Abstract
Purpose
The primary objective of the study was to assess the frequency and severity of visual field defects (VFD) in primary congenital glaucoma (PCG). The secondary objective was to ascertain any associated risk factors.
Methods
An ambispective review of patients with PCG on follow-up with a ‘target’ intraocular pressure (IOP) of ≤ 15 mmHg. Age, sex, laterality, duration of follow-up, baseline IOP, baseline cup-disc ratio (CDR), central corneal thickness (CCT), age during filtering surgery, second surgery if any, yearly IOP, glaucoma medications and best corrected visual acuity from 2013 (year 1) to the final review and final CDR were noted down. Children ≥ 5 years of age with best corrected visual acuity ≥ 6/60 were subjected to manual kinetic Goldmann perimetry, and visual field defects (VFD) were identified.
Results
Seventy-one of 90 eyes completed a reliable kinetic perimetry. The mean age of children was 12.34 ± 4.86 years, and the mean follow-up duration was 10.77 ± 4.69 years. Baseline IOP and CDR were 29.07 ± 8.83 mmHg and 0.66 ± 0.22, respectively. 86.67% of eyes underwent a trabeculotomy + trabeculectomy with mitomycin-C. Thirty-one eyes (34.44%) required a second surgery, 25 of which were bleb revisions and 3 trabeculectomies. Mean IOP and CDR during last visit were 10.23 ± 2.76 mmHg and 0.52 ± 0.25, p < 0.001 as compared with baseline. On Goldmann perimetry, 19 eyes, 26.76%, had defects, arcuate scotoma being most frequent. On the Fisher exact test, a baseline/final CDR > 0.8, undergoing just a trabeculectomy with MMC, needing ≥ 2 glaucoma medications on review or a repeat trabeculectomy was associated with greater severity of VFD. On univariate logistic regression, eyes that needed a bleb revision [OR, 95% CI 9.75 (2.66–35.67), p = 0.001], a repeat trabeculectomy with mitomycin-C [OR (CI) 18 (1.31–245.58), p = 0.03] and final CDR of > 0.8 [OR (CI) 23.1 (3.7–144.21), p = 0.001] were associated with VFD. On multivariable regression analysis, female sex [OR (CI) 18 (2.01–161.04), p = 0.01] was identified as the single most important risk factor for development of a VFD.
Conclusion
At a ‘target’ IOP of ≤ 15 mmHg, 26.76% of PCG eyes manifested a VFD over 10 years. Baseline and/or final CDR > 0.8, necessity for ≥2 medications or a repeat glaucoma surgery, and female sex were identified as risk factors for development and greater severity of glaucomatous VFD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary congenital glaucoma (PCG) has a varied incidence, from 1 in 10,000 to 1 in 68,000 live births in Caucasians to about 1 in 3300 live births in Indians [1, 2]. It contributes to 4.2% of all childhood blindness in India [2, 3]. Children with glaucoma report health-related quality of life equivalent to children born with severe congenital heart disease [4]. Their vision-related quality of life is also low, especially for those with bilateral disease, reduced best corrected visual acuity and for those who have undergone ≥ 3 glaucoma surgeries [5]. Visual disability in PCG is due to glaucomatous optic neuropathy and associated corneal astigmatism, myopia, anisometropia, corneal opacities and amblyopia [6,7,8]. For children with PCG, even after a successful glaucoma surgery at infancy, the health-related quality of life remained adverse due to complexity of the disease and need for lifelong monitoring [9]. However, it is still better than that for children with secondary childhood glaucomas [10] and is important to be preserved.
Most studies on congenital glaucoma characterize success in terms of intraocular pressure (IOP), with < 21 mmHg being the most common cut-off. However, such an IOP control in these children does not necessarily translate into functional benefits. Reports on visual field defects and biomarkers for these, in the PCG scenario, are few.
Therefore, this study was undertaken with the primary objective of assessing the frequency and severity of visual field defects (VFD) in primary congenital glaucoma. The secondary objective was to ascertain any associated risk factors.
Materials and methods
This was a single-centre, ambispective, observational study of consecutive PCG patients on regular follow-up at the Glaucoma Service of a tertiary care centre, who could perform kinetic perimetry. A written informed consent was obtained from the guardians of paediatric patients. All procedures were performed in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Inclusion criteria
A diagnosis of PCG was made if the child presented with photophobia, enlarged corneal diameters, corneal oedema/Haab striae, raised baseline IOP, increased axial length and glaucomatous optic nerve head cupping. When these features presented at > 2 years of age, a diagnosis of late-onset PCG was made.
Exclusion criteria
Children with incomplete ocular records, those lost to follow-up in between, those with any other ocular disorders and systemic anomalies likely to cause a visual field defect and guardians not willing for the study were excluded.
The study was done in two parts.
Part 1 – Patients’ ocular records were reviewed, and details such as age, sex, laterality, duration of follow-up, baseline IOP, baseline cup-disc ratio (CDR), central corneal thickness (CCT), age at first filtering surgery, second surgery if any, mean yearly IOP, number of glaucoma medications and best corrected visual acuity from the year 2013 to their last visit were noted down. Children underwent Goldmann applanation tonometry (when cooperative) or a Perkin’s applanation tonometry during examination under anaesthesia, performed using a standardized protocol of inhalational sevoflurane and laryngeal mask airway, without muscle relaxants, during regular scheduled visits. A ‘target’ IOP of ≤ 15 mmHg had been set. Both eyes of children were evaluated.
Part 2 – At the last review (2018–2019), final CDR was again noted on 90D examination as far as possible. IOP control was calculated by averaging at least 3 readings per year during the last 3 years. Children with best corrected visual acuity of ≥ 6/60 performed manual kinetic perimetry (Goldmann perimeter, Haag-Streit model 940-K7, Bern, Switzerland) with a single experienced technician. Children were instructed to maintain central fixation and, while the target was brought from the periphery, were advised to press the buzzer when the stimulus was first visible. A practice session to familiarize the child with the machine was undertaken, before the actual examination began. With refractive correction in place, the eye with better visual acuity was tested first. Kinetic stimuli of sizes I4e, III4e and V4e were used to test 24 meridians every 15° apart, from the non-seeing to seeing areas. Points where the stimulus was first seen in each meridian were marked to form the corresponding isopter. This was repeated at least twice for each meridian. Localized field defects were rechecked with smaller stimuli to better delineate them. Definitive glaucomatous visual field defects, VFD, were identified as per Aulhorn’s staging. A nasal step had to be an isopter defect at least 5° in width, while arcuate scotomas were identified between 10 and 20° away from fixation in Bjerrum’s area. These had to be reproducible.
The data was analysed by Stata 14 and presented in mean ± SD/median (range) and frequency (%). Categorical variables were compared by chi-square/Fisher’s exact tests. Continuous variables were compared among the groups by independent t test (following normal distribution) and the Wilcoxon rank sum test (non-normal distribution). Univariate logistic regression and stepwise multivariable logistic regression analysis were done to assess risk factors for development of a visual field defect. A p value < 0.05 was considered statistically significant.
Variables such as age, sex, laterality, diagnosis, baseline IOP, baseline CDR, CCT, type of management, age at 1st surgery, need for a second surgery, yearly IOP, number of glaucoma medications, total follow-up duration, IOP control and final CDR were evaluated to identify risk factors for development of a VFD.
Results
A total of 66 patients were screened, of which 10 cases were excluded: 6 due to incomplete ocular records, 2 due to an interim lost to follow-up, 1 case of Sturge-Weber syndrome and one whose parents were not willing to participate in the study. Both eyes of 56 patients were evaluated, of which a total of 90 eyes met inclusion criteria. Seventeen eyes (18.89%) had unilateral glaucoma, and 5 eyes were phthisical. The sex ratio (M:F) was 38:18, and with respect to number of eyes were 60:30. Seventy-one of 90 eyes completed reliable kinetic perimetry.
The mean age of children was 12.34 ± 4.86 (4–27) years, and they had completed a mean follow-up of 10.77 ± 4.69 (2–26) years. Fifty-one patients had early onset PCG (91.07%), and 5 had late-onset PCG (8.92%).
Intraocular pressure
The baseline IOP of the cohort was 29.07 ± 8.83 mmHg. The mean CCT was 528.10 ± 64.46 μ. Seventy-eight eyes (86.67%) underwent a trabeculotomy with trabeculectomy and mitomycin-C (MMC), 5 eyes (5.56%) only a trabeculectomy with MMC, and 7 eyes (7.78%) were on medical management. The median age at first surgery was 6 months (range, 0.5–72). Thirty-one eyes had required a second surgery, 25 (27.7%) of which were bleb revisions, 3 (3.33%) repeat trabeculectomies and 3 (3.33%) cataract surgeries. The mean IOP during year 2013 was 10.80 ± 3.78 mmHg. The mean IOP during last review was 10.23 ± 2.76 mmHg, significantly lower than the baseline value, p < 0.001. Over the last 3 years, 67 eyes, 74.44%, had an IOP control of ≤ 12 mmHg, 16 eyes, 17.78%, of 13–15 mmHg, 5 eyes, 5.56%, of 16–18 mmHg and 2 eyes, 2.22%, of ≥ 19 mmHg. On the last visit, 80.89% of the eyes had an IOP ≤ 12 mmHg, and 59.77% were free of glaucoma medications.
Best corrected visual acuity
The median first documented best corrected visual acuity (BCVA) was 0.3 (0–5), which remained stable until the last review, p = 0.62. On the last visit, 70.83% had a BCVA of ≤ 0.48 logMAR (Snellen equivalent 6/18).
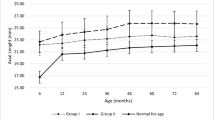
The distribution of IOP and BCVA through the period of review has been depicted in Fig. 1.
Cup-disc ratio over time
The baseline CDR of the cohort was 0.66 ± 0.22. The mean CDR during last review was 0.52 ± 0.25, significantly lower than the baseline, p < 0.001. A baseline CDR of ≤ 0.5 did not show any visual field defects till last review; however, a final CDR of ≤ 0.5 had VFD in 13.16%. A severe cupping of CDR > 0.8 at baseline was likely to present with a VFD in 62.5%, and a final CDR > 0.8 had a VFD in 77.8% (p = 0.002, p < 0.001 respectively) (Table 1).
Goldmann kinetic perimetry
Fifty-two eyes, 73.24%, had a normal visual field, while 19 eyes, 26.76%, had a definitive glaucomatous VFD. One eye (1.41%) showed a nasal step and another (1.41%) a Seidel’s scotoma, 3 eyes (4.23%) showed a single arcuate defect, 6 eyes (8.45%) showed defects in both hemispheres (3 binasal steps, 3 eyes had an arcuate and a nasal step), 6 eyes (8.45%) showed a biarcuate defect, and 2 eyes (2.82%) showed an advanced field defect with just a central island < 10°.
Possible risk factors for developing a VFD
In addition to a baseline or final CDR of > 0.8, undergoing a trabeculectomy with MMC (p = 0.04), needing ≥ 2 glaucoma medications on the first review year (p = 0.01) and eyes that required a repeat surgery, trabeculectomy or bleb revision (p < 0.001) was associated with the development of a VFD. On univariate logistic regression, eyes that needed a bleb revision [odds ratio (OR), 95% confidence interval (CI) 9.75 (2.66–35.67), p = 0.001], a repeat trabeculectomy with MMC [OR (CI) 18 (1.31–245.58), p = 0.03] and had a final CDR of > 0.8 [OR (CI) 23.1 (3.7–144.21), p = 0.001] were associated with development of a VFD. On stepwise multivariable logistic regression analysis, female sex [OR (CI) 18 (2.01–161.04), p = 0.01] was identified as the single risk factor for development of a VFD (Table 2). The median age at surgery for the males and females was 0.5 (6–72) and 0.5 (6–20) months respectively, p = 0.83.
Severities of VFD
The VFD were then categorized into mild (nasal step and Seidel scotoma), moderate (single arcuate scotoma), severe (bi-hemisphere defects and biarcuate scotoma) and very severe defects (central island < 10°), and the role of risk factors was studied. Undergoing an isolated trabeculectomy with MMC (p = 0.005), usage of ≥ 2 medicines on year 1 (2013) (p = 0.01), a need for repeat trabeculectomy (p = 0.001) and a baseline and final CDR > 0.8 (severe cupping) (p = 0.005 and p < 0.001, respectively) were associated with a severe to very severe VFD. A longer follow-up > 5 years (p = 0.01) was associated with nil visual field defects (Table 3). Figure 2 is a graph showing the distribution of VFD severity with respect to baseline and last visit IOP and CDR.
Discussion
Congenital glaucomas are a management challenge, demanding early diagnosis and lifelong control of IOP, to achieve best possible results. Studies on long-term outcomes of PCG are few and are mainly focussed upon IOP [11,12,13,14]. Even eyes with IOPs of < 21 mmHg have been reported to culminate in poor vision, primarily due to visual field loss, refractive errors, corneal opacities, amblyopia and lens pathologies [6, 14,15,16,17,18,19,20]. This can result in compromised functionality of the child and their caregivers [9, 21]. This study had a ‘target’ IOP of ≤ 15 mmHg, as the mean IOP in children is significantly lower than that of the adults [22, 23].
A normal visual field is an important component for activities of daily living, which can get compromised in all glaucomas. Identification of visual field defects in paediatric glaucomas is all the more important, to monitor and preserve function for over a duration of 60–70 years further. Reports on perimetry in PCG are few at present, and even fewer are those which studied the causal risk factors [6, 24,25,26,27,28,29]. This study assessed the clinical features of PCG, at baseline and over time, that could be contributory to long-term perimetric changes and their severity, as apparently stable congenital glaucomas may worsen anytime [11].
In our study, 27% of eyes had a localized VFD, over a mean follow-up period of 10 years. The most common scotomas were arcuate, complementing the findings of Robin et al., who showed that childhood glaucomas had an initial predilection for arcuate defects, which expanded further [27]. Morin and Bryars found that 39.21% of eyes with congenital glaucoma showed a VFD on tangent screen test and Goldmann perimetry [6]. Souza et al. identified localized VFD in 37.5% of eyes with congenital glaucoma on Goldmann perimetry and found VFD in bilateral congenital glaucomas only, with a nasal step being most common, followed by arcuate scotomas [19]. Filho et al. performed Humphrey perimetry in 66 PCG eyes and found localized VFDs in 22% of early and 44% of moderate/advanced glaucomas [25]. Sinha et al. performed Humphrey and Goldmann perimetry on PCG children and identified a definitive glaucomatous VFD in 41% of the eyes, with the most common being an arcuate scotoma [24]. Teijeiro and Dominguez treated children to an IOP of < 16 mmHg and found VFD in 15.2% [30]. The low occurrence of VFD in their study and ours could probably be attributed to the lower ‘target’ IOPs.
On analysing putative risk factors for visual field loss, this study identified three overarching aspects – optic nerve head damage (both baseline and final), inappropriate IOP and changes in IOP, and female gender.
A severe baseline glaucomatous neuropathy, CDR > 0.8, led to VFD in 62.5% of eyes, while a final CDR of > 0.8 was associated with VFD in 77.78% of eyes, and a CDR of < 0.5 at baseline resulted in no field defects. Therefore, a baseline grading of the CDR as ≤ 0.5, 0.6 to 0.8 and > 0.8 could be graded as mild, moderate and severe cupping in PCG eyes.
Ely et al. reported that the preoperative CDR predicted post-operative RNFL thickness better than the post-operative ‘reversed and smaller’ CDR [31]. In this study, eyes with a baseline CDR of ≤ 0.5 showed no VFD at final review, with a ‘target’ IOP of ≤ 15 mmHg. Meirelles et al. reported that reversal of cupping was less likely in children operated at > 1 year of age [13]. Given the possibility of CDR reversal when PCGs are treated promptly and adequately [13, 32], a baseline or final cup-disc ratio of over 0.8 should be considered as a biomarker for greater perimetric loss.
Patients undergoing an isolated trabeculectomy with MMC (without trabeculotomy), requiring ≥ 2 glaucoma medications in the first year of review and eyes that required a repeat trabeculectomy or bleb revision, had a higher risk of developing a VFD. Though trabeculectomy has been shown to be a good technique for congenital glaucoma [33], a combined trabeculotomy + trabeculectomy with MMC could be a better alternative and reported to be both useful and safe for uncomplicated PCGs [34]. A repeat trabeculectomy implies high IOPs requiring another surgery. After bleb revisions, their mean IOP was 10.55 ± 2.61 mmHg, which in itself was within the ‘target’; however, there was a rise from IOPs of 4–6 mmHg, and this change or fluctuations over time could have added to the glaucomatous optic neuropathy. Sampaolesi et al. also reported that congenital glaucomas which required re-surgeries presented with more severe damage to the optic disc and visual fields [35].
In this study, at the last follow-up visit, 81% of eyes had an IOP ≤ 12 mmHg, and 60% of the eyes were free of glaucoma medications. Patients who required additional glaucoma medications in the early years were significantly more likely to have a VFD, probably due to the rise of IOP being treated and possible fluctuations in IOP on medical therapy which is not easy to administer in children. On keeping the ‘target’ IOP as ≤ 15 mmHg, the IOP over time did not appear as a risk factor for the presence or severity of visual field defects.
Our study also found that a longer follow-up of > 5 years was protective, probably reflecting better compliance and long-term IOP monitoring and control. Moschos et al. did a retrospective study on the prognostic factors for paediatric glaucoma and concluded that late developmental glaucomas manifested poorer visual and IOP outcomes as compared with PCG [29]. On the other hand, Sinha et al. reported that PCG detected at ≤ 1 month of age and a baseline IOP of > 30 mmHg manifested a greater visual field loss [24]. Female sex was found to be associated with 18 times greater risk of developing a VFD and the only significant factor on multivariate analysis in this study. The baseline characteristics and median age at surgery were statistically similar between both sexes, and the cause for this difference remains unexplained at present. No other study has reported sex to be a risk factor for VFD in PCG.
The main limitation of this report is its ambispective design; however, only those patients with complete review data were analysed. Also, being from a tertiary referral centre, the cohort studied represents some of the most severe cases in the disease spectrum. IOP changes during examinations under anaesthesia may distort data; however, the protocol used in this study, sevoflurane induction and use of laryngeal mask airway without administration of muscle relaxants, is least likely to cause this effect [36]. Agarwal et al. had compared manual Goldmann perimetry and automated Humphrey perimetry in adult patients and concluded that, at a CDR of ≤ 0.8, Goldmann perimetry identified less VFD than the Humphrey analyser [37]. This could have affected the results of our study, overlooking defects in eyes with early cupping. However, the algorithms devised for Humphrey perimetry are primarily based on an adult normative database and hence could be inappropriate for children. Assessing perimetry in children is challenging due to their playfulness and short attention span. Patel et al. studied three different types of perimetry, the Goldmann, Octopus and Humphrey in children of 5–15 years of age, and concluded that Goldmann was the most reliable among the three, especially in children < 9 years of age [38]. Children could also be encouraged and guided, and their gaze positions continuously monitored by the observer during testing, to avoid their ‘trigger happy’ nature.
In conclusion, at a ‘target’ IOP of ≤ 15 mmHg, 26.76% of PCG eyes manifested a VFD over 10 years. Baseline and/or final CDR > 0.8, necessity for ≥ 2 medications or repeat surgery and female sex were identified as risk factors for development and greater severity of glaucomatous VFD. Also, a baseline grading of the CDR as ≤ 0.5, 0.6 to 0.8 and > 0.8 could be graded as mild, moderate and severe cupping with regard to later visual field loss in PCG eyes.
References
Moore DB, Tomkins O, Ben-Zion I (2013) A review of primary congenital glaucoma in the developing world. Surv Ophthalmol 58:278–285
Senthil S, Badakere S, Ganesh J, Krishnamurthy R, Dikshit S, Choudhari N, Garudadri C, Mandal AK (2019) Profile of childhood glaucoma at a tertiary center in South India. Indian J Ophthalmol 67:358–365
Sihota R, Midha N, Selvan H, Sidhu T, Swamy DR, Sharma A, Gupta A, Gupta V, Dada T, Chaudhary S (2017) Prognosis of different glaucomas seen at a tertiary center: a 10-year overview. Indian J Ophthalmol 65:128–132
Dahlmann-Noor A, Tailor V, Bunce C, Abou-Rayyah Y, Adams G, Brookes J, Khaw PT, Papadopoulos M (2017) Quality of life and functional vision in children with glaucoma. Ophthalmology 124:1048–1055
AlDarrab A, Al Qurashi M, Al Thiabi S, Khandekar R, Edward DP (2019) Functional visual ability and quality of life in children with glaucoma. Am J Ophthalmol 200:95–99
Morin JD, Bryars JH (1980) Causes of loss of vision in congenital glaucoma. Arch Ophthalmol 98:1575–1576
Patil B, Tandon R, Sharma N, Verma M, Upadhyay AD, Gupta V, Sihota R (2015) Corneal changes in childhood glaucoma. Ophthalmology 122:87–92
Badawi AH, Al-Muhaylib AA, Al Owaifeer AM, Al-Essa RS, Al-Shahwan SA (2019) Primary congenital glaucoma: an updated review. Saudi J Ophthalmol 33:382–388
Gothwal VK, Seelam B, Mandal AK (2019) Quality of life following surgery for congenital glaucoma: findings of the LVPEI congenital glaucoma registry. Eye (Lond) 33:659–667
Gothwal VK, Sharma S, Mandal AK (2020) Beyond intraocular pressure: visual functioning and quality of life in primary congenital glaucoma and secondary childhood glaucoma. Am J Ophthalmol 209:62–70
de Silva DJ, Khaw PT, Brookes JL (2011) Long-term outcome of primary congenital glaucoma. J AAPOS 15:148–152
Zagora SL, Funnell CL, Martin FJ, Smith JEH, Hing S, Billson FA, Veillard A-S, Jamieson RV, Grigg JR (2015) Primary congenital glaucoma outcomes: lessons from 23 years of follow-up. Am J Ophthalmol 159:788–796
Meirelles SHS, Mathias CR, Bloise RR, Stohler NSF, Liporaci SD, Frota AC, Simões CC (2008) Evaluation of the factors associated with the reversal of the disc cupping after surgical treatment of childhood glaucoma. J Glaucoma 17:470–473
Mandal AK, Chakrabarti D (2011) Update on congenital glaucoma. Indian J Ophthalmol 59(Suppl):S148–S157
Yu Chan JY, Choy BN, Ng AL, Shum JW (2015) Review on the management of primary congenital glaucoma. J Curr Glaucoma Pract 9:92–99
Richardson KT, Ferguson WJ, Shaffer RN (1967) Long-term functional results in infantile glaucoma. Trans Am Acad Ophthalmol Otolaryngol 71:833–837
Ben-Zion I, Tomkins O, Moore DB, Helveston EM (2011) Surgical results in the management of advanced primary congenital glaucoma in a rural pediatric population. Ophthalmology 118:231–235.e1
Soltani L, Ahammou H, Baroudi S, Essafi H, Hajji I, Moutaouakil A (2019) Congenital glaucoma: intraocular pressure and visual prognosis after trabeculectomy and functional rehabilitation for amblyopia. J Fr Ophtalmol 42:57–62
Yassin SA (2017) Long-term visual outcomes in children with primary congenital glaucoma. Eur J Ophthalmol 27:705–710
Chaudhary RS, Gupta A, Sharma A et al (2019) Long-term functional outcomes of different subtypes of primary congenital glaucoma. Br J Ophthalmol. https://doi.org/10.1136/bjophthalmol-2019-315131
Kantipuly A, Pillai MR, Shroff S, Khatiwala R, Raman GV, Krishnadas SR, Lee Robin A, Ehrlich JR (2019) Caregiver burden in primary congenital glaucoma. Am J Ophthalmol 205:106–114
Sihota R, Tuli D, Dada T, Gupta V, Sachdeva MM (2006) Distribution and determinants of intraocular pressure in a normal pediatric population. J Pediatr Ophthalmol Strabismus 43:14–18 quiz 36–37
Jaafar MS, Kazi GA (1993) Normal intraocular pressure in children: a comparative study of the Perkins applanation tonometer and the pneumatonometer. J Pediatr Ophthalmol Strabismus 30:284–287
Sinha G, Patil B, Sihota R, Gupta V, Nayak B, Sharma R, Sharma A, Gupta N (2015) Visual field loss in primary congenital glaucoma. J AAPOS 19:124–129
Lopes Filho JGG, Betinjane AJ, de Carvalho CA (2007) Automated perimetry in patients with primary congenital glaucoma. Arq Bras Oftalmol 70:37–40
de Souza EC, Berezovsky A, Morales PH, de Arruda Mello PA, de Oliveira Bonomo PP, Salomão SR (2000) Visual field defects in children with congenital glaucoma. J Pediatr Ophthalmol Strabismus 37:266–272
Robin AL, Quigley HA, Pollack IP, Maumenee AE, Maumenee IH (1979) An analysis of visual acuity, visual fields, and disk cupping in childhood glaucoma. Am J Ophthalmol 88:847–858
Marraffa M, Pucci V, Marchini G, Morselli S, Bellucci R, Bonomi L (1995) HPR perimetry and Humphrey perimetry in glaucomatous children. Doc Ophthalmol 89:383–386
Moschos MM, Nitoda E, Fenzel I, Song X, Langenbucher A, Kaesmann B, Seitz B, Gatzioufas Z (2019) Prognostic factors of pediatric glaucoma: a retrospective study. Int Ophthalmol 39:359–373
Teijeiro A, Dominguez A (1990) Perimetría computarizada en el glaucoma congénito. Arch Soc Esp Oftalmol 58:631–634
Ely AL, El-Dairi MA, Freedman SF (2014) Cupping reversal in pediatric glaucoma--evaluation of the retinal nerve fiber layer and visual field. Am J Ophthalmol 158:905–915
Strouthidis NG, Papadopoulos M (2013) Clinical evaluation of glaucoma in children. Curr Ophthalmol Rep 1:106–112
Turaçh ME, Aktan G, Idil A (1995) Medical and surgical aspects of congenital glaucoma. Acta Ophthalmol Scand 73:261–263
Mullaney PB, Selleck C, Al-Awad A, Al-Mesfer S, Zwaan J (1999) Combined trabeculotomy and trabeculectomy as an initial procedure in uncomplicated congenital glaucoma. Arch Ophthalmol 117:457–460
Sampaolesi R, Casiraghi J, Sampaolesi J (1994) A comparative study between differential light sensitivity perimetry and high pass resolution perimetry in congenital glaucoma. In: Perimetry update 1994–95 Proc. XIth Int. Perimetric Soc. Meet. Kugler Publications, New York, pp 189–195
Duman A, Ogün CO, Okesli S (2001) The effect on intraocular pressure of tracheal intubation or laryngeal mask insertion during sevoflurane anaesthesia in children without the use of muscle relaxants. Paediatr Anaesth 11:421–424
Agarwal HC, Gulati V, Sihota R (2000) Visual field assessment in glaucoma: comparative evaluation of manual kinetic Goldmann perimetry and automated static perimetry. Indian J Ophthalmol 48:301–306
Patel DE, Cumberland PM, Walters BC, Russell-Eggitt I, Rahi JS, OPTIC study group (2015) Study of optimal perimetric testing in children (OPTIC): feasibility, reliability and repeatability of perimetry in children. PLoS One 10:e0130895
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional Ethics Committee, All India Institute of Medical Sciences, New Delhi, India, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all enrolled individual participants or their legal guardians (of paediatric patients) for participation and publication of the data.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sihota, R., Selvan, H., Sharma, A. et al. Severity of visual field defects in primary congenital glaucoma and their risk factors. Graefes Arch Clin Exp Ophthalmol 258, 1483–1491 (2020). https://doi.org/10.1007/s00417-020-04677-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-020-04677-w