135(6):639–649. https://doi.org/10.1001/jamaophthalmol.2017.1141
Bhisitkul RB, Campochiaro PA, Shapiro H, Rubio RG (2013) Predictive value in retinal vein occlusions of early versus late or incomplete ranibizumab response defined by optical coherence tomography. Ophthalmology. 120(5):1057–1063. https://doi.org/10.1016/j.ophtha.2012.11.011
Gonzalez VH, Campbell J, Holekamp NM et al (2016) Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol 172:72–79. https://doi.org/10.1016/j.ajo.2016.09.012
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 310(20):2191–2194. https://doi.org/10.1001/jama.2013.281053
Scott IU, VanVeldhuisen PC, Ip MS et al (2017) SCORE2 Report 2: study design and baseline characteristics. Ophthalmology. 124(2):245–256. https://doi.org/10.1016/j.ophtha.2016.09.038
Diabetic Retinopathy Clinical Research Network Writing Committee (2014) Reproducibility of spectral-domain optical coherence tomography retinal thickness measurements and conversion to equivalent time-domain metrics in diabetic macular edema. JAMA Ophthalmol 132(9):1113–1122. https://doi.org/10.1001/jamaophthalmol.2014.169
Browning DJ, Glassman AR, Aiello LP et al (2007) Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 114(3):525–536. https://doi.org/10.1016/j.ophtha.2006.06.052
Bressler SB, Odia I, Maguire MG et al (2019) Factors associated with visual acuity and central subfield thickness changes when treating diabetic macular edema with anti–vascular endothelial growth factor therapy. An exploratory analysis of the protocol t randomized clinical trial. JAMA Ophthalmol 137(4):382–389. https://doi.org/10.1001/jamaophthalmol.2018.6786
Heier JS, Campochiaro PA, Yau L et al (2012) Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 119(4):802–809. https://doi.org/10.1016/j.ophtha.2011.12.005
Campochiaro PA, Sophie R, Pearlman J, RETAIN Study Group et al (2014) Long-term outcomes in patients with retinal vein occlusion treated with ranibizumab: the RETAIN study. Ophthalmology. 121(1):209–219. https://doi.org/10.1016/j.ophtha.2013.08.038
Scott IU, Oden NL, VanVeldhuisen PC et al (2019) Month 24 outcomes after treatment initiation with anti–vascular endothelial growth factor therapy for macular edema due to central retinal or hemiretinal vein occlusion SCORE2 report 10: a secondary analysis of the SCORE2 randomized clinical trial. JAMA Ophthalmol 137(12):1389–1398. https://doi.org/10.1001/jamaophthalmol.2019.3947
Acknowledgments
The Study of Comparative Treatments for Retinal Vein Occlusion 2 (SCORE2) Investigator Group members are SCORE2 Executive Committee: Ingrid U. Scott, (Study Chair), Penn State College of Medicine; Michael S. Ip (Study Co-Chair), Doheny Eye Center UCLA; Barbara A. Blodi (Principal Investigator), Fundus Photograph Reading Center; Sangeeta Bhargava (Director, Clinical Trials), National Eye Institute, NIH; Paul VanVeldhuisen (DCC Principal Investigator), The Emmes Company, LLC; Neal L. Oden (DCC Statistician), The Emmes Company, LLC; Maria J. Figueroa (DCC Project Director), The Emmes Company, LLC. SCORE2 Data and Safety Monitoring Committee: David C. Musch (Chair), University of Michigan; Sangeeta Bhargava, National Eye Institute, NIH; Dennis P. Han, Medical College of Wisconsin; Srinivas R. Sadda, Doheny Eye Institute; George A. Williams, Beaumont Eye Institute; Stephen Wisniewski, University of Pittsburgh. Penn State University: Ingrid U. Scott (Study Chair), Janelle Gaston (Research Project Manager). SCORE2 Data Coordinating Center (DCC): Paul VanVeldhuisen (Principal Investigator), Maria Figueroa (Project Director), Lena Bradley, Jodi De Stefano, Eileen Guan, Anne Hoehn, Alexa Irazabal, Sue Isman, Jacquie King, Radhika Kondapaka, Hamsa Kumar, Matt Lahut, Robert Lindblad, Robin Lovett, Neal Oden, Michael Patschak, Ro Shauna Rothwell, Rosemary Smith, Janet Van Dyke, Valerie Watson, Lauren Yesko. SCORE2 Fundus Photograph Reading Center (FPRC): Barbara A. Blodi (Principal Investigator), Amitha Domalpally (Research Director), Susan Reed (Research Program Manager), Pam Vargo (Lead Imaging Specialist). Institute for Personalized Medicine: Cynthia Reed (Patient Enrollment Specialist).
Sites are listed in alphabetical order. Personnel are listed by their primary role as PI (PI), subinvestigator (SI), clinic coordinator (CC), visual acuity technician (V), photographer (P), pharmacist (PH), and other (O). Black Hills Regional Eye Institute Prema Abraham (PI), Beth Bement-Stump (V), Leah Callahan (CC), Mindi Dockter (V), Kristi Livermont (CC), Robert Nixon (SI), Dan Parks (P), Nathan Steinle (SI), Alison Thompson (CC), Dylan Yu (SI); Casey Eye Institute-Oregon Health & Science University Steven Bailey (SI), Jordan Barth (P), J. Peter Campbell (SI), Christina Flaxel (SI), Christopher Howell (P), Jocelyn Hui (P), Thomas Hwang (SI), Shirley Ira (V), Andreas Lauer (PI), Phoebe Lin (SI), Ann Lundquist (CC), Susan Nolte (V), Scott Pickell (P), Dawn Ryan (P), Mitchell Schain (CC), Peter Steinkamp (P), Chiedozie Ukachukwu (P), Sara Blefgen (PH), Brad Fujisaki (PH), Jeanne Liming (PH), Frank Mistretta (PH), Jennifer Petrolati (PH); Center for Retina and Macular Disease Adam Berger (SI), Erika Darby (P), Vera Dilts (V), Richard Hamilton (SI), Laura Holm (V), Tammy McCarty (CC), Esmeralda Medina (CC), David Misch (SI), Suk Jin Moon (SI), Kari Owen (P), John Randolph (SI), Jason Strickland (P), Dawn Sutherland (CC), Michael Tolentino (PI), Nicole Varner (V); Charlotte Eye Ear Nose & Throat Andrew Antoszyk (PI), Uma Balasubramaniam (P), Swann Bojaj (P), David Browning (SI), Loraine Clark (P), Sarah Ennis (V), Christina Fleming (CC), Autumn Finch (P), Sherry Fredenberg (V), Angie Gentile (CC), Lisa Jackson (P), Donna McClain (P), Michael McOwen (P), Angela Price (CC), Omar Punjabi (SI), Beverly Rowland (P), Lynn Watson (P); Cumberland Valley Retina Consultants Ellis Bloom (P), Thomas Catapano (P), Mariia Chornobai (CC), Nicole Hollimon (P), Allen Hu (SI), Donna King (V), Christa McChancy (P), Wendy McCullough (V), Kristen Miller (CC), George Sanborn (SI), Ejiatu Sesay (P), Veronica Shetler (V), Paula Smith (CC), Becca Varner (P), Leslee Wallech (V), Shayla Ward (CC), David Warrow (SI), Cherie Wenger (P), Alison Wentz (CC), Kimberly Wood (P), John Wroblewski (PI); Dean McGee Eye Institute Shannon Almeida (CC), Vanessa Bergman (V), Joann Booth (P), Reagan Bradford (SI), Alisha Brewer (CC), Russ Burris (P), Mandi Butt (P), Vanessa Drummond (CC), Stephen Fransen (SI), Sonny Icks (V), Ronald Kingsley (PI), Robert Leonard (SI), Heather Miller (V), Vinay Shah (SI), Vicki Wolzen (P); Delaware Valley Retina Associates Krista Bayer (P), Lorraine Eggert (V), Susan Geraghty (P), Morgan Harper (V), Darmakusuma Ie (PI), Lauren Knezek (P), Jeffrey Lipkowitz (SI), Beverly Sannazzaro (CC), Kekul Shah (SI); East Bay Retina Consultants Inc. Renjini Balakrishnan (CC), Fazilla Bano (V), Daniel Brinton (SI), Denise Bustamante (V), Elyssa Casugay (CC), Roby Casupanan (V), Patty Chung (V), Heather Enyart (P), Caroline Frambach (V), Khaliah Jackson (V), Afsoon Jamali (P), Mae Kwan (V), Scott Lee (SI), Eugene Lit (PI), Joshua Machacon (V), Patty Madrigal (V), Scotty Renslow (CC), Helen Ricks (V), Soraya Rofagha (SI), Heidi Winje (CC); Elman Retina Group PA Jennifer Belz (CC), Theresa Cain (P), Teresa Coffey (V), Ashley Davis (V), Michael Elman (PI), Henry Leder (SI), Ashley Metzger (P), Peggy Orr (V), Dallas Sandler (V), Jennifer Simmons (V), Perel Simpson (V), Pamela Singletary (V), Peter Sotirakos (P), JoAnn Starr (CC), Amy Thompson (V); Emory University Eye Center Judy Brower (V), Blaine Cribbs (SI), Linda Curtis (CC), Jannah Dobbs (P), Deborah Gibbs (CC), Andrew Hendrick (PI), G. Baker Hubbard (SI), Debbie Jordan (P), Donna Leef (V), Darien Middleton (O), Ghazala O’Keefe (SI), Timothy Olson (SI), Samillya Pearson (P), Matthew Raeber (P), Joshua Robinson (SI), Susan Rogers (PH), Jiong Yan (SI); Florida Retina Consultants Jackie Andrews (V), Steve Carlton (P), Katrina Dawson (CC), Damanda Fagan (CC), Scott Friedman (PI), Tammi Marsh (CC), Allen McKinney (V), Nader Moinfar (SI), Karen Seyez (V), Paige Walters (V), Ceara Wendel (CC), Kimberly Williamson (CC); Georgia Retina Jini Jones (P), Leslie Jones (V), Scott Lampert (SI), Leslie Marcus (CC), Krishna Mukkamala (SI), Lindsey Neal (P), Lauren Nunn (CC), Maria Rogers (V), Atul Sharma (SI), Robert Stoltz (PI), Morgan West (P); Henry Ford Health System Megan Allis (P),Celia Benvenutti (CC), David Burley (P), Uday Desai (SI), Paul Edwards (PI), Hua Gao (SI), John Grybas (P), Melanie Gutkowski (V), Julianne Hall (CC), Thomas Hessburg (SI), Nitin Kumar (SI), Nicole Massu (P), Melina Mazurek (V), Mary Monk (CC), Janet Murphy (V), Jessica Nelson (P), Dinah Oude-Reimerink (V), Brian Rusinek (P), Jenny Shaheen (P), Jessica Staffne (P), Bradley Stern (P), Tracey Troszak (P), Katie Ventimiglia (V); Icahn School of Medicine at Mount Sinai Wanda Carrasquillo-Boyd (P), John Bo Soo Choi (V), Yuen Ping Chui (SI), Ronald Gentile (SI), Priya Mehta (V), Melissa Rivas (CC), Richard Rosen (PI), Katy Tai (CC), Jia Tan (V), Meliza Unson (V), Paul Whitten (P), Alex Yang (V); Illinois Eye and Ear Infirmary University of Illinois at Chicago Catherine Carroll (P), Felix Chau (SI), Mark Janowicz (P), Tametha Johnson (V), Yannek Leiderman (SI), Jennifer Lim (PI), Marcia Niec (CC), Yesenia Ovando (V), Kevin Reyes (P), Natasa Stankovic (V), Jie (Jessica) Sun (CC), Lauren Talasnik (CC), Lawrence Ulanski (SI); Loma Linda University Armand Assissini (P), Marvyn Cerdenio (CC), Joseph Fan (SI), Raquel Hernandez (CC), Lynn Huang (SI), William Kiernan (V), Jesse Knabb (P), Michael Rauser (PI), Gisela Santiago (CC), Mukesh Suthar (SI), Brandon Williams (P); Long Island Vitreoretinal Consultants Michael Chitjian (P), Kristen D’Amore (CC), Vincent Deramo (SI), David Fastenberg (SI), Philip Ferrone (PI), Barry Golub (SI), Kenneth Graham (SI), Sandra Jaya (V), Jonathan Jonisch (SI), David Rhee (SI), Juan Romero (SI), Brett Rosenblatt (SI), Marianne Schlameuss (CC), Jeffrey Shakin (SI), Eric Shakin (SI), Vasanti Sookhai (V), Jamie Szczepanski (V); Mayo Clinic Betsy Baker (CC), Sophie Bakri (PI), Andrew Barkmeier (SI), Jean Burrington (V), Gillian Currie (CC), Melissa Franzen (CC), Shannon Goddard (P), Denise Lewison (P), Jessica Morgan (V), Rebecca Nielsen (CC), Joan Overend (V), Heidi Rubin (CC), Wendy Smith (SI), Jamie Tesmer (P), Stephanie Thatcher (P), Diane Vogen (CC); Medical Center Ophthalmology Associates Rahen Ahmed (SI), Rene Andrade (P), Darren Bell (SI), Connie Bermea (V), Carrisa Bolado (CC), Jason Burns (SI), Melissa Dominguez (V), Catherine Ellis (CC), Rosa Escobar (P), Richard Evans (SI), Shelli Goel (P), Roxanne Gomez (V), Felicia Huron (V), Lita Kirschbaum (CC), Guillermo Montoya (CC), Cynthia Myrick (CC), Nellie Quiroga (P), Judy Rittimann (CC), Vincent Segovia (P), Michael Singer (PI); Medical College of Wisconsin Marriner Altmann (P), Vicki Barwick (V), Joe Beringer (P), Joseph Carroll (SI), Thomas Connor (SI), Eleanor Dorsey (CC), Mara Goldberg (P), Kristy Keller (P), Judy Kim (PI), Katie McKenney (CC), Stephanie Moebius (P), Krissa Packard (CC), Brittany Rego (V), Amber Roberts (V), Hannah Russell (P), Kimberly Stepien (SI), David Weinberg (SI), Vesper Williams (CC), Pat Winter (V), William Wirostko (SI); Mid Atlantic Retina Hannah Benfield (CC), Christina Centinaro (V), Allen Chiang (SI), Lauren Devine (V), Mitchell Fineman (SI), Michele Formoso (CC), Sunir Garg (SI), Cedric George (O), Elaine Gonzales (P), Lisa Grande (V), Omesh Gupta (SI), Allen Ho (SI), Samuel Houston (SI), Jason Hsu (SI), Maryann Jay (P), Richard Kaiser (SI), Brianna Kenney (CC), Lisa Lavetsky (P), Theresa Listner (CC), Joseph Maguire (SI), Sonia Mehta (SI), Jill Noble (O), Carl Park (SI), Ehsan Rahimy (SI), David Reed (SI), Carl Regillo (PI), Noga Senderowitsch (CC), Arunan Sivalingam (SI), Marc Spirn (SI), Jamilla Sudler (V); Midwest Eye Institute Erin Brown (CC), Thomas Ciulla (SI), Tammy Dale (V), Neil Finnen (PI), Charlotte Harris (P), Cindi Hood (P), Shyla Keesling (CC), Ingrid Kerr (CC), Raj Maturi (SI), Stephanie Morrow (P), Kristin Phillips (V), Ashley Radtke (V), Bethany Sink (CC), Tom Steele (P); National Ophthalmic Research Institute A. Thomas Ghuman (PI), Laura Greenhoe (CC), Cheryl Kiesel (CC), Ray Kiesel (P), Eileen Knips (P), Anita Leslie (V), Kristi Maro (CC), Crystal Peters (CC), Paul Raskauskas (SI), Cheryl Ryan (CC), Jessica Lynn Saez (CC), Ashish Sharma (SI), Natalie Torres (Crawford) (CC), Danielle Turnbo (V), Joseph Walker (SI), Glenn Wing (SI); New Jersey Medical School Tamara Berezina (P), Neelakshi Bhagat (PI), Kathryn Boschert (CC), Eileen Buroff (V), Janie Ellenberger (CC), Catherine Fay (CC), Marian Konop (P), Michael Lazar (P), Tatiana Mikheyeva (P), Monique Roy (SI), Marco Zarbin (SI); NJ Retina Howard Fine (SI), Laura Fox Gadless (CC), Eric Friedman (SI), Robyn Green (CC), Stuart Green (SI), Bruce Keyser (SI), Amy Leviton (V), Celeste Nelson (CC), Jennifer Pilato (CC), Jonathan Prenner (SI), Daniel Roth (SI), Alex Schlosser (P), Sumit Shah (PI), H. Matthew Wheatley (SI), David Yarian (SI); Northern California Retina Vitreous Assoc. Roxanne Arrivas (P), Alok Bansal (SI), Edwin Boldrey (SI), Jesus Borrillo (SI), Louis Chang (SI), Giovanna Corazzi (V), Amy Dennis (V), Sharon Depaz (CC), Andrea Gadda (V), Amy Goldstein (P), Rahul Khurana (PI), Diana Lam (CC), Celeste Obando (P), James Palmer (SI), Olga Sessions (P), Mark Wieland (SI); Orange County Retina Medical Group Trisha Ambrocio (CC), Marinel Casiano (CC), Eugene Chang (SI), Sanford Chen (PI), Millie Liu (V), Jessica Lowery (P), John Maggiano (SI), Rajiv Rathod (SI), Timothy You (SI); Paducah Retinal Center Carl Baker (PI), Tracey Caldwell (CC), Alecia Camp (P), Samantha Kettler (P), Lynnette Lambert (CC), Tracey Martin (V), Margaret Orr (V), Mary Jill Palmer (V), Ron Tilford (SI), Tana Williams (P); Palmetto Retina Center Ruth Bearden (O), Lloyd Clark (PI), Pamela Darlington (O), Rene Griggs (O), David Johnson (SI), Stephanie Karuza (CC), Peggy Miller (CC), Tiffany Ogbuewu (V), John Payne (SI), Robin Spivey (P), Ashley Studebaker (P), Tiffany Swinford (V), Mallie Taylor (CC), Deborah Watts (O), John Wells (SI), Amanda Wilson (O); Palmetto Retina Center, LLC Florence Krystal Canupp (CC), W. Lloyd Clark (SI), Ashley Floyd (V), Cassandra Garrison (CC), David Johnson (SI), Tyler Huggins (P), Crystal Parker (V), John Payne (PI), Erin Poston (P), Jessica Ross (P), Kristin Stokes (CC), John Wells (SI), Ashley Williams (P); Retina & Vitreous of Texas Diana Abdelgani (CC), Doug Blanchard (P), Colin Blank (P), Emmanuel Chang (SI), Deborah Fredrickson (V), Joseph Khawly (PI), Desiree Lopez (P), Donald Lowd (P), Lorena Martinez (V), Pam Miller (CC), Jason Muniz (P), Erica Pineda (V), Hassan Rahman (SI), Natalie Roncancio (P), Andrea Sales (P), Arthur Willis (SI); Retina Associates Lexie Ainley (CC), Ivan Batlle (PI), Karla Batlle (CC), Kiersten Bruce (V), Ryan Christensen (SI), Blake Cooper (SI), David Dyer (SI), Gregory Fox (SI), Amber Konrade (V), Samantha Perkins (P), Katherine Pippin (P), Ravi Singh (SI), Beatty Suiter (SI), Frank Yeager (P); Retina Associates of Cleveland Inc. Brett Amonett (CC), Cindy Boehm (P), Joseph Coney (SI), Jim Coyne (P), Tia Drugan (V), John DuBois (P), Gregg Greanoff (P), Mary Ilc (V), Aimee Kenska (P), Elizabeth McNamara (P), David Miller (SI), Michael Novak (SI), Llewelyn Rao (SI), Susan Rath (CC), Cecelia Rykena (V), Jerome Schartman (SI), Lawrence Singerman (PI), Veronica Smith (CC), Vivian Tanner (CC); Retina Associates of Kentucky Lisa Bicknell (V), Michelle Buck (P), Vernie Daniels (CC), Diana Holcomb (CC), Ricky Isernhagen (SI), John Kitchens (SI), Andrew Moshfeghi (PI), Bryan Noel (P), Ed Slade (P), Thomas Stone (SI), Jeanne Van Arsdall (V), Brenda VanHoose (V), William Wood (PI); Retina Associates of Western New York Mindy Burgess (V), Brian Connolly (SI), Mary Jo Doran (CC), Ernest Guillet (SI), Edward Hall (PI), Tiffany Heard (P), Anne Reynard (V), Steven Rose (SI), Joseph Territo (P), Margaret Whelehan (P), Meg Yagoda (CC); Retina Centers Dyonne Bachmann (V), Sally Brandon (CC), Kassandra Burdick (V), Jennifer Carreon (CC), Crystal Duncan (P), Susana Flores (V), Stacey Halper (P), Henry Hudson (SI), Amy Kan (CC), Shree Kurup (SI), Claudia Mendoza (P), Brianna Morris (V), George Novalis (SI), Patricia Wilkins (CC), Ryan Wong (SI), Martin Worrall (PI); Retina Consultants of Houston PA Belinda Almanza (V), Matthew Benz (SI), Meredith Berry (CC), David Brown (PI), JoLene Carranza (CC), Eric Chen (SI), Brenda Dives (V), Lauren Epp (CC), Richard Fish (SI), Nikki Franks (CC), Debbie Gillaspia (CC), Amy Hutson Hernandez (CC), Eric Kegley (P), Rosa Kim (SI), Nubia Landaverde (CC), James Major (SI), Elizabeth Quellar (V), Mary Reagan (CC), Beau Richter (P), Tressa Royse (V), Amy Schefler (SI), Robert Smith (O), Veronica Sneed (V), Cary Stoever (P), Sushma Vance (SI), Tien Wong (SI), Charles Wykoff (SI); Retina Consultants of Nevada Kelly Anderson (CC), Thomas Arambula (P), Linda Cortes (CC), Jennie Fabro (V), Joe Galura (P), Nikki Garcia (V), Leide Gomes (V), Marcy Henry (P), Rodney Hollifield (SI), Roy Loo (SI), Janet Marchese (CC), Ifeyinwa Okeke (V), Matthew Pezda (SI), Javier Rendorio (P), Treseelyn Spencer (P), Allen Thach (PI), Irene Voo (SI), Jason Wickens (SI), Meher Yepremyan (SI); Retina Group of Florida Scott Anagnoste (SI), Jaclyn Brady-Pyka (CC), Mario del Cid (PI), Mandeep Dhalla (SI), Lawrence Halperin (SI), Monica Hamlin (CC), Ann Marie Lamb (V), Angelia Mannarelli (P), Jamie Mariano (V), Linda O’Koren (V), Krista Rosenberg (SI), Barry Taney (SI), W. Scott Thompson (SI), Eduardo Uchiyama (SI), Rita Veksler (P); Retina Group of New England Heather Casey (V), Nauman Chaudhry (PI), Justin Cocilo (P), Alison Fontecchio (V), Emma German (CC) Samantha Gunter (V); Retina Northwest Ashley Adamo (CC), Peggy Charpentier (V), Josh Cohen (P), Michele Connaughton (P), Richard Dreyer (SI), Inessa Flato (V), Stephanie Ho (V), Stephen Hobbs (CC), Christine Hoerner (P), Marcia Kopfer (CC), Michael Lee (SI), Joseph Logan (P), Colin Ma (SI), Amanda Milliron (V), Apurva Patel (SI), Mark Peters (PI), Paul Tlucek (SI); Retina Research Institute of Texas Leah Adams (V), Cecilia Escamilla (O), Geneva Espinoza (P), Kristen Garcia (CC), Grant Janzen (SI), Seong Lee (SI), Priscilla Moreno (V), Sunil Patel (PI), Cindy Petty (CC), Gary Rickert (P), Deisy Villarreal (CC), Eric Zavaleta (SI); Retina Vitreous Consultants Robert Bergren (PI), Jennifer Chamberlin (CC), Paul Conrad (SI), Dawn DiPerna (P), Bernard Doft (SI), James Eadie (SI), Amanda Fec (P), Moryssa Grossman (P), Keith McBroom (P), Lori Merlotti (CC), Karl Olsen (SI), Pamela Rath (SI), Christina Schultz (V), David Steinberg (P), Lois Stepansky (V), Avni Vyas (SI), Julie Walter (V); Retina Vitreous Surgeons of Central New York PC Jeffrey Barker (P), Brandi Bellows (V), Jamin Brown (SI), Teresa Deforge (P), Stefanie DeSantis (P), Christine Dorr (CC), Viki Gabris (CC), Cindy Grinnell (CC), Christy Hall (CC), G. Robert Hampton (PI), Peter Hay (P), Lynn Kwasniewski (V), Michelle Manley (V), Abigail Miller (P), Nicole Robarge (P), Kevin Rosenberg (SI), Rajeev Seth (SI), Laurie Sienkiewycz (CC), Lisa Spuches (V); Retinal and Ophthalmic Consultants P.C. Kim Clark (P), Brett Foxman (SI), Scott Foxman (SI), Natalie Mahan (CC), Thomas Margolis (PI), Chastity Mendez (V), Felisha Morales (P), Suzie Post (P), Julie Rosenthal (SI), Qahdirah Torres (V); Retinal Consultants Medical Group Margaret Chang (SI), Robert Equi (SI), Danny Lopez (P), Erin Nickerman (CC), Arun Patel (SI), Joel Pearlman (PI), Nanette Podesta (CC), J. Brian Reed (SI), David Telander (SI), Tony Tsai (SI), Brooke Waller (V), Robert Wendel (SI), Kimberlee Wong (V); Retinal Consultants of AZ Sandra Arenas (V), Mark Barakat (SI), Dayna Bartoli (P), Jermain Byers (CC), Deedra Caraveo (P), Pravin Dugel (SI), David Goldenberg (SI), L. Milad Haak (SI), Ronald Hawkins (CC), Valerie Hoback (CC), Sujit Itty (SI), Karim Jamal (SI), Norma Jimenez (P), Peter Khoury (V), Derek Kunimoto (PI), Ashleigh Levison (SI), Georgina Lopez-Wood (CC), Elena Marcos (V), John Martin (P), Sachin Mehta (SI), Neal Palejwala (SI), Edward Quinlan (SI), Nohemi Ramirez (V), Ignacio Torres (P), Diania Joy Wilson (CC), Heather Yoshimura (CC); Retinal Consultants of San Antonio Lydia Adams (CC), Jaynee Baker (CC), Elaine Castillo (V), Moises Chica (SI), Sarah Holy (SI), Lita Kirschbaum (CC), Richard Gary Lane (SI), Calvin Mein (PI), Tori Moore (V), Brenda Nakoski (P), C. Sean Wienecke (P); Sabates Eye Centers Kelli Burge (V), Michael Cassell (PI), Yin Chen (CC), Heather Elliott (P), Gary Gallimore (P), Kristen Kietzman (CC), Nancy Kunjukunju (SI), Jeanne Niblock (V), Abraham Poulose (SI), Felix Sabates (PI), Nelson Sabates (SI), April Snider (V); Sarasota Retina Institute Melvin Chen (PI), Evelyn Inlow (CC), Peggy Jelemensky (CC), Marc Levy (SI), Rosa Miller (V), Tara Raphael (V), Mark Sneath (P); Scheie Eye Institute Jim Berger (P), Alexander Brucker (PI), Dominique Caggiano (CC), Judy Chen (CC), Sheri Drossner (V), Joan DuPont (CC), Armin Farazdaghi (V), Benjamin Kim (SI), Sara Morales (P), Jessica Morgan (SI), Beth Serpentine (P), Brian VanderBeek (SI); Southeast Retina Center Thomas Bailey (V), Amina Farooq (CC), Allison Foster (CC), Ken Ivey (P), Robert Lalane (SI), Dennis Marcus (PI), Siobhan Ortiz (CC), Bryan Rivera (V), Harinderjit Singh (SI), Kim Tuey (CC), Michele Woodward (CC); Southeastern Retina Associates PC Julie Asher (V), Patricia Coppola (V), Joseph Googe (SI), Raul Lince (P), Lisa Lovelady (CC), Tod McMillan (SI), Steve Morris (CC), Sarah Oelrich (P), Kristina Oliver (CC), R. Keith Shuler (PI), Justin Walsh (V), Jerry Whetstone (P); Southern California Desert Retina Isela Aldana (V), Clement Chan (PI), Tonya Gieser (CC), Tiana Gonzales (CC), Kenneth Huff (P), Maziar Lalezary (SI), Steven Lin (SI), Lenise Myers (V), Kim Walther (CC); Tennessee Retina Everton Arrindell (SI), Carl Awh (PI), Michelle Bowers (P), Amanda Briley (V), Brandon Busbee (SI), Talisha Campbell (V), Narey Cooper (CC), John Delaney (CC), Dezirae Elkins (P), Tara Farmer (P), Amanda Ferrell (CC), Casi Fleischman (P), Paul (Dustin) Freeman (P), Courtney Harris (V), Sarah Hines (CC), Kenneth Moffat (SI), Stephanie Morrow (P), Sue Rataski (CC), Franco Recchia (SI), David Reichstein (SI), Kris Rose (V), Eric Schneider (SI), Natalie Schwebel (V), Shari Scott (V), Gina Smith (CC), Peter Sonkin (SI), Cora Sonnier (CC), Kyra Stepney (V), Katlyne Thompson (V), Caleb Walker (P), R. Trent Wallace (SI), Susan Wiser (CC), Julia Wray (P); Texas Retina Associates-Arlington Melissa Alva (CC), Bob Boleman (P), David Callanan (SI), Jodi Creighton (V), Michelle Curry (CC), Chris Dock (P), Sandy Lash (V), Wayne Solley (SI), Patrick Williams (PI); Texas Retina Associates-Dallas Rajiv Anand (SI), Sally Arceneaux (V),Erik Arredondo-Perez (P), Daniel Carpintero (P), Deborah Chong (SI), Lori Coors (SI), Karl Csaky (SI), Karen Duignan (CC), Gary Fish (PI), Dwain Fuller (SI), Nick Hesse (P), Diana Jaramillo (CC), Tiffany Keaton (P), Michael Mackens (P), Amy Madrid (P), Gary Maines (P), Samantha Marin (P), James Nguyen (P), Brenda Sanchez (V), Kimberly Stanfel (P), Tina Stanley (P), Robert Wang (SI); The Ohio State University William Bloom (CC), PJ Fish (P), Alan Letson (PI), Barbara Mihalik (V), Matthew Ohr (SI), Jerilyn Perry (V), Jill Salerno (CC); The Retina Group of Washington Tanya Alexander-Snowden (CC), Sekou Alou (P), Daniel Berinstein (PI), Thomas Blondo (V), Jennifer Camia (V), Maggie Cashion (CC), Ashley Chancey (CC), Clete Clark (O), Justin Davis (P), William Deegan (SI), Vanessa Denny (V), Irene Fanous (CC), Richard Garfinkel (SI), Bryan Gallerson (V), Smitha Gopakumar (CC), Sarah Hanselman (V), Jessica Iglesias (O), Yury Iraheta (V), Thomas Johnson (SI), Molly Kalisch (V), Andrew King (V), Michael Lai (SI), Myradis Marin-Quinones (O), Alexander Melamud (SI), Howard Morris (O), Bryan Murphy (P), Robert Murphy (SI), Stacie Orencia (P), Michael Osman (SI), Jennifer Phosaksee (V), Steve Rauch (P), Gayatri Reilly (SI), Pamela Renteria (V), Michael Rivers (SI), James Russell (V), Reginald Sanders (SI), Nicole Schorer (V), Mona Shah (V), Manali Shah (CC), Maya Talatory (CC), Tiffany Tam (V), Manfred von Fricken (SI), Danielle Walker (V); The Retina Institute Kevin Blinder (PI), Lynda Boyd (V), Dana Gabel (P), Erika Hoehn (CC), Stephanie Guevara (V), Ginny Nobel (CC), Kelly Pepple (V), Brooke Pulliam (V), Diana Reardon (V), Steve Schremp (P), Gaurav Shah (SI), Bradley Smith (SI), Maria Stuart (V), Rhonda Weeks (CC), Jarrod Wehmeier (P), Tim Wright (P); The Retina Research Center Brian Berger (PI), Chelsey Bravenec (V), Saradha Chexal (SI), Boris Corak (V), Ivana Gunderson (V), Kimberly Hosein (CC), Chirag Jhaveri (SI), Ginger Manhart (CC), Brandon Nguyen (V), Ryan Reid (V), Yong Ren (P), Tina Seidu (CC), Chandler Stovall (P); TLC Eyecare & Laser Centers Kristi Ballard (P), Ashley Blaisdell (P), April Clay (CC), Shellie Fuentes (CC), Stephanie Gillispie (CC), Carmelina Gordon (PI), Heather Hill (V), Hailey Litzer (P), Elaine Lok (P), Bonnie Minier (CC), Susan Partridge (V), Surendar Purohit (SI), Tiffany Westgate (P), Jennifer Wireman (CC), Celia Wohlscheid (CC); University of California-Davis Marie Andrada (PH), Ashley Bryant (PH), Dominique Cargill (PH), Karishma Chandra (P), Sashi Deo (P), Katrina Imson (CC), Lawrence Morse (SI), Ala Moshiri (SI), Susanna Park (PI), Ellen Redenbo (P), Marisa Salvador (V), Nadir Sarwary (PH), Igor Slabosnitskiy (P), Peter Trovitch (PH), Jessica Turner (PH), Cindy Wallace (CC); University of California-San Francisco Jacque Duncan (PI), Don Eubank (CC), Scott Fields (PH), Betty Hom (P), Mary Lew (V), Andrew Light (P), Arshia Mian (CC), John Peterson (P), Jay Stewart (SI); University of Florida Kakarla Chalam (PI), Ashley Cowart (V), Sandeep Grover (SI), Shailesh Gupta (SI), Shamim Haji (V), Ghulam Hamdani (CC), Sherri Hart (P), Kumar Sambhav (CC), Jazzmin Smith (P); University of Kentucky Miroslava Aprelkov (V), Peter Blackburn (SI), Claire Fraser (SI), Mike Hanson (P), Eric Higgins (SI), Mark Kleinman (SI), Deborah McDonald (P), P. Andrew Pearson (PI), Susan Phillips (O), Lyudmila Pigulko (V), Michele Reg (CC), Stephen Sitzlar (PH), Lakisha Smith (V); University of Rochester Flaum Eye Institute Rachel Aleese (V), Mina Chung (PI), Arielle Conrad (V), Andrea Czubinski (V), David DiLoreto (PI), Tanya Forbes (CC), Gary Gagarinas (V), Rachel Hollar (P), Lisa Latchney (CC), Katherine Nedrow (CC), Taylor Pannell (P), Brittany Richardson (P); University of Wisconsin Angie Adler (CC), Michael Altaweel (PI), Barbara Blodi (SI), Shirley Craanen (P), Kristine Dietzman (V), Justin Gottlieb (SI), Michael Ip (SI), Denise Krolnik (P), Jennie Perry-Raymond (CC), Sandie Reed (P), Christopher Smith (V), John Peterson (P); University of Nebraska Medical Center Truhlsen Eye Institute Bryan Angle (PI), Lola Berg (P), Maria Blaiotta (V), Christopher Blodi (PI), Kunal Dansingani (PI), Diana Do (SI), Lisa Greer (CC), Fayez Jawed (V), Kristi Miller (CC), Donna Neely (P), Quan Nguyen (PI), Julie Sigmund (CC); University of Washington Medicine Eye Institute Brad Clifton (P), Patricia Ernst (V), Ron Jones (P), James Kinyoun (PI), Soojin Kyle (P), Jim Leslie (P), Juli Pettingill (V), Jeffrey Purcell (PH), Sue Rath (CC), Kasra Rezaei (SI), Gurunadh Vemulakonda (SI), Bao-Chau Vo (O); Valley Retina Institute Rohit Adyanthaya (SI), Lazaro Aguero (P), Samuel Alonso (P), Deyla Anaya (CC), Isaac Cabrera (V), Monica Cantu (V), Tabatha Deluna (V), Roberto Diaz-Rohena (SI), Santos Garza (P), Victor Gonzalez (PI), Hector Jasso (P), Karina Miranda (V), Jessica Muniz (P), Nehal Patel (SI), Jessica Rodriguez (CC), Rachel Rodriguez (V), Kethsaly Salinas (CC), Nancy Salinas (CC), Yesenia Salinas (CC), Juan Santiago (SI), Lissete Villanueva (CC); Vanderbilt Eye Institute Tony Adkins (P), Anita Agarwal (SI), Milam Brantley (PI), Joshua Carlson (SI), Edward Cherney (SI), Anthony Daniels (SI), Gowtham Jonna (SI), Stephen Kim (SI), Janice Law (SI), Rocky Munn (P), Sandy Owings (CC), Shriji Patel (SI), Scott Ruark (CC), Richard Skellie (P), Paul Sternberg (SI), Maryann Taylor-Ward (V); Virginia Eye Institute Lea Anderlini (V), Mark Bartlett (P), James Combs (SI), Eleanore Ebert (SI), Michael Hughes (P), Byron Ladd (PI), Kristin McKeever (V), Lynn Roderick (P), George Sanborn (SI), Karen Sullivan (CC), Melissa Vaughan (CC); Vitreoretinal Associates of Washington A. Samuel Barloon (SI), Charles Birnbach (SI), Andrea Blustein (V), Robert Francis (SI), Renee Gagnon (CC), Pamela Goldman (P), Ray Hunt (V), Frances Kipp (V), Todd Klesert (SI), DeShawn Minnis (P), Robert Nash (SI), Tetyana Nazarchuk (CC), David Saperstein (PI), Craig Wells (SI); Vitreoretinal Surgery Steven Bennett (SI), Tamra Bogolin (V), Carmen Chan-Tram (CC), Holly Cheshier (P), John Davies (SI), Sundeep Dev (PI), Julianne Enloe (CC), Peggy Gilbert (V), Timothy Gromov (V), Jill Johnson (SI), Tori Jones (P), Robert Mittra (SI), Neal Oestreich (CC), D. Wilkin Parke (SI), Polly Quiram (SI), Robert Ramsay (SI), Carol Rogers (V), Joy Royle (V), Trenise Steele (P), Jessica Tonsfeldt (P), David Williams (SI).
Funding
Supported by the National Eye Institute (National Institutes of Health, Department of Health and Human Services) grants U10EY023529, U10EY023533, and U10EY023521. Support also provided in part by Regeneron, Inc. and Allergan, Inc. through donation of investigational drug. This work was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the University of Wisconsin Madison Department of Ophthalmology and Visual Sciences and to the Jules Stein Eye Institute and Doheny Eye Institute, Department of Ophthalmology at the University of California Los Angeles, CA.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Neal Oden and Paul VanVeldhuisen and staff at the SCORE2 Data Coordinating Center. The first draft of the manuscript was written by Rahul Khurana, MD and Neal Oden and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Rahul N. Khurana, M.D.: serves as a consultant for Allergan (Irvine, CA), Apellis (Waltham, MA), Bausch + Lomb (Rochester, NY), Genentech (South San Francisco, CA), Merck & Co (Kenilworth, NJ), and Regeneron (Tarrytown, NJ); and has grant support from Allergan (Irvine, CA), Chengdu Kanghong (Shanghai, China), Clearside Biomedical (Alpharetta, GA), Roche (Basil, Switzerland), and Santen (Tokyo, Japan).
Neal L. Oden, Ph.D.: work supported by grant 1U10EY023529 from the National Eye Institute of the National Institutes of Health.
Paul C. VanVeldhuisen, Ph.D.: work supported by grant 1U10EY023529 from the National Eye Institute of the National Institutes of Health.
Ingrid U. Scott, M.D., M.P.H.: serves as Principal Investigator and Chair of SCORE2, which is funded by the National Eye Institute, and has served on the Data and Safety Monitoring Committee of clinical trials sponsored by Novartis (Basel, Switzerland).
Barbara A. Blodi, M.D.: No financial disclosures.
Michael S. Ip, M.D.: Dr. Ip is a consultant for the following: Novartis (Basel, Switzerland), Genentech (South San Francisco, CA), Allergan (Irvine, CA), Regeneron (Tarrytown, NJ), RegenxBio (Rockville, MD), Apellis (Waltham, MA), Aerie Pharmaceuticals (Durham, NC), Alimera Sciences (Alpharetta, GA), Amgen (Thousand Oaks, CA), Cell Lineage Therapeutics (Carlsbad, CA), Clearside Biomedical (Alpharetta, GA).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khurana, R.N., Oden, N.L., VanVeldhuisen, P.C. et al. Association between early and late response in eyes with central or hemiretinal vein occlusion treated with anti-VEGF agents. Graefes Arch Clin Exp Ophthalmol 259, 1839–1851 (2021). https://doi.org/10.1007/s00417-020-05018-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-05018-7