Abstract
Purpose
To investigate periostin (PN) and tenascin-C (TNC) expression in the aqueous humor and trabeculectomy specimens of patients with neovascular glaucoma (NVG) secondary to proliferative diabetic retinopathy (PDR).
Methods
This study enrolled 37 eyes of 37 patients who were grouped into (1) NVG secondary to PDR (NVG; n = 8); (2) PDR without NVG (PDR; n = 9); (3) primary open-angle glaucoma (POAG; n = 11); and (4) cataract surgery patients as a control group (CG; n = 9). Aqueous humor samples were collected from the anterior chamber at the start of surgery or intravitreal injection of anti-VEGF drug. The concentrations of PN, TNC, VEGF, and TGF-β2 (transforming growth factor-beta 2) were measured by ELISA. Sclerostomy tissues containing trabecular meshwork were obtained from two NVG patients and a POAG patient who underwent trabeculectomy surgery. Immunohistochemical analyses were performed to determine the localization of PN and TNC expression in the sclerostomy tissues.
Results
PN and TNC-C levels were below detection threshold in the POAG and CG groups. The NVG group had significantly higher levels of PN and TNC compared with the PDR group (84.7 ng/ml vs 2.2 ng/ml and 18.5 ng/ml vs 4.6 ng/ml, respectively; p < 0.05). There was a significant correlation between the levels of PN and TNC-C in the NVG group (r = 0.86, p < 0.05). We found significant expression of PN in the trabecular meshwork and Schlemm’s canal of sclerostomy tissues excised from patients with NVG.
Conclusions
Increased PN and TNC expression suggests their possible involvement in the pathogenesis of NVG secondary to PDR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neovascular glaucoma (NVG) secondary to proliferative diabetic retinopathy (PDR) is a potentially devastating condition, often refractory to medical treatment [1]. Retinal ischemia due to PDR can lead to neovascularization of the iris and anterior chamber angle and subsequent formation of fibrovascular membranes (FVMs). The condition may eventually obstruct the trabecular meshwork and cause angle closure, resulting in an intractable elevation of the intraocular pressure (IOP) [2].
The FVMs consist of stromal tissues and cellular components, such as proliferated myofibroblasts and vascular endothelial cells that can contribute to the development of fibrovascular proliferation [3, 4]. As the aqueous humor provides nutrients to tissues in the anterior segments and drains through the outflow pathway, which includes the trabecular meshwork (TM) and Schlemm’s canal (SC), several molecules in the aqueous humor are considered to play roles in the pathophysiology of NVG. Previous studies have indicated possible involvement of growth factors and cytokines, such as vascular endothelium growth factor (VEGF), transforming growth factor-beta 1 (TGF-β1) and transforming growth factor-beta 2 (TGF-β2), hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), and pigment epithelium-derived factor (PEDF) [5,6,7,8,9], in the fibrovascular proliferation associated with NVG, by showing that their levels were elevated in the aqueous humor. However, the molecular mechanisms regulating FVM formation have not been fully determined.
We previously performed genome-wide gene expression profiling of human pre-retinal FVMs excised from patients with PDR who underwent vitrectomy surgery. This study found significant upregulation of matricellular proteins, such as periostin (PN) and tenascin-C (TNC) [10]. Matricellular proteins are characterized by their potential to bind to both extracellular matrix and cell surface receptors, which can enhance tissue remodeling in conditions such as wound healing. We subsequently demonstrated that PN and TNC were significantly upregulated in the vitreous humor of patients with PDR and proliferative vitreoretinopathy, and facilitated both retinal angiogenesis and fibrosis in the vitreoretinal diseases [11,12,13,14]. The involvement of PN and TNC in NVG pathology has not been studied.
Here, we investigate PN and TNC expression in human specimens obtained from patients with NVG secondary to PDR to explore their possible involvement in NVG pathogenesis.
Methods
Patients and sample collection
This cross-sectional study was approved by the Ethics Committee of the Kyushu University Hospital, and the surgical specimens were handled in accordance with the ethical standards of the 1989 Declaration of Helsinki. Participants with NVG, PDR, primary open-angle glaucoma (POAG), or cataract who visited Kyushu University Hospital between May 2017 and November 2018 were enrolled. We aimed to obtain ten subjects per study group. Inclusion criteria were tractional retinal detachment and vitreous hemorrhage with active retinal neovascularization associated with PDR. NVG was diagnosed by neovascularization of the iris and/or iridocorneal angle, with a history of IOP of more than 21 mmHg. Patients who had undergone intraocular surgery within 6 months prior to sample collection were excluded. Aqueous humor samples were collected from the anterior chamber at the start of surgery for cataract and glaucoma, or at intravitreal injection of anti-VEGF drug for PDR according to our previous report. Briefly, at the start of surgery, each patient was placed in a supine position on the bed. A 50–100 μl volume of aqueous humor was obtained through limbal paracentesis using a syringe attached with a 30-gauge needle (Nipro, Osaka, Japan). For control, aqueous humor samples were collected from patients with primary open-angle glaucoma (POAG) or cataract without diabetes mellitus. During trabeculectomy surgery, we first fashioned a superficial scleral flap. After which, we excised a deeper scleral block to create a sclerostomy channel. The excised block (sclerostomy tissues) included the sclera, trabecular meshwork, and cornea. We obtained such sclerostomy tissues from a POAG patient and two NVG patients who underwent previous retinal photocoagulation but not anti-VEGF therapy. The dissected specimens were immediately fixed in 4% paraformaldehyde at 4 °C for 24 h and subsequently underwent histopathological analysis.
Enzyme-linked immunosorbent assay (ELISA)
PN and TNC concentrations were measured with a sandwich ELISA kit as described previously [14, 15]. VEGF and TGF-β2 concentrations were measured with human VEGF and TGF-β2 immunoassay kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. Concentrations were determined by measuring absorbance at 450 nm wavelength using a microplate reader (PerkinElmer, Waltham, MA, USA).
Immunohistochemistry and immunofluorescence staining
Fixed sclerostomy tissues were embedded in paraffin and cut at a 3-μm thickness. After removing the paraffin, the sections were rehydrated, blocked, and incubated with primary antibodies (Abs) for 24 h. For immunohistochemistry, the bound Ab was visualized using an aminoethylcarbazole kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. Nuclei were counterstained using a hematoxylin and eosin (H&E) staining procedure. For immunofluorescence analyses, the sections were incubated with secondary Abs for 1 h at room temperature. Nuclei were counterstained with Hoechst 33342 (Molecular Probes, Eugene, OR, USA). The specimens were flat-mounted in Crystal/Mount (Biomeda Corp., Foster City, CA, USA) and examined by fluorescence microscopy (BZ-9000; Keyence, Osaka, Japan). The primary Abs were PN (MAB3548 1 g/ml; R&D Systems), TNC (NB110-68136 1:50 dilution; Novus Biologicals, Centennial, CO, USA), and FITC-labeled alpha-smooth muscle actin (α-SMA) (F3777 1:250 dilution; Sigma-Aldrich). The secondary Ab was Alexa Fluor 647 (A21247 1:800 dilution; Molecular Probes).
Statistical analysis
Statistical analyses were performed using JMP V13 software package (SAS Institute, Cary, NC, USA). Tukey–Kramer honestly significant difference tests were performed to compare the growth factor levels between the subgroups for multiple variables. Differences were considered significant at values of p < 0.05.
Pearson product-moment correlation coefficients were calculated to determine the correlations between the aqueous humor levels.
Results
Patient demographics
Aqueous humor samples were collected from 37 eyes of 37 patients who were grouped into (1) NVG secondary to PDR (NVG; n = 8); (2) PDR without NVG (PDR; n = 9); (3) primary open-angle glaucoma (POAG; n = 11); and (4) cataract surgery patients as a control group (CG; n = 9). None of the PDR patients underwent advanced anti-vascular endothelial growth factor (VEGF) therapy. Characteristics of the patients and the eyes are summarized in Table 1.
Concentrations of PN, TNC, VEGF, and TGF-β2 in the aqueous humor
We quantified the concentrations of PN and TNC in aqueous humor samples from patients with NVG, PDR, POAG, or CG. VEGF is known to promote neovascularization of the iris and trabecular meshwork resulting in high IOP [7, 8]. TGF-β2 contributes to intraocular fibrosis of PDR [16] and is elevated in the aqueous humor of NVG [6, 17]. Therefore, we measured the concentration of VEGF and total (activated and latent) TGF-β2 in the same aqueous humor samples that were used for PN and TNC measurements.
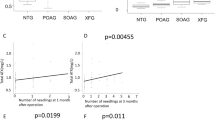
PN and TNC levels were below detection threshold in the POAG and CG groups. The NVG group had significantly higher levels of PN and TNC compared with the PDR group (84.7 ng/ml vs 2.2 ng/ml (p < 0.05) and 18.5 ng/ml vs 4.6 ng/ml (p < 0.05), respectively). The average levels of VEGF and TGF-β2 were higher in NVG compared with the PDR group with no significant difference (4064 ng/ml vs 209 pg/ml (p = 0.1) and 2548 ng/ml vs 1643 pg/ml (p = 0.1), respectively) (Fig. 1).
Levels of periostin, tenascin-C, VEGF, and total TGF-β2 in the aqueous humor. The concentrations were measured by ELISA. NVG secondary to PDR (NVG), n = 8; PDR without NVG (PDR), n = 9; primary open-angle glaucoma (POAG), n = 11; and cataract surgery patients as a control group (CG), n = 9. Bars show mean levels. *p < 0.05, Tukey–Kramer method
There was a significant correlation between PN and TNC levels (r = 0.86, p < 0.05) and VEGF and TGF-β2 levels (r = 0.7314, p < 0.05) in the NVG group. No significant correlations were seen between the levels of PN and VEGF and TGF-β2 and between TNC and VEGF and TGF-β2. In the NVG group, there were significant relationships between peripheral anterior synechiae (PAS) indices and PN and TNC levels (r = 0.84 and 0.82, p < 0.05 and p < 0.05, respectively), but not between IOP and PN and TNC levels.
PN and TNC expression in the trabecular meshwork and Schlemm’s canal of sclerostomy tissues from NVG patients
Immunohistochemical analysis of sclerostomy tissues excised from patients with NVG showed that prominent PN expression could be extensively observed in the Schlemm’s canal (SC) and trabecular meshwork (TM) (Fig. 2a (cases 1 and 2)), whereas relatively weak TNC expression could be seen in the SC and partially in the TM (Fig. 3a (cases 1 and 2)). No apparent signal of PN and TNC was seen in the SC and the TM of the POAG eye (Figs. 2a and 3a (case 3)). We found no significant signal in sections stained with control IgG antibody (data not shown).
Localization of periostin in the trabecular meshwork and Schlemm’s canal in the eyes of patients with NVG secondary to PDR and POAG. Histological section of the trabecular meshwork (TM) and Schlemm’s canal (SC) in the sclerostomy tissues excised from patients with NVG (cases 1 and 2) or POAG (case 3). (a) Periostin immunohistochemical staining. Nuclei are stained blue. Scale bars: 50 μm. (b) Triple-immunofluorescence staining for α-SMA (green) and periostin (red). Nuclei are counterstained with DAPI (blue). Scale bars: 50 μm.
Localization of tenascin-C in the trabecular meshwork and Schlemm’s canal in the eyes of patients with NVG secondary to PDR and POAG. Histological section of the trabecular meshwork (TM) and Schlemm’s canal (SC) in the sclerostomy tissues excised from patients with NVG (case 1 and 2) or POAG (case 3). (A) Tenascin-C immunohistochemical staining. Nuclei are stained blue. Scale bars, 50 μm. (b) Triple-immunofluorescence staining for α-SMA (green) and tenascin-C (red). Nuclei are counterstained with DAPI (blue). Scale bars, 50 μm.
Next, to determine the cellular expression of PN and TNC in the SC and the TM, we stained the sections with Abs against PN, TNC, and α-SMA. α-SMA is a marker for myofibroblasts that have been reported as the main sources of PN and TNC in intraocular fibrovascular diseases [12,13,14,15, 18]. Immunofluorescence analyses showed that PN was co-expressed with α-SMA-positive cells in the SC and the TM (Fig. 2b). In contrast, TNC was not co-expressed with α-SMA-positive cells (Fig. 3b). These findings indicate that PN but not TNC can be produced by or bound to myofibroblasts in the SC and the TM of eyes with NVG associated with PDR.
Discussion
This study introduces for the first time the expression and localization of PN and TNC proteins in the aqueous humor and in the TM and SC of sclerostomy tissues from NVG patients.
Since PN and TNC are upregulated in the vitreous of PDR patients [12, 14], PN and TNC expression in the aqueous humor of PDR patients could result from diffusional reflux from the vitreous cavity. In contrast, the significant higher levels of PN and TNC in the aqueous humor in NVG compared with PDR without NVG could be caused by increased local production and/or hyperpermeability of the iris neovessels that may cause influx of PN and TNC into the anterior ocular segments in NVG patients. It has been shown that matricellular proteins including PN, TNC, and fibronectin cooperate with each other to form the extracellular matrix (ECM) meshwork architecture during the process of scaffold remodeling in angiogenesis and fibrosis, such as in bronchial asthma and intrahepatic cholangiocarcinoma [19,20,21]. Thus, the significant correlation between PN and TNC levels in NVG aqueous humors could imply their cooperation in ECM formation in eyes with NVG.
Our observation that PN but not TNC signal was localized in α-SMA-positive cells in the TM suggests possible local production of one or both and possible binding of PN at the site. TM cells normally function to maintain IOP by regulating aqueous outflow resistance, and under stress conditions, TM cells display different behaviors typical of endothelia, macrophages, fibroblasts, and smooth muscle that characterizes the clinical features of glaucoma [22]. The fibroblasts and smooth muscle–like phenotypes have the potential to secrete a number of ECM proteins [23]. We previously reported that PN is released from α-SMA-positive cells (myofibroblasts) and acts in an autocrine fashion to promote fibrovascular proliferation in proliferative vitreoretinal disease [13, 14]. Taken together, our findings suggest that α-SMA-positive cells in TM could produce PN that is released into the aqueous humor to promote formation of FVMs in the anterior chamber angle of NVG eyes.
Although TNC level was increased in aqueous humor, cellular expression of TNC was not evident in the TM and SC of NVG eyes. Our previous report demonstrated that TNC was produced and secreted from vascular smooth muscle cells in FVMs excised from PDR patients [12]. Similarly, in the eyes of NVG patients, the source of TNC may be vascular smooth muscle cells in the FVMs in the anterior chamber; however, further study is required to identify TNC-secreting cells.
VEGF and TGF-β are considered to play major roles in the neovascularization and fibrosis in the pathophysiology of NVG, respectively [6,7,8, 17]. PN and TNC act downstream of TGF-β2, and significant correlation has been seen between PN and TGF-β2 levels in the vitreous in PDR and PVR [13, 24, 25]. However, our results showed no correlation in aqueous humors of NVG patients. For VEGF, we previously found a weak correlation between TNC and VEGF in the vitreous in PDR [12]; however, the present study did not find correlations between PN and TNC and VEGF. These results suggest that TGF-β2 and VEGF might not regulate PN and TNC expression in the aqueous humor of NVG. Since a wide variety of cytokines and growth factors are elevated in NVG eyes [9, 26], simultaneous measurements of multiple factors would be needed to seek upstream regulators of PN and TNC in the aqueous humor of NVG eyes.
A limitation of our current study is its small sample size. Although we found statistically higher PN and TNC levels in the NVG group compared with the other groups, the levels were highly variable. The clinical significance of this variation should be considered with a larger sample size. In addition, while the mean VEGF concentration in the aqueous humor of NVG eyes was high and consistent with previous reports, it was not significantly different compared with the other groups [7, 8]. This could possibly be due to the small sample sizes in our study.
As NVG progresses, neovessels can appear over the angle structures and PAS can ultimately occur, and this is accompanied by FVM formation and subsequently leads to complete angle closure. It has been reported that intravitreal anti-VEGF drug injection in stage 1 and 2 NVG patients with open iridocorneal angle could have beneficial effects on IOP control and regression of the iris and angle neovascularization [27]. However, anti-VEGF treatment is not effective for lowering IOP after occurrence of angle closure by PAS, and surgical intervention is usually inevitable. Thus, in the management of PDR, it is important to control ischemic stimuli primarily by panretinal photocoagulation and/or anti-VEGF treatment for preventing subsequent development of NVG. In addition, development of novel therapeutic agents against fibrovascular proliferation is also required to prevent the consequent angle closure. Our group proposed PN and TNC as important players in retinal fibrosis and showed that their blockade could suppress generation of subretinal and pre-retinal fibrous membrane in animal models [13, 15]. The present study showing expressions of PN and TNC in NVG eyes and the relationships between PAS indices and PN and TNC levels may suggest their role in fibrovascular proliferation in NVG pathogenesis. Taken together, PN and TNC might be candidate therapeutic targets to inhibit FVM development in the treatment of NVG.
References
Havens SJ, Gulati V (2016) Neovascular glaucoma. Dev Ophthalmol 55:196–204
Shazly TA, Latina MA (2009) Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol 24(2):113–121
Kohno R, Hata Y, Mochizuki Y, Arita R, Kawahara S, Kita T, Miyazaki M, Hisatomi T, Ikeda Y, Aiello LP, Ishibashi T (2010) Histopathology of neovascular tissue from eyes with proliferative diabetic retinopathy after intravitreal bevacizumab injection. Am J Ophthalmol 150(2):223–229 e221
John T, Sassani JW, Eagle RC Jr (1983) The myofibroblastic component of rubeosis iridis. Ophthalmology 90(6):721–728
Miao H, Hou X, Hwang DK, Tao Y (2018) Vascular endothelial growth factor, basic fibroblast growth factor, and pigment epithelium-derived factor expression in the neovascular iris in retinal diseases. J Ophthalmol 2018:8025951
Yu XB, Sun XH, Dahan E, Guo WY, Qian SH, Meng FR, Song YL, Simon GJ (2007) Increased levels of transforming growth factor-betal and -beta2 in the aqueous humor of patients with neovascular glaucoma. Ophthalmic Surg Lasers Imaging 38(1):6–14
Tripathi RC, Li J, Tripathi BJ, Chalam KV, Adamis AP (1998) Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology 105(2):232–237
Sone H, Okuda Y, Kawakami Y, Hanatani M, Suzuki H, Kozawa T, Honmura S, Yamashita K (1996) Vascular endothelial growth factor level in aqueous humor of diabetic patients with rubeotic glaucoma is markedly elevated. Diabetes Care 19(11):1306–1307
Ohira S, Inoue T, Shobayashi K, Iwao K, Fukushima M, Tanihara H (2015) Simultaneous increase in multiple proinflammatory cytokines in the aqueous humor in neovascular glaucoma with and without intravitreal bevacizumab injection. Invest Ophthalmol Vis Sci 56(6):3541–3548
Ishikawa K, Yoshida S, Kobayashi Y, Zhou Y, Nakama T, Nakao S, Sassa Y, Oshima Y, Niiro H, Akashi K, Kono T, Ishibashi T (2015) Microarray analysis of gene expression in fibrovascular membranes excised from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 56(2):932–946
Nakama T, Yoshida S, Ishikawa K, Kubo Y, Kobayashi Y, Zhou Y, Nakao S, Hisatomi T, Ikeda Y, Takao K, Yoshikawa K, Matsuda A, Ono J, Ohta S, Izuhara K, Kudo A, Sonoda KH, Ishibashi T (2017) Therapeutic effect of novel single-stranded RNAi agent targeting periostin in eyes with retinal neovascularization. Mol Ther Nucleic Acids 6:279–289
Kobayashi Y, Yoshida S, Zhou Y, Nakama T, Ishikawa K, Arima M, Nakao S, Sassa Y, Takeda A, Hisatomi T, Ikeda Y, Matsuda A, Sonoda KH, Ishibashi T (2016) Tenascin-C promotes angiogenesis in fibrovascular membranes in eyes with proliferative diabetic retinopathy. Mol Vis 22:436–445
Ishikawa K, Yoshida S, Nakao S, Nakama T, Kita T, Asato R, Sassa Y, Arita R, Miyazaki M, Enaida H, Oshima Y, Murakami N, Niiro H, Ono J, Matsuda A, Goto Y, Akashi K, Izuhara K, Kudo A, Kono T, Hafezi-Moghadam A, Ishibashi T (2014) Periostin promotes the generation of fibrous membranes in proliferative vitreoretinopathy. FASEB J 28(1):131–142
Yoshida S, Ishikawa K, Asato R, Arima M, Sassa Y, Yoshida A, Yoshikawa H, Narukawa K, Obika S, Ono J, Ohta S, Izuhara K, Kono T, Ishibashi T (2011) Increased expression of periostin in vitreous and fibrovascular membranes obtained from patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 52(8):5670–5678
Kobayashi Y, Yoshida S, Zhou Y, Nakama T, Ishikawa K, Kubo Y, Arima M, Nakao S, Hisatomi T, Ikeda Y, Matsuda A, Sonoda KH, Ishibashi T (2016) Tenascin-C secreted by transdifferentiated retinal pigment epithelial cells promotes choroidal neovascularization via integrin alphaV. Lab Investig 96(11):1178–1188
Kita T, Hata Y, Kano K, Miura M, Nakao S, Noda Y, Shimokawa H, Ishibashi T (2007) Transforming growth factor-beta2 and connective tissue growth factor in proliferative vitreoretinal diseases: possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes 56(1):231–238
Min SH, Lee TI, Chung YS, Kim HK (2006) Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J Ophthalmol 20(3):162–165
Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Zhou Y, Nakao S, Sassa Y, Oshima Y, Takao K, Shimahara A, Yoshikawa K, Hamasaki T, Ohgi T, Hayashi H, Matsuda A, Kudo A, Nozaki M, Ogura Y, Kuroda M, Ishibashi T (2015) Inhibition of choroidal fibrovascular membrane formation by new class of RNA interference therapeutic agent targeting periostin. Gene Ther 22(2):127–137
Kudo A, Kii I (2018) Periostin function in communication with extracellular matrices. J Cell Commun Signal 12(1):301–308
Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A (2010) Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 285(3):2028–2039
Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K (2006) Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118(1):98–104
Stamer WD, Clark AF (2017) The many faces of the trabecular meshwork cell. Exp Eye Res 158:112–123
Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS (2009) Extracellular matrix turnover and outflow resistance. Exp Eye Res 88(4):676–682
Norris RA, Potts JD, Yost MJ, Junor L, Brooks T, Tan H, Hoffman S, Hart MM, Kern MJ, Damon B, Markwald RR, Goodwin RL (2009) Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev Dyn 238(5):1052–1063
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A (1999) Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res 14(7):1239–1249
Chono I, Miyazaki D, Miyake H, Komatsu N, Ehara F, Nagase D, Kawamoto Y, Shimizu Y, Ideta R, Inoue Y (2018) High interleukin-8 level in aqueous humor is associated with poor prognosis in eyes with open angle glaucoma and neovascular glaucoma. Sci Rep 8(1):14533
SooHoo JR, Seibold LK, Pantcheva MB, Kahook MY (2015) Aflibercept for the treatment of neovascular glaucoma. Clin Exp Ophthalmol 43(9):803–807
Funding
This study was funded by the Japan Society for the Promotion of Science (KAKENHI grants 17H05101 and 18K09450).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Kyushu University Hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishikawa, K., Kohno, Ri., Mori, K. et al. Increased expression of periostin and tenascin-C in eyes with neovascular glaucoma secondary to PDR. Graefes Arch Clin Exp Ophthalmol 258, 621–628 (2020). https://doi.org/10.1007/s00417-019-04574-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04574-x