Abstract
Purpose
To compare objective accommodation of phakic and pseudophakic eyes between two different age groups.
Methods
Eighty-three eyes (83 participants aged ≥ 40 years) with a visual acuity of 20/25 or better, and refractive error < spherical − 1.0 diopters (D) and cylindrical 1.0 D, were included. Forty-four patients had undergone phacoemulsification and monofocal intraocular lens implantation and were examined 6 months post-surgery. Participants were divided into groups 1 (pseudophakic, age < 60 years), 2 (pseudophakic, ≥ 60 years), 3 (phakic, < 60 years), and 4 (phakic, ≥ 60 years). Objective accommodation and pupil diameter to 2.0- and 3.0-D stimuli were measured with a binocular open-field autorefractor.
Results
The mean objective accommodation was 0.29 ± 0.47 D, 0.01 ± 0.21 D, 1.00 ± 0.88 D, and 0.01 ± 0.13 to a 2.0-D stimulus, and 0.26 ± 0.51 D, − 0.06 ± 0.21 D, 1.42 ± 1.21 D, and − 0.06 ± 0.21 to a 3.0-D stimulus in groups 1, 2, 3, and 4, respectively. For both stimuli, the values in group 1 exceeded those in groups 2 and 4, and were smaller than those in group 3, while the values in group 3 exceeded those in groups 2 and 4. The mean pupillary diameter was − 0.5 ± 0.8 mm, − 0.3 ± 0.8 mm, − 0.6 ± 0.5 mm, and − 0.6 ± 0.9 mm to a 2.0-D stimulus, and − 0.6 ± 0.8 mm, − 0.6 ± 0.8 mm, − 0.9 ± 0.5 mm, and − 1.0 ± 1.1 mm to a 3.0-D stimulus in groups 1, 2, 3, and 4, respectively. There was significant correlation between objective accommodation and changes of pupil size for both stimuli.
Conclusion
Age seems to play a role in objective accommodation among relatively young pseudophakic patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accommodation refers to the change of a human eye’s refractive status when focusing on nearby objects. The anterior radial muscle fibers of the ciliary muscle are responsible for the accommodation which contract toward sclera, and increasing tension on the equatorial zonules which are the active component in determining the optical power change of the lens [1]. With aging, accommodative power decreases due to progressive hardening of the lens and weakening of the ciliary muscles—a phenomenon called presbyopia [2]. Restoring accommodation by surgical means in presbyopic eyes is one of the hot topics in current ophthalmology. Thus, measuring accommodation accurately is important for assessing the effects of surgical procedures on presbyopic eyes.
Subjective and objective methods are used to measure accommodation [3,4,5,6]. Accommodation in phakic patients measured with subjective methods has been reported to be greater than that measured using objective methods [7], implying that subjective accommodation could be affected by not only ciliary muscle contraction but also static optical features, such as corneal astigmatism [8], corneal multifocality [9, 10], and pupil size [10,11,12,13]. Objective accommodation measured with a wavefront aberrometer was also reported to be affected by pupil size and spherical aberration that accommodative amplitude reduced with larger pupil and increased amount of spherical aberration reduction during accommodation [14, 15].
Apparent accommodation is a concept used in the literature to explain why pseudophakic patients have relatively good visual acuity over certain distance ranges [4, 12]. In pseudophakic patients, apparent accommodation as measured by subjective methods (0.5 D) was also reported to be greater than that measured by objective methods (0.12–0.23 D) [10]. Two studies have revealed that the amplitude of apparent accommodation in pseudophakic patients with monofocal intraocular lenses (IOLs) (0.93 D) was virtually equivalent to that of normal accommodation in phakic patients older than 60 years of age (0.76 D) [3, 16].
True accommodation, the actual change in the eye’s dioptric power, can only be measured by objective methods. Previous studies have reported that there was no clinically significant anterior chamber depth (ACD) shifts or objective accommodation in pseudophakic patients [10,11,12,13, 16, 17].
Our goal was to investigate the amplitude of accommodation, measured objectively with a binocular open-field autorefractor/keratometer, in phakic and pseudophakic patients and to compare this objective accommodation among age groups in pseudophakic patients, as well as phakic patients, as a control group. In addition, we concurrently measured changes in the pupil size to analyze the correlation with objective accommodation.
Methods
This study was approved prospectively by the Institutional Review Board of Severance Hospital (No. 4–2017-1205) and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Informed consent was obtained from all patients after the purposes and possible consequences of the study were explained to them.
Patients who visited Severance Hospital, a tertiary referral center in Seoul, Republic of Korea, were recruited. The inclusion criteria were patients 40 years of age or older whose eyes with a refractive error less than spherical −1.0 diopter (D) and cylindrical 1.0 D, a minimum best corrected distance visual acuity of 20/25, with the Snellen equivalent, no previous intraocular surgery except cataract surgery, no pathological condition except cataract, and no history of ocular trauma. Exclusion criteria were evidence of wearing contact lenses during the preceding 6 months and any intraoperative or postoperative complications. One eye per patient was selected randomly, using a computer-generated randomization envelope. Pseudophakic patients who had undergone cataract surgery approximately 6 months prior to the examination were divided into two groups including group 1 for patients younger than 60 years of age and group 2 for patients 60 years of age or older. Phakic participants were also divided into two groups, including group 3 for patients younger than 60 years of age and group 4 for patients 60 years of age or older. The size of each patient group was set to be around 20.
Pseudophakic patients had undergone a standard phacoemulsification procedure on both eyes by a single surgeon (TIK) via a temporal clear corneal incision under topical anesthesia. A single-piece intraocular lens, TECNIS PCB00 (Abbott Medical Optics Inc.) was implanted in a capsular bag using a preloaded IOL delivery system.
Refractive measurements were conducted with a WAM-5500 binocular open-field autorefractor/keratometer (GrandSeiko Co., Ltd., Tokyo, Japan) that allows infrared pupillometry. The lighting in the examination room was between 10 and 15 lx at eye level, as measured with a GOSSEN MAVOLUX 5032 (GOSSEN Foto-und Lichtmesstechnik GmbH, Nürnberg, Germany). The seated participant was asked to look at a target through a view window with both eyes. This target did not have a built-in light source. The distant target was placed 4 m from the window. The participant looked at a black dot on a white sheet at 50 and 33 cm distance from the window as intermediate and near targets, respectively. The participant viewed a target binocularly while only one eye was examined at a time, and the participant did not know which eye was being examined. The operator manipulated the joystick to keep the pupil in focus on the screen during examination. When the pupil was centrally adjusted, measurements were obtained automatically. The operator performed three repeated measurements. Spherical and cylindrical refractive results, with the axis, were recorded. Accommodative power was calculated as the difference of spherical equivalent measured with a WAM-5500 between the one with the distant target and the one with the near (33 cm) or intermediate target (50 cm).
Statistical analysis
Statistical analysis was performed with SPSS Statistics 16 (IBM Co., Ltd., Armonk, NY, USA). Statistical results were described as mean ± standard deviation (SD). The normality of the data was checked with the Kolmogorov-Smirnov test. If the normality was not rejected (P ≥ 0.05), analysis of variance (ANOVA) with Bonferroni adjustment for post hoc comparisons were used. If normality was rejected (P < 0.05), nonparametric tests were used. The Kruskal-Wallis test was used for comparisons among the four groups, and the Mann-Whitney U test for comparisons between two groups. The Spearman rank test was used to assess correlation. The chi-square test and the Fisher exact probability test were used for comparisons of categorical variables, while the Wilcoxon-signed-rank test was used for comparisons between two matched samples. A P value less than 0.05 was considered statistically significant.
Results
A total 83 eyes of 83 patients were included. All surgeries were uneventful, and all IOLs were implanted in the capsular bag. Patient characteristics are shown in Table 1.
The mean age of all subjects was 60.0 ± 12.2 years (range 40 to 82 years). The mean age of group 1 was not statistically different from group 3 (P = 0.08). No statistically significant differences were found between groups in terms of the ratio of males and females (P = 0.97), the ratio of the left and right eyes in each group (P = 0.90), spherical equivalent (P = 0.61), or the postoperative interval between surgery and examination (P = 0.94). The mean resting pupillary diameter was greater in group 1 than in group 2 (P = 0.02) and group 4 (P < 0.01), and was greater in group 3 than in group 2 (P = 0.02) and group 4 (P < 0.01). There was no significant difference between groups 1 and 3 and between groups 2 and 4.
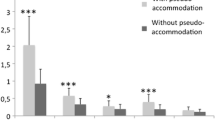
The results of objective accommodation measurement to 2.0- and 3.0-D stimulus are shown in Table 2. The mean accommodative response to a 2.0-D stimulus in group 1 was greater than that in group 2 (P = 0.02) and group 4 (P = 0.02), and was smaller than that in group 3 (P < 0.01), while that in group 3 was greater than that in group 2 (P < 0.01) and group 4 (P < 0.01) (df = 3, F = 17.5) (Fig. 1). The mean accommodative response to a 3.0-D stimulus in group 1 was greater than that in group 2 (P = 0.02) and group 4 (P = 0.02) and was smaller than that in group 3 (P < 0.01), and that in group 3 was greater than that in group 2 (P < 0.01) and group 4 (P < 0.01) also (df = 3, F = 21.0) (Fig. 2).
With a 2.0-D stimulus, the mean changes in pupillary diameter was − 0.5 ± 0.8 mm (range − 2.8 to 0.5 mm) in group 1, − 0.3 ± 0.8 mm (range − 2.6 to 2.4 mm) in group 2, − 0.6 ± 0.5 mm (range − 2.3 to 0.2 mm) in group 3, and − 0.6 ± 0.9 mm (range − 2.9 to 0.2 mm) in group 4 (Fig. 3). With a 3.0-D stimulus, the mean changes in pupillary diameter was − 0.6 ± 0.8 mm (range − 2.8 to 0.3 mm) in group 1, − 0.6 ± 0.8 mm (range − 2.8 to 0.0 mm) in group 2, − 0.9 ± 0.5 mm (range − 2.2 to 0.1 mm) in group 3, and − 1.0 ± 1.1 mm (range − 3.5 to 0.0 mm) (Fig. 4). Differences in the mean changes in pupillary diameter to 2.0- and 3.0-D stimuli among all groups were not statistically significant.
In groups 1, 2, and 4, both objective accommodation to 2.0- and 3.0-D stimuli showed no significant correlation with age. In group 3, objective accommodation to 2.0- and 3.0-D stimuli showed a negative correlation with age (r = − 0.76, P < 0.01; r = −0.86, P < 0.01, respectively). Objective accommodation to both 2.0- and 3.0-D stimuli in all groups showed no significant correlation with pupillary diameter changes.
Discussion
Restoring accommodation in presbyopic eyes is one of the big challenges in current ophthalmology. The present study sought to obtain insight into the objective accommodation of pseudophakic eyes. In this study, we showed that pseudophakic patients younger than 60 years had a greater amplitude of objective accommodation than that shown by phakic and pseudophakic patients aged 60 years or older.
Changes in the crystalline lens are considered to play a major role in the accommodative process, while movement of the lens also plays an important role [2]. Some pseudophakic patients with monofocal IOLs demonstrate apparent accommodation (pseudoaccommodation), showing good near visual acuity with distance correction [4, 12]. Various methods have been suggested for measuring apparent accommodation either subjectively or objectively. The pushup test and the minus-lenses-to-blur test are subjective methods frequently used for measuring apparent accommodation [18, 19]. Hartinger coincidence refractometry [7], wavefront aberrometry [20], dynamic retinoscopy, and open-field autorefractors are used to measure accommodation objectively [21]. In open-field autorefractors, infrared light is used to measure accommodation and pupil size. This allows the measurement of binocular physiological accommodation and changes in pupil size under more physiological circumstances. We measured binocular accommodation without occluding one eye. A monocular closed-view format was reported to cause problems such as instrument myopia [22]. Other factors like ocular dominance, binocular rivalry, and the difference in ocular alignment might also affect measuring refractive status under monocular condition. Several studies have considered Grand Seiko autorefractors to be a reliable method for measuring objective accommodation [5, 6, 23, 24].
Subjectively measured accommodation tends to be greater than that measured objectively. According to Nemeth et al. [10], the mean subjective accommodation in pseudophakic eyes was 0.5 D, while the mean objective accommodation was 0.23 and 0.13 to 2.0 and 3.0-D stimuli. The actual change in dioptric power during accommodation in pseudophakic eyes was found not to be clinically significant [10], and no significant ACD shifts in pseudophakic eyes were seen during near fixation or after pharmacologic ciliary muscle relaxation in previous studies [17]. However, previous objective accommodation studies have considered pseudophakic patients as a single group, regardless of their age. In this study, we divided pseudophakic patients into two groups according to their age. We decided on a cutoff of 60 years old, as the amplitude of accommodation in normal phakic eyes gradually decreases and becomes clinically less meaningful from that age. Interestingly, we found that pseudophakic patients under 60 years of age showed a greater accommodative response than pseudophakic patients 60 years of age or older. It is possible that ciliary muscle function is partially retained during accommodation in pseudophakic patients under 60 years of age when focusing on a target in the intermediate or near distance. However, the accommodative response in pseudophakic patients under 60 years of age was significantly smaller than that in control phakic participants under 60 years of age. In addition, the objective accommodation measured in the relatively young group of pseudophakic patients in our study was smaller than the mean subjective accommodation of pseudophakic patients (0.5 D) in the previous study [10]. It is difficult to determine the clinical implications of 0.29 and 0.26 D objective accommodation on intermediate and near distance in pseudophakic patients under the age of 60 years. However, significant differences in the amplitude of objective accommodation between pseudophakic patients of different ages could help in understanding accommodation in pseudophakic patients under the age of 60 years.
Among patients in group 1, there was an outlier whose accommodative response to 2.0- and 3.0-D stimuli was 1.63 and 1.50 D. Without this 56-year-old patient, the mean objective accommodation of group 1 to 2.0- and 3.0-D stimuli was 0.22 ± 0.37 and 0.20 ± 0.44 D. Although the outlier affected the mean accommodative amplitudes of group 1, mean accommodations without the outlier were still around 0.2 D.
Amplitude of objective accommodation in pseudophakic patients under the age of 60 years did not increase with a 3.0-D stimulus compared to a 2.0-D stimulus, showing no statistically significant difference. As a minimum measurement step of the autorefractor we used was 0.25 D, accommodative response smaller than 0.25 D cannot be measured. An autorefractor with a smaller minimum measurement step may detect difference of accommodative response between a 2.0-D stimulus and 3.0-D stimulus.
In this study, we used physiological stimuli to measure accommodation. Pharmacologically stimulated accommodation has been reported to overestimate the accommodative effect of an IOL [19, 25, 26] as the maximum potential accommodation does not necessarily reflect accommodation driven by physiological stimuli.
There was no significant correlation between objective accommodation and amount of pupillary miosis. Accommodative amplitude of subjects aged 22 to 40 measured monocularly with an aberrometer was reported to be smaller with low ambient light than with high ambient light, implying that a larger pupil size is associated with reduced accommodative amplitude [14]. Compared to the previous study, we used the binocular autorefractor, limited stimuli of vergence (only 2.0 and 3.0-D) to measure accommodative response among relatively older patients including pseudophakia. In addition, we only analyzed pupil miosis during accommodation and accommodative response instead of modifying room light condition. It is hard to determine the impact of pupil size on objective accommodation in pseudophakic patients aged older than 40 years from our results. Although changes in pupillary diameter did not correlate with objective accommodation in all groups in our study, a smaller pupil size in patients aged 60 years or more can help to improve subjective accommodation [10,11,12].
Spherical aberration was reported to affect objective accommodation [15]. The authors explained that the human eye accommodates to optimize image quality by selecting the best image plane which can be affected by spherical aberration during accommodation. In addition, depth of focus, the variation in image distance of a lens, or an optical system which can be tolerated without incurring an objectionable lack of sharpness in focus, can also affect objective accommodation in a way that the eye accommodates the minimum amount to place the target within its depth-of-focus to see the target clearly [27]. However, we focused on assessing accommodative response in a simple, objective way by using a binocular autorefractor. Measuring objective accommodation concurrently with other factors such as spherical aberration and depth of focus in pseudophakic patients will help to understand objective accommodation in pseudophakia. Other limitations of our study include its relatively small sample size and a lack of anterior segment measurements during accommodation. In addition, the autorefractor we used in our study has a minimum measurement step of 0.25 D. Objective accommodation in relatively young pseudophakic patients to intermediate and near stimulus was similar to this value (0.29 and 0.26 D). As we did not include anterior segment changes in our study, there is no clear evidence to support our suggestion of remaining ciliary muscle function among young pseudophakic patients. The small number of accommodative stimuli and the minimum measurement scale of the autorefractor mean that we are unable to provide evidence in support of our suggestion. To investigate evidence of remaining ciliary muscle function among young pseudophakic patients, measuring objective accommodation with a more sensitive device to more stimulus along with ACD shifts would be helpful.
The objective accommodation measured using an open-field autorefractor/keratometer in the pseudophakic patients younger than 60 years was greater than that in phakic and pseudophakic patients aged 60 years or older, both at intermediate and near distance. However, the objective accommodation in the pseudophakic group under the age of 60 years at both distances (50 and 33 cm) was smaller than that in the similarly aged phakic group. No previous report has compared objective accommodation in pseudophakic patients according to their age. Age seems to play a key role in objective accommodation in pseudophakic patients as well as in phakic patients. Our study may imply a clinical possibility of preserving objective accommodation partly in relatively young pseudophakic patients.
References
Schachar RA (2006) The mechanism of accommodation and presbyopia. Int Ophthalmol Clin 46:39–61
Koretz JF, Cook CA, Kaufman PL (1997) Accommodation and presbyopia in the human eye. Changes in the anterior segment and crystalline lens with focus. Invest Ophthalmol Vis Sci 38:569–578
Hayashi K, Hayashi H (2006) Comparison of amplitude of apparent accommodation in pseudophakic eyes with that of normal accommodation in phakic eyes in various age groups. Eye (Lond) 20:290–296
Hayashi K, Hayashi H, Nakao F, Hayashi F (2003) Aging changes in apparent accommodation in eyes with a monofocal intraocular lens. Am J Ophthalmol 135:432–436
Win-Hall DM, Glasser A (2009) Objective accommodation measurements in pseudophakic subjects using an autorefractor and an aberrometer. J Cataract Refract Surg 35:282–290
Win-Hall DM, Ostrin LA, Kasthurirangan S, Glasser A (2007) Objective accommodation measurement with the grand Seiko and Hartinger coincidence refractometer. Optom Vis Sci 84:879–887
Wold JE, Hu A, Chen S, Glasser A (2003) Subjective and objective measurement of human accommodative amplitude. J Cataract Refract Surg 29:1878–1888
Sawusch MR, Guyton DL (1991) Optimal astigmatism to enhance depth of focus after cataract surgery. Ophthalmology 98:1025–1029
Fukuyama M, Oshika T, Amano S, Yoshitomi F (1999) Relationship between apparent accommodation and corneal multifocality in pseudophakic eyes. Ophthalmology 106:1178–1181
Nemeth G, Lipecz A, Szalai E, Berta A, Modis L Jr (2013) Accommodation in phakic and pseudophakic eyes measured with subjective and objective methods. J Cataract Refract Surg 39:1534–1542
Nakazawa M, Ohtsuki K (1983) Apparent accommodation in pseudophakic eyes after implantation of posterior chamber intraocular lenses. Am J Ophthalmol 96:435–438
Nakazawa M, Ohtsuki K (1984) Apparent accommodation in pseudophakic eyes after implantation of posterior chamber intraocular lenses: optical analysis. Invest Ophthalmol Vis Sci 25:1458–1460
Ravalico G, Baccara F (1990) Apparent accommodation in pseudophakic eyes. Acta Ophthalmol 68:604–606
Lara F, Bernal-Molina P, Fernandez-Sanchez V, Lopez-Gil N (2014) Changes in the objective amplitude of accommodation with pupil size. Optom Vis Sci 91:1215–1220
Lopez-Gil N, Fernandez-Sanchez V (2010) The change of spherical aberration during accommodation and its effect on the accommodation response. J Vis 10:12
Hayashi K, Yoshida M, Manabe S, Hayashi H (2010) Comparison of visual function between phakic eyes and pseudophakic eyes with a monofocal intraocular lens. J Cataract Refract Surg 36:20–27
Tsorbatzoglou A, Nemeth G, Math J, Berta A (2006) Pseudophakic accommodation and pseudoaccommodation under physiological conditions measured with partial coherence interferometry. J Cataract Refract Surg 32:1345–1350
Atchison DA, Capper EJ, McCabe KL (1994) Critical subjective measurement of amplitude of accommodation. Optom Vis Sci 71:699–706
Langenbucher A, Huber S, Nguyen NX, Seitz B, Gusek-Schneider GC, Kuchle M (2003) Measurement of accommodation after implantation of an accommodating posterior chamber intraocular lens. J Cataract Refract Surg 29:677–685
Lopez-Gil N, Fernandez-Sanchez V, Legras R, Montes-Mico R, Lara F, Nguyen-Khoa JL (2008) Accommodation-related changes in monochromatic aberrations of the human eye as a function of age. Invest Ophthalmol Vis Sci 49:1736–1743
Pugh JR, Winn B (1988) Modification of the canon auto ref R1 for use as a continuously recording infra-red optometer. Ophthalmic Physiol Opt 8:460–464
Tsuneyoshi Y, Negishi K, Tsubota K (2017) Importance of accommodation and eye dominance for measuring objective refractions. Am J Ophthalmol 177:69–76
Sheppard AL, Davies LN (2010) Clinical evaluation of the grand Seiko auto ref/keratometer WAM-5500. Ophthalmic Physiol Opt 30:143–151
Win-Hall DM, Houser J, Glasser A (2010) Static and dynamic accommodation measured using the WAM-5500 autorefractor. Optom Vis Sci 87:873–882
Findl O (2005) Intraocular lenses for restoring accommodation: hope and reality. J Refract Surg 21:321–323
Kriechbaum K, Findl O, Koeppl C, Menapace R, Drexler W (2005) Stimulus-driven versus pilocarpine-induced biometric changes in pseudophakic eyes. Ophthalmology 112:453–459
Wang B, Ciuffreda KJ (2006) Depth-of-focus of the human eye: theory and clinical implications. Surv Ophthalmol 51:75–85
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number, HI18C1111).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was presented as e-poster in 36th Congress of the ESCRS on 22nd to 26th September 2018.
Rights and permissions
About this article
Cite this article
Chung, B., Choi, S., Ji, Y.W. et al. Comparison of objective accommodation in phakic and pseudophakic eyes between age groups. Graefes Arch Clin Exp Ophthalmol 257, 575–582 (2019). https://doi.org/10.1007/s00417-019-04249-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04249-7