Abstract
Purpose
To evaluate the surgical outcomes of the 27-gauge (G) vitrectomy system for the treatment of primary rhegmatogenous retinal detachment (RRD).
Methods
This retrospective consecutive series multicenter study involved a total of 410 eyes of 406 patients who underwent 3-port transconjunctival 27G pars plana vitrectomy (PPV) for RRD between November 2014 and December 2016 and who were followed for a minimum of 3 months postoperative. The main outcome measure was primary reattachment, with the secondary outcome measures being final reattachment, improvement of visual acuity (VA), intraocular pressure (IOP), intraoperative and postoperative complications, and surgery time.
Results
Of the 410 treated eyes, primary reattachment was achieved in 392 (95.6%) and final reattachment was achieved in 410 (100%). In 226 eyes (55.1%) with macula-on RRD, the mean logarithm of the minimum angle of resolution (logMAR) VA improved from 0.16 ± 0.51 pre-surgery to 0.02 ± 0.14 post-surgery (P = 0.11). In 184 eyes (44.9%) with macula-off RRD, logMAR VA improved from 1.06 ± 0.77 pre-surgery to 0.26 ± 0.35 post-surgery (P < 0.001). Following surgery, the mean IOP was highest at 1 day (15.7 ± 7.0 mmHg) postoperative. In all eyes, surgery was concluded without the use of sutures or the need of conversion to a larger-gauge instrument. Although hypotony was observed in 14 (3.4%) of the 410 treated eyes at 1 day postoperative, it spontaneously resolved within 1 week without additional surgical intervention. No postoperative complications such as infectious endophthalmitis were observed throughout the follow-up period.
Conclusion
Our findings show that 27G PPV is both safe and effective for the treatment of primary RRD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For patients undergoing vitrectomy surgery, the use of small incisions is one of the latest innovative surgical techniques and is a method that is currently widespread. Compared with traditional 20-gauge (G) vitrectomy, sutureless posterior segment surgery provides numerous potential advantages, such as faster wound healing, a lesser amount of conjunctival scarring, improved patient comfort, decreased postoperative inflammation with faster visual recovery, and reduced postoperative astigmatic change [1,2,3,4]. Moreover, the elimination of sutures also shortens the opening and closing times during surgery [5].
Sutureless tunnel-based vitrectomy in vitreoretinal surgery was initially proposed by Chen in 1996 [6]. In 2002, Fujii et al. [7, 8] introduced the 25G vitrectomy system for sutureless transconjunctival self-sealing sclerotomy, and in 2005, Eckardt [9] introduced sutureless 23G vitrectomy, which offers a compromise between the 20G and 25G systems. In a 2010 pilot study, Oshima et al. [10] described the initial feasibility and safety of a novel 27G microincision vitrectomy surgery (MIVS) system (i.e., currently the smallest-gauge MIVS system and one that effectively results in self-sealing sclerotomies) and reported excellent visual and anatomic outcomes. Since then, the use of 27G sutureless vitrectomy surgery has been on the increase, yet mostly for the treatment of macula disease [10,11,12,13,14]. However, it should be noted that there have been several arguments against the efficiency, as well as the structural stiffness and integrity, of the 27G instruments.
Due to the recent improvements in surgical methods and the development of more advanced instruments, as well as the increased understanding of vitreoretinal pathology, postoperative outcomes have dramatically improved. The development of bright light sources and structurally rigid instruments has expanded the indications for 23G and 25G MIVS to include complex challenging cases such as rhegmatogenous retinal detachment (RRD) and vitreoretinal disorders such as proliferative vitreoretinopathy (PVR) [5, 15, 16]. In 1981, Kloti was the first to report using vitrectomy for superior retinal detachment [17]. Since 1985, an adequate series of pars plana vitrectomy (PPV) procedures have been used for the treatment of RRD, with a primary success rate of 79% [18], and the subsequent favorable success rates have made PPV a popular primary treatment option for RRD. Studies have shown that 25G vitrectomy is both safe and effective for the treatment of RRD, with a primary anatomical success rate for primary RRD ranging between 84% and 96.3% [19,20,21]. However, it remains unclear as to whether or not RRD can be successfully treated using a 27G instrument. In order to fully treat RRD, surgeons must perform extensive and thorough shaving up to the pars plana, and a few previous studies have reported the potential of 27G vitrectomy for the treatment of primary RRD [14, 22, 23].
The purpose of this present study was to conduct a retrospective multicenter survey involving a large number of patients to evaluate the safety and efficacy of 27G PPV for the treatment of primary RRD.
Subjects and methods
This retrospective multicenter survey study was performed in accordance with the requirements for a parallel Institutional Review Board (IRB; Registration No. UMIN000031103) and involved patients who underwent 27G PPV for primary RRD with a minimum 3-month postoperative follow-up period at 1 of the following 13 institutions from November 2014 to December 2016: Kyoto Prefectural University of Medicine, Kyoto, Japan; Oshima Eye Clinic, Takatsuki, Japan; St. Marianna University School of Medicine, Kawasaki, Japan; Kobe University Graduate School of Medicine, Kobe, Japan; Jikei University School of Medicine, Tokyo, Japan; Department of Ophthalmology, Okanami General Hospital, Iga, Japan; Osaka University Graduate School of Medicine, Osaka, Japan; MIE Eyecare Clinic, Yotsukaichi, Japan; Kozawa Eye Hospital and Diabetes Center, Mito, Japan; Hyogo Prefectural Amagasaki General Medical Center, Amagasaki, Japan; Keiyu Hospital, Yokohama, Japan; Fukui Red Cross Hospital, Fukui, Japan; and Mikawa Eye Clinic, Saga, Japan. The study protocols were performed in accordance with the tenets set forth in the Declaration of Helsinki and were carried out by a total of 21 surgeons (K.Y., T.Y., K.K., K.N., H.T., Y.O., S.A., H.I., S.K., T.N., A.W., T.G., J.K., H.O., K.S., A.K., J.M., S.O., Y.M., T.N., and T.H.)
Subjects
This multicenter survey study involved a total of 410 eyes of 406 consecutive patients who underwent 3-port transconjunctival 27G PPV for primary RRD. Recurrent RRD, PVR, traumatic retinal detachment, and macular-hole retinal detachment in highly myopic eyes were excluded from the survey.
Surgical technique
In all 406 patients, PPV was performed via the use of a 27G vitrectomy system. Typical intraoperative views of transconjunctival 27G vitrectomy are shown in Fig. 1, and Supplemental Digital Content 1. Prior to surgery, all patients underwent peribulbar or subconjunctival anesthesia using 2% lidocaine. As needed, additional local anesthesia was topically administered during surgery. Although the instrument setting for transconjunctival 27G MIVS is similar to that of a 23G or 25G system and follows the standard 3-port configuration, 27G trocars were designed for simple perpendicular insertion into the pars plana. In addition, chandelier illumination was set up for peripheral vitreous shaving. MIVS was performed using the Constellation® Vision System (Alcon Laboratories, Inc., Fort Worth, TX, USA) or the EVA® Phaco-vitrectomy System (Dutch Ophthalmic Research Center International (D.O.R.C.), Zuidland, the Netherlands). All surgeons used the wide-angle viewing system of contact or noncontact lens and the cutter, i.e., the UltraVit® Vitrectomy Probe (Alcon Laboratories) or a twin duty cycle (TDC) vitreous cutter (D.O.R.C.), in accordance with the surgeon’s personal preference. When indicated, triamcinolone acetonide was used as a surgical adjuvant. All eyes underwent a core vitrectomy and an extensive and thorough shaving up to the pars plana using a high-speed (7500 or 8000 cycles/min) vitreous cutter. Any vitreous traction on the retinal breaks was removed with special caution. Perfluorocarbon liquid (PFCL) was injected to flatten the detached retina as needed, and fluid-gas exchange was performed for retinal breaks. At the determination of the operating surgeon, air or 20% sulfur hexafluoride (SF6), 14% octafluoropropane (C3F8), or silicon oil was introduced as a tamponade agent. Retinal breaks were treated with laser endophotocoagulation or cryotherapy according to the hole location. Phacoemulsification and aspiration was performed in patients with a cataract (patient age: over 45 years). A foldable acrylic intraocular lens (IOL) was inserted through a 2.2-mm clear corneal wound using an injector system. At the end of surgery, the cannulas were removed and moderate pressure was applied to the sclerotomy sites with a cotton-tipped applicator. If apparent wound leakages were found, transconjunctival sutures were placed to close the wound. All patients maintained an appropriate “face down” (prone) position after surgery, depending on the location of the tear, for approximately 3 to 7 days.
Images showing typical intraoperative views of transconjunctival 27-guage (G) vitrectomy for primary rhegmatogenous retinal detachment (RRD). Placement of the transscleral microcannulas through the pars plana in the superonasal, superotemporal, and inferotemporal quadrants. Surgery in this case was performed with a 27G VIVID chandelier (Moria Japan KK) (a). Core vitrectomy and peripheral vitreous shaving were performed under the contact-type wide-angle viewing system (d). Thorough vitreous shaving on the detached retina was performed with scleral indentation (b). Fluid-gas exchange was performed for retinal breaks (e). After fluid-gas exchange was completed, laser photocoagulation was performed (c). The surgery was concluded without sutures, and the conjunctiva remained stable with limited bleeding (f)
A 72-year-old female patient underwent 27G vitrectomy for primary RRD with cataract surgery. Kazuhito Yoneda performed the surgery using the Constellation® Vision System. (MP4 81436 kb)
Statistical analysis
The following parameters of each subject were collected: patient age, gender, lens status, preoperative macula status, the location of primary tears, the method of tamponade, the surgical time, primary success rate, and final success rate (final success being defined as reattachment at 3 months postoperative). Best-corrected visual acuity (BCVA) and intraocular pressure (IOP) were examined at baseline and at 1 day, 1 week, 1 month, and 3 months postoperative. Visual acuity (VA) was recorded in Snellen VA ratios and then converted to the logarithm of the minimum angle of resolution (logMAR) equivalents for statistical analysis. Intraoperative and postoperative complications were also collected and reviewed. Hypotony was defined as an IOP of less than 6 mmHg. Where appropriate, the analysis of variance (ANOVA), a pairwise Turkey-Kramer honest significant difference test, and Student’s t test were used to compare the differences among the groups. Statistical analysis was performed using IBM SPSS® Statistics 24.0 (SPSS, Inc., Chicago, IL, USA) statistical software. A P value of < 0.05 was considered statistically significant.
Results
A total of 410 eyes (259 male eyes and 151 female eyes) of 406 consecutive patients were analyzed. The preoperative baseline characteristics of the patients are summarized in Table 1. The mean age at the time of surgery was 59.4 ± 11.5 years (range 29 to 86 years). The mean follow-up period was 4.4 ± 2.3 months (range 3 to 12 months); follow-up data for more than 3 months postoperative was available. Of the 410 treated eyes, 325 (79.3%) were phakic and 85 (20.7%) were pseudophakic.
The preoperative findings and intraoperative factors of the 410 treated eyes are summarized in Table 2. The macula was attached in 226 eyes (55.1%), yet detached in 184 eyes (44.9%), and 102 eyes (24.9%) had more extensive RRD with three or four quadrants of involvement. As for the location of the primary tears, 203 eyes (49.5%) had retinal tears in the superior quadrants, 107 eyes (26.1%) had retinal tears in the inferior quadrants, and 71 eyes (17.3%) had retinal tears in the superior and inferior quadrants. Preoperative hypotony was observed in 13 eyes (3.2%), and choroidal detachment was complicated in 4 eyes (1.0%). Preoperative vitreous hemorrhage was observed in 16 eyes (3.9%). The intraoperative tamponade was performed with 20% SF6 gas (n = 309 eyes), 14% C3F8 gas (n = 8 eyes), air (n = 88 eyes), and silicone oil (n = 5 eyes). Of the 5 silicone-oil tamponade eyes, 3 were giant retinal tears while the other 2 were previous RRD with choroidal detachment. All surgeries were completed using the 27G system without any need of conversion to a larger-gauge instrument. At the end of surgery, all sclerotomies were self-sealed without suture placement after simple removal of the cannulas. The surgical time and anatomical success rates are shown in Table 3. The mean surgery time was 59.3 ± 33.4 min for the 133 eyes that underwent PPV and 62.1 ± 24.7 min for the 277 eyes that underwent PPV, phacoemulsification and aspiration, and IOL implantation. Of the total 410 eyes, the mean surgical time was 61.2 ± 27.8 min, and the primary success rate was 95.6% (n = 392 eyes). The final anatomical success rate was 100%.
The IOP outcomes are summarized in Fig. 2. Compared with the mean preoperative IOP of 13.1 ± 4.0 mmHg (mean ± SD, range 1–40 mmHg), IOP at 1 day, 1 week, 1 month, and 3 months postoperative was 15.7 ± 7.0 mmHg (range 1–52 mmHg) (P < 0.001), 15.0 ± 6.8 mmHg (range 3–32 mmHg) (P < 0.001), 14.6 ± 4.2 mmHg (range 5–31 mmHg) (P < 0.01), and 13.7 ± 3.3 mmHg (range 5–28 mmHg) (P = 0.61), respectively. No significant differences were observed between the mean IOP prior to surgery and that at 3 months postoperative. At 1 day postoperative, the percentage of eyes with ocular hypertension (i.e., an IOP of >30 mmHg) was 3.2% (n = 13 eyes).
The postoperative complications are summarized in Table 4. Primary surgical failure was observed in 18 eyes (4.4%). Of the 18 eyes with retinal redetachment, the detachment in 8 eyes was associated with other retinal breaks, probably due to the retinal breaks being missed by the operating surgeon, while in 7 eyes, it involved a reopening of the original breaks. In 3 eyes, it involved a combination of both. Of the 18 eyes, 3 eyes had progressed PVR within 1 month postoperative. As for 15 eyes of the redetachment cases, excluding the PVR cases, 11 eyes underwent PPV and a 20% SF6 gas tamponade, 1 eye underwent PPV and a 14% C3F8 gas tamponade, and 3 eyes underwent PPV and a silicone oil tamponade. Final reattachment was achieved in 13 eyes with secondary surgical intervention and with a third surgical intervention in the other 2 eyes. Of the 3 eyes with posterior PVR, 1 eye underwent 27G PPV with a 20% SF6 gas tamponade, 1 eye underwent 25G PPV with a silicone oil tamponade around the encircling buckle placement, and 1 eye required 4 operations, the largest number of operations, and that eye was finally managed by undergoing 25G PPV with a silicone oil tamponade.
Postoperative hypotony was observed in 14 eyes (3.4%) at 1 day postoperative. Of those, 3 eyes (0.7%) were complicated with postoperative choroidal detachment. Of the total 14 eyes, the mean surgical time was 55.6 ± 12.8 min. However, hypotony spontaneously resolved within 1 week and the choroidal detachment cases were gradually improved within 1 month. In all 14 eyes, no additional sutures were required post surgery. Although vitreous hemorrhage occurred in 9 eyes (2.2%) post surgery, it was spontaneously absorbed within 1 month without any need of additional surgical intervention. There were no cases of endophthalmitis.
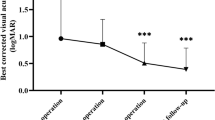
The preoperative and 3-month postoperative BCVA (logMAR) data of the eyes with macula-on RRD (n = 226 eyes) is shown in Fig. 3a. At 3 months postoperative, the BCVA was improved in 78 eyes (34.5%), was unchanged in 131 eyes (58.0%), and was decreased in 17 eyes (7.5%). In the group with improved VA, 58 eyes were vitrectomy combined with cataract surgery and 9 eyes were observed with preoperative vitreous hemorrhage. In the group with decreased VA, 6 eyes had undergone lens-sparing vitrectomy and 4 eyes were redetachment cases. In 3 of the redetachment cases, BCVA decreased by 3 or more lines. Compared to the mean preoperative BCVA of 20/29 (logMAR 0.16 ± 0.51 (mean ± SD)), that at 1 month and 3 months postoperative was 20/22 (logMAR 0.04 ± 0.20) (P = 0.19) and 20/21 (logMAR 0.02 ± 0.14) (P = 0.11), respectively.
a Pre- and postoperative best-corrected visual acuity (BCVA) data with macula-on RRD (n = 226 eyes). Scatterplot showing the changes in pre- and postoperative BCVA measured prior to surgery and at the 3-month postoperative follow-up visit. Mean logMAR improved from 0.16 ± 0.51 preoperative to 0.02 ± 0.14 postoperative (P = 0.11). b Pre- and postoperative best-corrected visual acuity (BCVA) data with macula-off RRD (n = 184 eyes). Scatterplot showing the changes in pre- and postoperative BCVA measured prior to surgery and at the 3-month postoperative follow-up visit. Mean logMAR improved from 1.06 ± 0.77 preoperative to 0.26 ± 0.35 postoperative (P < 0.001)
The preoperative and 3-month postoperative BCVA (logMAR) data of the eyes with macula-off RRD (n = 184 eyes) is shown in Fig. 3b. At 3 months postoperative, BCVA was improved in 164 eyes (89.1%), unchanged in 16 eyes (8.7%), and decreased in 4 eyes (2.2%). Only 2 redetachment cases resulted in a decreased VA of 3 or more lines, 1 of those cases being a PVR patient. Compared to the mean preoperative BCVA of 20/230 (logMAR 1.06 ± 0.77 (mean ± SD)), that at 1 month and 3 months postoperative had improved to 20/44 (logMAR 0.34 ± 0.41) (P < 0.001) and 20/36 (logMAR 0.26 ± 0.35) (P < 0.001), respectively.
Discussion
In this multicenter retrospective study involving the evaluation of the data of a large number of primary RRD patients (410 eyes) seen at 13 eye institutes, we observed a very high primary success rate of 95.6% and a final anatomical success rate of 100%. Compared with the published literature, and to the best of our knowledge, this is the largest study to date on 27G vitrectomy being used for the treatment of primary RRD. In the above-described 2010 pilot study by Oshima et al. [10], the authors reported the initial feasibility and safety of a novel 27G MIVS system, and the use of that system for a variety of diseases has subsequently been proposed in previous studies [10,11,12,13,14, 22, 23].
A primary concern in regard to developing a 27G system is the management of complex challenging cases, such as RRD and PVR, without the need to convert to a larger-gauge instrument, as those diseases require extensive peripheral vitreous shaving and manipulations. In addition, wide-field viewing and structurally stiff instruments are considered necessary when treating those diseases with a 27G system. When performing PPV using the conventional viewing systems, i.e., those that are not the wide-field type system, it was sometimes necessary to tilt the patient’s eyes to observe and shave the peripheral vitreous via the use of vitreous cutter probes. However, it has been speculated that tilting and controlling the eyes with weak-structure 27G instruments is difficult. Recently, some types of wide-field viewing systems have become commonly used, and all of the surgeons in this present study used contact or noncontact wide-angle viewing systems. By using those systems, a broad view can be secured during surgery and PPV can be performed without the need of tilting the eye of the patient. It should be noted that all surgeons in this study did not require conversion to larger-gauge devices, instruments, or infusion. These results can be attributed to the wide-viewing systems, and the fact that no conversion to larger instruments was needed indicates that the overall strength of some vitreous surgery equipment has been improved.
There are several apparent advantages to using the 27G instrument instead of the standard 25G instrument for PPV. First, the integrity of the smaller wound is thought to be one advantage, and all eyes in this study were self-sealed without suture placement. Previous studies reported that the rate of intraoperative suture for wound closure was 1.3 to 36.4% in the initial series of 23G and 25G systems [5, 14, 24]. Compared with other vitrectomy systems which utilize a different incision size, e.g., the 25G or 23G systems, the 27G system induces minimal ocular trauma. Moreover, the postoperative irritation from exposed sutures is eliminated. Second, a 27G instrument may have the advantage of producing less postoperative inflammation. Moreover, it has been reported that compared with 25G vitrectomy, aqueous flare values and cell counts recover to the baseline levels earlier following 27G vitrectomy [11]. Third, the 27G cutter is the safest device, comparatively, because the 27G probe is a smaller-gauge instrument. Although the distance from the outer edge to the center hole of the 27G probe is the same as that of a 25G probe, the 27G probe (diameter of thickness 0.40 mm) is smaller than a 25G probe (diameter of thickness 0.50 mm). As stated in the study conducted by Dugel et al. [25], “The sphere of influence on surrounding tissue was greatest with large-gauge vitrectomy probes.” Therefore, we theorize that a 27G probe has a smaller sphere of influence during tissue attraction than a larger-gauge probe. Based on the above findings, we performed PPV with a core vitrectomy and an extensive and thorough shaving up to the pars plana in all eyes.
In our review of the literature, we noted a trend and summarized the single-operation success rate and final success rate over the last 5 years (Table 5) [19, 21,22,23, 26, 27]. In addition, we compared our findings with those in a previously reported large-scale study of 20G vitrectomy for primary RRD of 205 eyes [28] and found that our results were equivalent or even better. Regarding the use of the 27G system for RRD, only a few previous studies have investigated the safety and feasibility. Khan et al. [14] described the longer-term outcomes of 27G PPV in cases of posterior segment disease. That multicenter study involved a series of 390 eyes, i.e., the largest number of 27G cases previously reported. Although the results in that study mainly consisted of macula diseases such as epiretinal membrane, there were a few complicated cases, i.e., primary RRD (n = 18 cases) and recurrent PVR-related RRD (n = 17 cases). Recent comparative studies of 27G versus 25G for the treatment of RRD reported that the safety and efficacy of 27G for RRD appears similar to that of 25G PPV [22, 23]. However, those findings were limited due to the small number of patients. In this current study, we obtained a large number of patients and the primary success rate was 95.6%. Our results are comparable to those in other studies, thus illustrating that the 27G vitrectomy system is as safe and effective as larger-gauge vitrectomy systems.
In this present study, the mean surgery time of 61.2 min for RRD using 27G was longer than that previously reported when using 25G [20, 22]. It was also initially reported that the mean time of vitreous cutting for epiretinal membrane by using 27G was significantly longer than by using 25G [11]. Since flow rates and aspiration are both regulated by Poiseuille’s law, the 27G probe has the smallest internal diameters, so it is known that the 27G flow rate is smaller than that of 25G. Recently, some companies have released new cutting system cutters, such as a TDC vitreous cutter that makes the core vitrectomy time faster than that of a standard single-action cutter at similar cut speeds [29]. Thus, the TDC cutter is one surgical optional. In the current study, more than half of the surgeons used UltraVit® probes that are not the TDC cutter, so it can be assumed that by using such a double-action surgical cutting probe the surgical time may decrease.
In regard to postoperative IOP, it has been reported that hypotony post surgery ranges between 0 and 25% in cases undergoing 23G and 25G vitrectomy [5, 30,31,32,33]. In this present study, hypotony was observed in 14 eyes (3.4%) at 1 day postoperative, and choroidal detachment was complicated in only 3 of those eyes. However, the transient hypotony in those eyes spontaneously improved within 1 week postoperative. It should be noted that none of the eyes in this study required suturing, due to the fact that no apparent wound leakage occurred. However, we are unable to conclude that all sclerotomies were closed, because all sclerotomies were not evaluated in detail via the use of anterior-segment optical coherence tomography. Usually, when using the 27G system, we try, as much as possible, to not perform suturing in order to reduce suture-related irritation. Thus, at the end of each surgery, we removed the cannulas and moderate pressure was applied to the sclerotomy sites with a cotton-tipped applicator, a technique that may have contributed to the low rate of hypotony. Oshima et al. [10] reported that the overall mean IOP before and after surgery when using the 27G system was stable, with no significant differences among the examined time points (i.e., at 1-, 7-, and 30 days postoperative), and they found that the mean IOP at 1 day postoperative in eyes without a gas tamponade was lower than that in the eyes with it. Moreover, Yamane et al. [34] found that in patients undergoing 25G PPV, the IOP at 1 day postoperative was significantly higher in the gas-filled eyes than in the fluid-filled eyes. Similarly, the findings in this present study showed that the mean IOP was highest at 1 day postoperative. It is reported that air-gas exchange helps seal sclerotomies from the interior due to different surface tensions between the air-gas and the fluid [10]. Moreover, it is reported that extensive manipulation of the intraocular instrument can lead to wound leakage by enlarging the sclerotomy incision and possibly causes the gaping wound and leakage [5]. Hence, we speculated that the perpendicular incisions would seal easily when using the 27G MIVS due to the small gauge size [35].
LogMAR VA in the macula-on RRD cases (n = 226 eyes) improved from 0.16 ± 0.51 prior to surgery to 0.02 ± 0.14 at 3 months postoperative (P = 0.11). Although there was no statistically significant improvement of VA, cataract surgery simultaneously performed in 139 (62.0%) of these eyes may have partially contributed to the visual recovery. To prevent postoperative cataract progression, vitrectomy combined with cataract surgery is reportedly useful [36]. However, in 6 eyes that underwent lens-sparing vitrectomy, VA worsened by cataract progression after surgery.
LogMAR VA in the macula-off RRD cases (n = 184 eyes) improved from 1.06 ± 0.77 prior to surgery to 0.26 ± 0.35 at 3 months postoperative (P < 0.001). Similar to the findings in this present study, it has been reported that postoperative VA in macula-off RRD cases significantly improves in comparison to the preoperative VA [37]. In this present study, a decrease of BCVA at 3 months postoperative was observed in only 4 eyes (2.2%), including the cases that underwent redetachment.
Another major concern associated with MIVS is surgical complications, such as endophthalmitis. However, it should be noted that in this present study, endophthalmitis occurred in none of the cases, even though several previous studies have reported on the incidence of MIVS-related endophthalmitis [38,39,40]. In a previous multicenter survey, Oshima et al. [40] reported that the rates of acute-onset endophthalmitis after MIVS (including the 23G and 25G procedures) were higher than those associated with the conventional 20G procedure; however, the differences were not statistically significant (0.054% vs. 0.034%). Moreover, it has been reported that wound-related complications such as wound leakage and hypotony are risk factors of endophthalmitis [39, 41]. In previous studies, it has been reported that in many cases, liquid remains in eyes with endophthalmitis at the end of vitrectomy surgery [40, 42]. However, in this present study, postoperative hypotony was observed in only 14 eyes (3.4%), and all treated eyes were filled with air, gas, or silicone oil. Thus, we theorized that the no incidence of endophthalmitis was due to these results.
It should be noted that this current study did have several limitations. First, this was a retrospective study. Second, in this study, we did not quantify the refraction of the treated eye. Third, the selection of surgical procedure, as well as the specific surgical devices and probes that were used, was determined by each surgeon based on personal preference. Thus, the data in this study presented limitations due to the variability in sample sizes, the surgical indication criteria, and the surgical procedures that were performed. In the future, a large prospective comparative study is needed for a more detailed evaluation of 27G vitrectomy for RRD.
In conclusion, this current multicenter study confirmed the feasibility of using the 27G instrument system for transconjunctival sutureless MIVS in all cases. The favorable wound-sealing structures, as well as the limited number of postoperative complications, clearly demonstrate that the 27G system is both safe and feasible for the treatment of primary RRD.
References
Assi AC, Scott RA, Charteris DG (2000) Reversed self-sealing pars plana sclerotomies. Retina 20:689–692
Yanyali A, Celik E, Horozoglu F, Nohutcu AF (2005) Corneal topographic changes after transconjunctival (25-gauge) sutureless vitrectomy. Am J Ophthalmol 140:939–941. https://doi.org/10.1016/j.ajo.2005.05.042
Okamoto F, Okamoto C, Sakata N, Hiratsuka K, Yamane N, Hiraoka T, Kaji Y, Oshika T (2007) Changes in corneal topography after 25-gauge transconjunctival sutureless vitrectomy versus after 20-gauge standard vitrectomy. Ophthalmology 114:2138–2141. https://doi.org/10.1016/j.ophtha.2007.01.034
Kellner L, Wimpissinger B, Stolba U, Brannath W, Binder S (2007) 25-Gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol 91:945–948. https://doi.org/10.1136/bjo.2006.106799
Lakhanpal RR, Humayun MS, de Juan E Jr, Lim JI, Chong LP, Chang TS, Javaheri M, Fujii GY, Barnes AC, Alexandrou TJ (2005) Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology 112:817–824. https://doi.org/10.1016/j.ophtha.2004.11.053
Chen JC (1996) Sutureless pars plana vitrectomy through self-sealing sclerotomies. Arch Ophthalmol 114:1273–1275
Fujii GY, De Juan E Jr, Humayun MS, Pieramici DJ, Chang TS, Awh C, Ng E, Barnes A, Wu SL, Sommerville DN (2002) A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology 109:1807–1812 discussion 1813
Fujii GY, De Juan E Jr, Humayun MS, Chang TS, Pieramici DJ, Barnes A, Kent D (2002) Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology 109:1814–1820
Eckardt C (2005) Transconjunctival sutureless 23-gauge vitrectomy. Retina 25:208–211
Oshima Y, Wakabayashi T, Sato T, Ohji M, Tano Y (2010) A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology 117:93–102.e2. https://doi.org/10.1016/j.ophtha.2009.06.043
Mitsui K, Kogo J, Takeda H, Shiono A, Sasaki H, Munemasa Y, Kitaoka Y, Takagi H (2016) Comparative study of 27-gauge vs 25-gauge vitrectomy for epiretinal membrane. Eye (Lond) 30:538–544. https://doi.org/10.1038/eye.2015.275
Rizzo S, Barca F, Caporossi T, Mariotti C (2015) Twenty-seven-gauge vitrectomy for various vitreoretinal diseases. Retina 35:1273–1278. https://doi.org/10.1097/iae.0000000000000545
Yoneda K, Morikawa K, Oshima Y, Kinoshita S, Sotozono C (2017) Surgical outcomes of 27-gauge vitrectomy for a consecutive series of 163 eyes with various vitreous diseases. Retina 37:2130–2137. https://doi.org/10.1097/iae.0000000000001442
Khan MA, Kuley A, Riemann CD, Berrocal MH, Lakhanpal RR, Hsu J, Sivalingam A, Ho AC, Regillo CD (2018) Long-term visual outcomes and safety profile of 27-gauge pars plana vitrectomy for posterior segment disease. Ophthalmology 125:423–431. https://doi.org/10.1016/j.ophtha.2017.09.013
Fine HF, Iranmanesh R, Iturralde D, Spaide RF (2007) Outcomes of 77 consecutive cases of 23-gauge transconjunctival vitrectomy surgery for posterior segment disease. Ophthalmology 114:1197–1200. https://doi.org/10.1016/j.ophtha.2007.02.020
Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, Tano Y (2009) Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 116:927–938. https://doi.org/10.1016/j.ophtha.2008.11.005
Kloti R (1981) Retinal holes - cause and therapeutical key of retinal detachments. Iatrogenic holes (author’s transl). Klinische Monatsblatter fur Augenheilkunde 179:276–279. https://doi.org/10.1055/s-2008-1057310
Escoffery RF, Olk RJ, Grand MG, Boniuk I (1985) Vitrectomy without scleral buckling for primary rhegmatogenous retinal detachment. Am J Ophthalmol 99:275–281
Kobashi H, Takano M, Yanagita T, Shiratani T, Wang G, Hoshi K, Shimizu K (2014) Scleral buckling and pars plana vitrectomy for rhegmatogenous retinal detachment: an analysis of 542 eyes. Curr Eye Res 39:204–211. https://doi.org/10.3109/02713683.2013.838270
Susskind D, Neuhann I, Hilgers RD, Hagemann U, Szurman P, Bartz-Schmidt KU, Aisenbrey S (2016) Primary vitrectomy for rhegmatogenous retinal detachment in pseudophakic eyes: 20-gauge versus 25-gauge vitrectomy. Acta Ophthalmol 94:824–828. https://doi.org/10.1111/aos.13133
Mohamed YH, Ono K, Kinoshita H, Uematsu M, Tsuiki E, Fujikawa A, Kitaoka T (2016) Success rates of vitrectomy in treatment of rhegmatogenous retinal detachment. J Ophthalmol 2016:2193518. https://doi.org/10.1155/2016/2193518
Rizzo S, Polizzi S, Barca F, Caporossi T, Virgili G (2017) Comparative study of 27-gauge versus 25-gauge vitrectomy for the treatment of primary rhegmatogenous retinal detachment. J Ophthalmol 2017:6384985. https://doi.org/10.1155/2017/6384985
Otsuka K, Imai H, Fujii A, Miki A, Tagami M, Azumi A, Nakamura M (2018) Comparison of 25- and 27-gauge pars plana vitrectomy in repairing primary rhegmatogenous retinal detachment. J Ophthalmol 2018:7643174. https://doi.org/10.1155/2018/7643174
Bourla DH, Bor E, Axer-Siegel R, Mimouni K, Weinberger D (2010) Outcomes and complications of rhegmatogenous retinal detachment repair with selective sutureless 25-gauge pars plana vitrectomy. Am J Ophthalmol 149:630–634.e631. https://doi.org/10.1016/j.ajo.2009.11.003
Dugel PU, Zhou J, Abulon DJ, Buboltz DC (2012) Tissue attraction associated with 20-gauge, 23-gauge, and enhanced 25-gauge dual-pneumatic vitrectomy probes. Retina 32:1761–1766. https://doi.org/10.1097/IAE.0b013e3182456f4d
Wong CW, Wong WL, Yeo IY, Loh BK, Wong EY, Wong DW, Ong SG, Ang CL, Lee SY (2014) Trends and factors related to outcomes for primary rhegmatogenous retinal detachment surgery in a large Asian tertiary eye center. Retina 34:684–692. https://doi.org/10.1097/IAE.0b013e3182a48900
Mikhail MA, Mangioris G, Casalino G, McGimpsey S, Sharkey J, Best R, Chan WC (2017) Outcome of primary rhegmatogenous retinal detachment surgery in a tertiary referral centre in Northern Ireland - a regional study. Ulster Med J 86:15–19
Schmidt JC, Rodrigues EB, Hoerle S, Meyer CH, Kroll P (2003) Primary vitrectomy in complicated rhegmatogenous retinal detachment--a survey of 205 eyes. Ophthalmologica 217:387–392. https://doi.org/10.1159/000073067
Pavlidis M (2016) Two-dimensional cutting (TDC) vitrectome: in vitro flow assessment and prospective clinical study evaluating core vitrectomy efficiency versus standard vitrectome. J Ophthalmol 2016:3849316. https://doi.org/10.1155/2016/3849316
Ibarra MS, Hermel M, Prenner JL, Hassan TS (2005) Longer-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol 139:831–836. https://doi.org/10.1016/j.ajo.2004.12.002
Byeon SH, Lew YJ, Kim M, Kwon OW (2008) Wound leakage and hypotony after 25-gauge sutureless vitrectomy: factors affecting postoperative intraocular pressure. Ophthalmic Surg Lasers Imaging 39:94–99
Woo SJ, Park KH, Hwang JM, Kim JH, Yu YS, Chung H (2009) Risk factors associated with sclerotomy leakage and postoperative hypotony after 23-gauge transconjunctival sutureless vitrectomy. Retina 29:456–463. https://doi.org/10.1097/IAE.0b013e318195cb28
Teixeira A, Allemann N, Yamada AC, Uno F, Maia A, Bonomo PP (2009) Ultrasound biomicroscopy in recently postoperative 23-gauge transconjunctival vitrectomy sutureless self-sealing sclerotomy. Retina 29:1305–1309. https://doi.org/10.1097/IAE.0b013e3181b09487
Yamane S, Kadonosono K, Inoue M, Kobayashi S, Watanabe Y, Arakawa A (2011) Effect of intravitreal gas tamponade for sutureless vitrectomy wounds: three-dimensional corneal and anterior segment optical coherence tomography study. Retina 31:702–706. https://doi.org/10.1097/IAE.0b013e3181f0d2e6
Yomoda R, Sasaki H, Kogo J, Shiono A, Jujo T, Sekine R, Tokuda N, Kitaoka Y, Takagi H (2018) Comparative study of straight vs angled incision in 27-gauge vitrectomy for epiretinal membrane. Clin Ophthalmol 12:2409–2414. https://doi.org/10.2147/OPTH.S183456 eCollection 2018
Oshima Y, Ohji M, Tano Y (2006) Surgical outcomes of 25-gauge transconjunctival vitrectomy combined with cataract surgery for vitreoretinal diseases. Ann Acad Med Singap 35:175–180
Miller DM, Riemann CD, Foster RE, Petersen MR (2008) Primary repair of retinal detachment with 25-gauge pars plana vitrectomy. Retina 28:931–936. https://doi.org/10.1097/IAE.0b013e31816b313a
Scott IU, Flynn HW Jr, Dev S, Shaikh S, Mittra RA, Arevalo JF, Kychenthal A, Acar N (2008) Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina 28:138–142. https://doi.org/10.1097/IAE.0b013e31815e9313
Czajka MP, Byhr E, Olivestedt G, Olofsson EM (2016) Endophthalmitis after small-gauge vitrectomy: a retrospective case series from Sweden. Acta Ophthalmol 94:829–835. https://doi.org/10.1111/aos.13121
Oshima Y, Kadonosono K, Yamaji H, Inoue M, Yoshida M, Kimura H, Ohji M, Shiraga F, Hamasaki T (2010) Multicenter survey with a systematic overview of acute-onset endophthalmitis after transconjunctival microincision vitrectomy surgery. Am J Ophthalmol 150:716–725.e1. https://doi.org/10.1016/j.ajo.2010.06.002
Bamonte G, Mura M, Stevie Tan H (2011) Hypotony after 25-gauge vitrectomy. Am J Ophthalmol 151:156–160. https://doi.org/10.1016/j.ajo.2010.06.042
Wu L, Berrocal MH, Arevalo JF, Carpentier C, Rodriguez FJ, Alezzandrini A, Farah ME, Roca JA, Maia M, Saravia M, Morales-Canton V, Graue-Wiechers F, Cardillo JA (2011) Endophthalmitis after pars plana vitrectomy: results of the Pan American Collaborative Retina Study Group. Retina 31:673–678. https://doi.org/10.1097/IAE.0b013e318203c183
Acknowledgments
The authors wish to thank John Bush for reviewing the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this retrospective multicenter survey study involving human participants were in accordance with the ethical standards of the requirements for a parallel Institutional Review Board (IRB; Registration No. UMIN000031103) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Appendix
Appendix
The 27G Vitrectomy Study Group is comprised of the following participating investigators: Yoichiro Shinkai, Kazuhito Yoneda, Chie Sotozono (Kyoto Prefectural University of Medicine, Kyoto, Japan), Yusuke Oshima (Oshima Eye Clinic, Takatsuki, Japan), Jiro Kogo (Department of Ophthalmology, St. Marianna University School of Medicine, Kanagawa, Japan), Hisanori Imai (Kobe University Graduate School of Medicine, Kobe, Japan), Akira Watanabe (Department of Ophthalmology, Jikei University School of Medicine, Tokyo, Japan), Yoshitsugu Matsui (Mie University Graduate School of Medicine, Mie, Japan), Kotaro Suzuki (Department of Ophthalmology, Keiyu Hospital, Kanagawa, Japan), Shinsuke Ataka (Department of Ophthalmology, Osaka University Graduate School of Medicine, Osaka, Japan), Shunsuke Osawa (Department of Ophthalmology, the MIE Ganka, Yotsukaichi, Japan), Tsukasa Hanemoto (Department of Ophthalmology, Kozawa Eye Hospital and Diabetes Center, Mito Japan), Hideyasu Oh (Department of Ophthalmology, Hyogo Prefectural Amagasaki General Medical Center, Hyogo, Japan), Akira Kobori (Department of Ophthalmology, Fukui Red Cross Hospital, Fukui, Japan), and Tomohisa Nishimura (Mikawa Eye Clinic, Saga, Japan).
Rights and permissions
About this article
Cite this article
Shinkai, Y., Oshima, Y., Yoneda, K. et al. Multicenter survey of sutureless 27-gauge vitrectomy for primary rhegmatogenous retinal detachment: a consecutive series of 410 cases. Graefes Arch Clin Exp Ophthalmol 257, 2591–2600 (2019). https://doi.org/10.1007/s00417-019-04448-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04448-2