Abstract
Purpose
To report the clinical and visual outcome and the therapeutical management in a large cohort of endophthalmitis patients.
Methods
In a monocentric, observational study, we retrospectively analyzed the data of all patients with endophthalmitis who were referred to the department of ophthalmology of the Carl Gustav Carus Hospital Dresden between 2006 and 2018.
Results
In total, data of 104 patients (49 female, 55 male) were included in the present analysis. The most frequent clinical scenario for endophthalmitis was postcataract surgery (30.8%). The most frequent treatment at presentation was a pars plana vitrectomy (ppV) in 42 patients, followed by an intravitreal antibiotic in 41 patients. Out of 41 patients who received an intravitreal antibiotic, 35 patients (85%) needed additional treatment. In contrast, out of 42 patients who received a ppV as the initial treatment, 19 patients (42%) needed additional therapy, which was significantly different (p < 0.0001). The best presenting visual acuity improved significantly after treatment (p < 0.0001).
Conclusion
The results of the present study suggest that, compared with an intravitreal antibiotic, a ppV as the initial therapy of endophthalmitis might reduce the number of additional treatments. From our data, it can be hypothesized that a ppV should be performed as early as possible to achieve the best visual outcome in most endophthalmitis patients. Prospective studies are now needed to address this issue in greater detail and to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endophthalmitis represents a severe and potentially sight-threatening complication which is caused either exogenously (e.g., after surgery or injury) by an infectious process or endogenously by septicemia spreading to the inner of the eye [1]. The randomized endophthalmitis vitrectomy study (EVS), conducted more than 20 years ago, investigated the role of different treatment approaches (pars plana vitrectomy [ppV], intravenous antibiotics and tab biopsy) on the outcome of postoperative endophthalmitis patients [2]. The results of the EVS showed that patients who presented with hand motion or better might be treated with tab biopsy and patients presenting with light perception only were recommended for immediate ppV. Other more recent studies discussed a broader use of vitrectomy in postoperative endophthalmitis patients [1, 3, 4]. In contrast, other authors favored a vitreous tab only [5]. These reports indicate a lack of consensus regarding the “correct” treatment modality for endophthalmitis patients at the initial time point of the presentation. Hence, it was the aim of the present study to report and give an update on the clinical experiences of the clinical/visual outcome and the therapeutical management of endophthalmitis in a large cohort of patients.
Methods
The present study was conducted as a retrospective, monocentric study. It was based on a review of medical data of all patients with endophthalmitis who were referred to the Department of Ophthalmology, Carl Gustav Carus University Hospital Dresden, for further treatment from January 2006 to December 2018. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines were applied. The study followed the tenets of the Declaration of Helsinki of the World Medical Association. A review of medical records was performed based on all patients who were allocated to the ICD-10 diagnosis codes of endophthalmitis (H 44.0, H 44.1, H 45.1, H 59.8, A54.3, B 69.1, B 73, B 83). Data analysis included the following major parameters: patient demographics, clinical scenario, initial treatment, the necessity of additional treatments, visual outcome before and after the intervention, and microbiological results. The clinical diagnosis of endophthalmitis was ensured through the loss of vision by inflammation of the anterior chamber and the vitreous opacity in the B-scan ultrasound. Determined by the individual physician, the treatment strategies did not follow a standardized protocol. Additionally, all physicians took a sample of the anterior chamber or vitreous for microbiological investigations prior to surgery. Classification of pathogens was executed by the department of microbiology of our university hospital. Snellen best-corrected visual acuity (BCVA) was converted to logarithm of the minimum angle of resolution (LogMAR) equivalents. Visual acuity of no light perception, light perception, hand motion, or counting fingers was converted to LogMAR values of 3.0, 2.7, 2.3, and 1.9, respectively [6]. IBM Statistical Package for the Social Sciences 25.0 (SPSS Inc., Chicago, Illinois, USA) was used for statistical analysis. Cross tabulations were used to evaluate the prevalence of the performed interventions. The chi-square test was applied to compare the prevalence of the different initial treatments. The paired student’s t test was applied to compare the visual acuity (in LogMAR) at baseline (preVA) with the visual acuity after an intervention (postVA). One-way analysis of variance (ANOVA) with least significant difference (LSD) post-hoc analysis was implemented to evaluate the differences of the results within the numerical groups. A p value of ≤ 0.05 was considered as statistically significant. The numerical data are listed as mean values and standard errors of the mean (SEM). Only the time between the trigger of endophthalmitis and the diagnosis was displayed as the median.
Results
Demographics
In total, data of 104 patients (49 female, 55 male) were included in the present analysis. The mean age of all patients was 69.3 ± 1.7 years. The median time between the exogenous endophthalmitis-causing event and the diagnosis was 4 days. The mean duration of anamnesis, describing the time from the onset of symptoms to the consultation in our hospital, was 3.37 ± 0.735 days. The time between the admission to hospital and the operation day in all patients was 1.35 ± 0.241 days (Table 1). The time between the admission to hospital and the operation day in the nonsurgical group was 6.18 ± 1.548 days. This was significantly longer than in the groups of patients who received either an intravitreal injection of antibiotics (0.51 ± 0.111 days) or a ppV (0.79 ± 0.121 days) as the initial treatment (p < 0.001). There was no statistically significant difference in the time between the admission to hospital and the operation day among the intravitreal injection of antibiotics or a ppV as the initial treatment (p = 0.489).
Clinical scenario
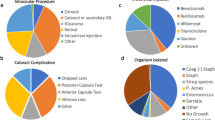
The most frequent clinical scenario for an endophthalmitis was postcataract surgery (30.8%), followed by postintravitreal injections (15.4%), postcombined cataract/retinal surgery (13.5%), postglaucoma surgery (10.6%), and postretinal surgery (4.8%). Miscellaneous surgeries (12.5%) included the following surgical interventions: five cases of perforating injuries, three cases of corneal suture removals, two cases of perforating keratoplasties, two cases of amnion membrane transplantations, and one case of orbital phlegmon surgery. Among all patients, 13 (12.5%) presented with endogenous endophthalmitis (Table 1).
Initial treatment and necessity of additional treatment
The most frequent treatment at presentation was a ppV (in 42 patients), followed by an intravitreal injection of antibiotics (in 41 patients). Out of the 41 patients who received an intravitreal injection of antibiotics, 35 (85%) needed additional treatment. In contrast, among the 42 patients who received a ppV as the initial treatment, 19 (45%) needed an additional therapy, which was significantly different (p < 0.0001) (Table 2).
The nonsurgical treatment approach consisted of a subconjunctival depot with gentamicin and dexamethasone in combination with a local and systemic (intravenous) antibiotic therapy. Other initial treatments, apart from the three strategies described before, comprised: anterior chamber irrigation in seven cases (4× with vancomycin, 2× with cefotaxime, and 1× without antibiotics), a corneal crosslinking in one case, a perforating keratoplasty à chaud in combination with explantation of an Ahmed valve in one case and an orbital decompression another case.
There was no statistically significant difference in the rate of additional treatments in exogenous or endogenous cases of endophthalmitis patients (Table 2). From the 91 patients who had exogenous endophthalmitis, 64 (70%) needed an additional treatment and from the 13 patients who had an endogenous endophthalmitis, ten (77%) needed an additional therapy which was not significantly different (p = 0.449).
For all intravitreal antibiotic injections, 1 mg vancomycin in 0.1 ml sodium chloride solution was used. The second intravitreal antibiotic was either cefotaxime or cefuroxime or ceftazidime. In case of strong clinical suspicion of mycotic endophthalmitis or positive mycotic pathogen verification, amphotericin B or voriconazole were used as an intravitreal injection.
Visual outcome
Regardless of the clinical scenario, the best presenting visual acuity was 2.36 ± 0.03 LogMAR and the best visual outcome after the intervention was 1.29 ± 0.10 LogMAR which was significantly improved (p < 0.0001). For completeness, the best presenting visual acuity and the best visual outcome after each clinical scenario are displayed separately in Table 3.
Microbiological results
The pathogens were classified as Gram-positive bacteria in 45 patients (72.6%), Gram-negative bacteria in seven patients (11.3%), fungal infections in five patients (8.1%), and multiple infections with bacteria and fungi in five patients (8.1%). The most frequent pathogens were Staphylococcus epidermidis (in 15 patients) and Staphylococcus aureus (in 11 patients). Candida albicans was the most common fungal pathogen (in six patients). Altogether, culture-positive pathogens were tested in 62 of 104 cases (59.6%). Some of the patients had multiple attested pathogens.
Discussion
Endophthalmitis is a severe and potentially sight-threatening ocular complication. The aim of the present study was to give an update on the clinical experience in a large cohort of endophthalmitis patients in a university setting. The data of 104 patients were retrospectively analyzed. The mean age of all patients was 69.3 years which is comparable with a mean age of 70.3 years in 67 endophthalmitis cases in a recent study of Karacal and coworkers [7]. The reported median time between the exogenous endophthalmitis causing event and the diagnosis was 4 days and are in line with the findings of Yannuzzi et al. observing a median time of 6 days between cataract surgery and diagnosis of endophthalmitis [8]. The mean duration of anamnesis in the present study was 3.37 days. Mayer et al. reported a median time interval between the onset of symptoms and the initiation of therapy of 4 days in 17 patients with endogenous endophthalmitis [9]. Additionally, the time between the admission to hospital and the operation day was 1.35 days, which is comparable with the findings of Dave et al. who accounted a median interval between vitreoretinal surgery and endophthalmitis of 1.5 days in 20 patients [10]. Considering the time from admission to hospital and the operation day, in our cohort, 11 of 104 patients were treated with a nonsurgical therapy approach first. This substantially prolonged the delay in performing surgery. The time between the admission to hospital and the operation day in the nonsurgical group was 6.18 ± 1.548 days, which was significantly longer than in the groups of patients who received either an intravitreal injection of antibiotics (0.51 ± 0.111 days) or a ppV (0.79 ± 0.121 days) as the initial treatment. Taking together, the demographic data in the present study are broadly comparable with the literature.
Approximately 12.5% of all patients in our study presented with endogenous endophthalmitis which is consistent with the endophthalmitis rates of Yospaiboon et al. (15.6%) [11] and Mayer and Loos (13.2%) [12]. In contrast, other studies described wide ranges of endophthalmitis rates from 3.2 [13] to 25.4% [7]. The reason for the wide range of endophthalmitis rates might be due to the diversity of the countries from which these data were acquired. The present data analysis was performed in a large tertiary referral center and revealed a rate of 87.5% postoperative endophthalmitis cases. This is comparable with data of other large referral centers in industrial countries (e.g., 74.3% postoperative endophthalmitis cases in Munich, Germany [12], 74.6% in St. Louis, USA [7], and 85.7% in Miami, USA [13]). In contrast, there are rates of postoperative endophthalmitis cases of 6.9 [14] to 35.6% [11] in developing or threshold countries. Additionally, the year of data acquisition has to be taken into account. Nobe et al. detected a rate of 56% postoperative endophthalmitis cases in a retrospective analysis from 1972 to 1985 indicating that, due to an increase in ocular surgeries in industrial countries over time, there is also an increase of infectious complications [15].
Thirty-five of 41 patients (85.4%) receiving an intravitreal injection of antibiotics as their initial therapy at presentation needed an additional treatment due to deterioration of their clinical findings. In contrast, only 19 of 42 patients (45.2%) who received a ppV as the initial treatment needed additional therapy, which was significantly better.
The largest prospective study investigating clinical and therapeutical findings in endophthalmitis patients is the EVS from 1995, which included 420 patients. Thirteen percent of the patients received a tap-biopsy underwent additional procedures, compared with 8% of patients receiving a ppV as their initial therapy [2, 16]. Some further small retrospective studies indicated the superiority of a ppV in comparison to an intravitreal injection of antibiotics [13, 17]. Unfortunately, comparability is limited to the heterogeneity of study designs and inclusion/exclusion criteria of the different studies. Kuriyan et al. showed a statistically significant advantage of a ppV as an initial therapy. Thirty-four of 49 patients (69.4%) receiving an intravitreal injection of antibiotics as their initial therapy needed additional treatment, compared with four of 14 patients (28.6%) who received a ppV as the initial treatment [13]. Siqueira and coworkers also hinted at the superiority of a ppV as a first-line therapy. Thirteen of 24 patients (54.2%) receiving an intravitreal injection of antibiotics as their initial therapy regressed. In contrast, none of the 11 patients who received a ppV as the initial treatment deteriorated [17]. Considering the sample sizes of the studies of Kuriyan et al. (63 patients) and Siqueira et al. (35 patients) and the large number of 104 patients in the present study, these data emphasize the superiority of performing a ppV as the initial treatment of endophthalmitis patients. In the present study, the rates of additional treatments were generally higher than rates in previous studies of Kuriyan et al. and Siqueira et al., independent of the initial therapy (intravitreal injection of antibiotics or ppV). The reason for the higher rates of additional treatments remains unknown to us. It is possible that the patterns of infectious agents in these studies were different from those in our study.
In contrast to these findings, Monnet and coworkers displayed an equal benefit of either ppV or intravitreal antibiotics as the initial treatment of endophthalmitis. These authors highlighted a possible superiority of an intravitreal injection of antibiotics compared with a ppV due to a minimal surgical trauma. However, the sample size in their study (15 patients) was relatively small [5]. Other study groups postulated that treatment of an infectious eye should depend on the clinical picture and course of the disease of the individual patient. In cases of clinical deterioration and availability of a vitreoretinal surgeon vitrectomy should be performed [4, 18]. In extrapolation, Kaynak and Bali et al. recommended a more radical intervention, especially the use of silicone oil tamponades, to decrease the number of additional procedures and to increase the chance of surgical success in endophthalmitis eyes [19, 20].
Our nonsurgical treatment approach consisted of a subconjunctival depot with gentamicin and dexamethasone in combination with a local and systemic (intravenous) antibiotic therapy. We propose that the strategy of using systemic antibiotic therapy should be reconsidered since also the EVS group yielded no statistical difference in the clinical or functional outcome between patients who received systemic antibiotics or not [2]. In this context, in our study, all 11 patients who received systemic antibiotics (and topical therapy) only deteriorated and needed additional treatments in the further course of their disease. The reason for not performing surgery in these 11 endophthalmitis patients of various origins was the only mild inflammation in the anterior chamber and the only soft vitreous infiltration in the B-scan ultrasound. On the other hand, these patients had a very reluctant attitude to undergoing surgery to their eye. In animal experiments, Engelbert et al. concluded that there was no statistical difference in treating a Staphylococcus aureus–associated endophthalmitis, either with a combination of intravitreal and intravenous antibiotics or intravitreal injection of antibiotics only [21]. These findings raise questions about the rationale for using systemic antibiotics in the treatment of endophthalmitis patients. As described in the review of Barry et al., intravitreal injections of antibiotics provide the highest drug concentration in the infectious eye. However, Barry et al. recommended the continuous use of systemic antibiotics aware of the fact that antibiotic levels after an intravitreal injection only remain in the eye for a limited time. Hence, an additional systemic antibiotic therapy with the same drugs used for intravitreal therapy should be applied in cases of a severe acute purulent endophthalmitis or a component of vasculitis. Antibiotic therapy might be modified according to the clinical response and the antibiotic sensitivity profile of the cultured microorganisms [1]. Taken together, the use of systemic antibiotics remains controversial. Furthermore, especially more sophisticated experimental studies to investigate the bioavailability of systemic antibiotics in the ocular tissue are certainly needed to elucidate this topic in more detail.
In the present study, the primary intravitreal antibiotic was vancomycin. Either cefotaxime, cefuroxime, or ceftazidime was used additionally. In cases of a mycotic origin of endophthalmitis, intravitreal amphotericin B or voriconazole was applied. According to Barry et al., the first choice of treating endophthalmitis by an intravitreal antibiotic should be the combination of vancomycin and ceftazidime [1]. Other study groups have echoed this recommendation, covering a wide spectrum of Gram-positive and Gram-negative bacteria [11, 13]. Nevertheless, even under the correct antibiotic regime, the ocular inflammation habitually becomes worse before improving [1].
Regardless of the clinical scenario, the best presenting visual acuity in our study was 2.36 ± 0.03 LogMAR, and the best visual outcome after the intervention was 1.29 ± 0.10 LogMAR, which was statistically significantly different. Comparing our visual acuity outcomes with other studies is very difficult. First, many studies have categorized visual acuities only in a subset of their patients. Also, visual acuity was only assessed using the Snellen’s chart [2, 3, 5, 7, 8, 11, 13, 22,23,24,25]. However, the results of Mayer and Loos are comparable with our study. These authors collected data from 141 patients with various endophthalmitis origins. The initial visual acuity was 1.3 LogMAR, and the post interventional visual acuity was 0.6 LogMAR [12]. Mayer et al. reported even better visual outcomes, with a visual acuity of 2.0 LogMAR at presentation and 0.1 LogMAR after the intervention [9]. Dave and colleagues reported a presenting visual acuity of 2.16 LogMAR and a postoperative visual acuity of 1.7 LogMAR, which is comparable to the results of our study. However, due to the limited number of patients (n = 20), changes in visual acuity did not reach statistical significance [10].
Microbiological culture testing revealed positive pathogens in 62 of 104 cases (59.6%), which is within the range of other studies showing positive testing from 52 to 69% [10, 15, 26, 27]. In our study, Gram-positive bacteria were detected in 45 patients (72.6%), Gram-negative bacteria in seven patients (11.3%), fungal infections in five patients (8.1%), and multiple infections with bacteria and fungi in five patients (8.1%). Lin et al. found a similar rate of 71% Gram-positive bacteria in 29 endophthalmitis of different origins [23]. Endophthalmitis after cataract surgery was associated with a rate of more than 90% of Gram-positive bacteria [25, 27].
Limitations of the present study are its monocentric and retrospective study design. A prospective, randomized study design would have been more desirable. Also, the treatment of patients by different surgeons might have affected the postoperative outcome. A further limitation might be the variable duration of follow-up periods, eventually restricting the best-corrected postoperative visual acuity.
Taken together, the results of the present study showed, that performing a ppV as the initial therapy of endophthalmitis could reduce the number of additional treatments. Compared with an intravitreal antibiotic as the initial treatment, the rate of additional treatments was significantly lower in patients who underwent a ppV as the first treatment. From our data, it can be hypothesized that a ppV should be performed as early as possible to achieve the best visual outcome in most endophthalmitis patients. Further prospective and randomized studies are now needed to address this issue in greater detail and to confirm our findings.
References
Barry P, Cordovés L, Gardner S (2013) ESCRS guidelines for prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions. The European Society for Cataract & Refractive Surgeons, Ireland, Dublin, pp 1–52
Endophthalmitis Vitrectomy Study Group (1995) Results of the endophthalmitis vitrectomy study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 113:1479–1496
Kuhn F, Gini G (2005) Ten years after... are findings of the endophthalmitis vitrectomy study still relevant today? Graefes Arch Clin Exp Ophthalmol 243:1197–1199
Kuhn F, Gini G (2006) Vitrectomy for endophthalmitis. Ophthalmology 113:714
Monnet D, Labetoulle M, Lautier-Frau M, Offret H, Frau E (2002) Therapeutic strategy in delayed postoperative endophthalmitis: a report on 15 cases. J Fr Ophtalmol 25:599–603
Bach M, Schulze-Bonsel K, Feltgen N, Burau H, Hansen L (2007) Author response: numerical imputation for low vision states. Invest Ophthalmol Vis Sci eLetter:1–3
Karacal H, Kymes SM, Apte RS (2007) Retrospective analysis of etiopathogenesis of all cases of endophthalmitis at a large tertiary referral center. Int Ophthalmol 27:251–259
Yannuzzi NA, Si N, Relhan N, Kuriyan AE, Albini TA, Berrocal AM, Davis JL, Smiddy WE, Townsend J, Miller D, Flynn HW Jr (2017) Endophthalmitis after clear corneal cataract surgery: outcomes over two decades. Am J Ophthalmol 174:155–159
Mayer C, Loos D, Feucht N, Zapp D, Prahs PM, Tandogan T, Khoramnia R (2018) [Endogenous endophthalmitis: epidemiology, clinic, therapy and visual outcome]. Klin Monbl Augenheilkd
Dave VP, Pathengay A, Basu S, Gupta N, Basu S, Raval V, Das T, Sharma S, Mathai A, Narayanan R, Chhablani J, Sharma P, Tyagi M, Balakrishnan D, Jalali S, Rani PK, Pappuru RR (2016) Endophthalmitis after pars plana vitrectomy: clinical features, risk factors, and management outcomes. Asia Pac J Ophthalmol (Phila) 5:192–195
Yospaiboon Y, Meethongkam K, Sinawat S, Laovirojjanakul W, Ratanapakorn T, Sanguansak T, Bhoomibunchoo C (2018) Predictive factors in the treatment of streptococcal endophthalmitis. Clin Ophthalmol 12:859–864
Mayer CS, Loos DA (2016) Posttraumatic endophthalmitis: complication following severe eye injury. Ophthalmologe 113:478–483
Kuriyan AE, Weiss KD, Flynn HW Jr, Smiddy WE, Berrocal AM, Albini TA, Miller D (2014) Endophthalmitis caused by streptococcal species: clinical settings, microbiology, management, and outcomes. Am J Ophthalmol 157:774–780
Yang XB, Liu YY, Huang ZX, Mao Y, Zhao L, Xu ZP (2018) Clinical analysis of 1593 patients with infectious endophthalmitis: a 12-year study at a tertiary referral center in Western China. Chin Med J 131:1658–1665
Nobe JR, Gomez DS, Liggett P, Smith RE, Robin JB (1987) Post-traumatic and postoperative endophthalmitis: a comparison of visual outcomes. Br J Ophthalmol 71:614–617
Doft BH, Kelsey SF, Wisniewski SR (1998) Additional procedures after the initial vitrectomy or tap-biopsy in the endophthalmitis vitrectomy study. Ophthalmology 105:707–716
Siqueira RC, Gil AD, Canamary F, Minari M, Jorge R (2009) Pars plana vitrectomy and silicone oil tamponade for acute endophthalmitis treatment. Arq Bras Oftalmol 72:28–32
Clarke B, Williamson TH, Gini G, Gupta B (2018) Management of bacterial postoperative endophthalmitis and the role of vitrectomy. Surv Ophthalmol 63:677–693
Kaynak S, Oner FH, Kocak N, Cingil G (2003) Surgical management of postoperative endophthalmitis: comparison of 2 techniques. J Cataract Refract Surg 29:966–969
Bali E, Huyghe P, Caspers L, Libert J (2003) Vitrectomy and silicone oil in the treatment of acute endophthalmitis. Preliminary results. Bull Soc Belge Ophtalmol:9–14
Engelbert M, Mino de KH, Thiel M, Grasbon T, Ta CN, Schulze-Schwering M, Klauss V, Kampik A (2004) Intravitreal vancomycin and amikacin versus intravenous imipenem in the treatment of experimental Staphylococcus aureus endophthalmitis. Graefes Arch Clin Exp Ophthalmol 242:313–320
Zghal I, Souguir A, Fekih O, Chebbi A, Romdhane O, Bouguila H, Nacef L (2017) Postoperative endophthalmities: therapeutic results and early vitrectomy. Tunis Med 95:172–178
Lin M, Zhang W, Liu Y, Wang L, Ding Y, Wu X, Shi Y, Sun L, Li Y (2011) Nosocomial acute-onset postoperative endophthalmitis at a university teaching hospital in China. J Hosp Infect 79:323–327
Aaberg TM Jr, Flynn HW Jr, Schiffman J, Newton J (1998) Nosocomial acute-onset postoperative endophthalmitis survey. A 10-year review of incidence and outcomes. Ophthalmology 105:1004–1010
Pijl BJ, Theelen T, Tilanus MA, Rentenaar R, Crama N (2010) Acute endophthalmitis after cataract surgery: 250 consecutive cases treated at a tertiary referral center in the Netherlands. Am J Ophthalmol 149:482–487
McCannel CA (2011) Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina 31:654–661
Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, Kelsey SF (1996) Spectrum and susceptibilities of microbiologic isolates in the endophthalmitis vitrectomy study. Am J Ophthalmol 122:1–17
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study (retrospectively study), formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Kitsche, M., Herber, R., Pillunat, L.E. et al. Clinical and visual outcome of endophthalmitis patients: a single-center experience. Graefes Arch Clin Exp Ophthalmol 258, 183–189 (2020). https://doi.org/10.1007/s00417-019-04480-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04480-2