Abstract
Purpose
To measure the thickness of the inferior oblique muscle (IOM) among Japanese by magnetic resonance imaging (MRI) using a new technique.
Methods
This retrospective observational study included 78 patients (36 males and 42 females) who underwent MRI for detection of a unilateral orbital lesion or examining causes of unilateral retrobulbar pain. The thickness of the IOM was measured on the side without the orbital lesion or symptom. On the quasi-sagittal plane through the optic nerve, the major and minor axes of the cross-section of the IOM were measured. On the coronal plane, the maximum thickness perpendicular to the course of the IOM was measured. All measurements were performed using the digital caliper tool of the viewing software.
Results
The major and minor axes on the quasi-sagittal plane and the maximum IOM thickness on the coronal plane were 8.00 ± 1.83 mm, 2.98 ± 0.55 mm, 3.04 ± 0.55 mm respectively. There were no significant differences in IOM thickness measurements between sexes and sides (P > 0.050, Student’s t-test). No significant correlation with the major axis (r = 0.064, P = 0.576), minor axis (r = −0.065, P = 0.573) or the maximum thickness on the coronal plane (r = −0.099, P = 0.387) was found in relation to age (Pearson’s correlation coefficient).
Conclusions
The normative IOM thickness in Japanese was presented on MRI, which were similar among all ages irrespective of sex and side. The new technique we used is easily applicable, and the results may serve as a guide to detect IOM involvement in inflammatory and neoplastic conditions of the orbit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The involvement of the inferior oblique muscle (IOM) has been reported in certain inflammatory and neoplastic conditions affecting the orbit. Although relatively uncommon, thyroid-associated orbitopathy [1, 2], sarcoidosis [3, 4], IgG4-related ophthalmic disease (IgG4-ROD) [5], and lymphomas [6] are among the disease entities with documented enlargement of the IOM.
Knowledge of normal IOM size can help elucidate this phenomenon. Our previous study examined the normal thickness of the IOM in Japanese, in which measurements were taken from the quasi-sagittal plane of the orbits of cadaveric specimens [7]. As tissue shrinkage is expected after formalin fixation [8], in-vivo measurements are still more reliable for clinical application.
Magnetic resonance imaging (MRI) is currently the best method to determine extraocular muscle (EOM) size in vivo as it provides better soft tissue contrast compared to other radiologic modalities [9]. This is especially important since the oblique muscles are very small and are subsequently the most difficult to measure in any plane [9, 10]. Three previous studies measured the normative IOM size by MRI using its cross-sectional area and/or volume [10,11,12]; however, the measurement techniques that were used are not always available. An ideal measurement method should be accessible, readily available, easy to perform, and have a short learning curve [9]. A study on Caucasians determined the normative values of IOM thickness on coronal MRI [13]. Although the measurement method used was easily accessible, IOM thickness varies depending on where the slice is made on the coronal plane. The sagittal plane, on the other hand, better visualizes the maximum thickness of the IOM along the axis perpendicular to the orbital floor. Moreover, significant differences in EOM thickness exist among different races [14, 15].
This study examined the normal thickness of the IOM in Japanese by MRI through the quasi-sagittal and coronal planes using a new technique.
Materials and methods
Study design, patients, and ethics approval
This study was a retrospective chart review of consecutive Japanese patients who underwent MRI for detection of a unilateral orbital lesion, including an orbital tumor, orbital haematoma, or orbital cellulitis, or for examining causes of unilateral retrobulbar pain at our department between May 2013 and October 2017. Patients with unilateral carotid cavernous fistula who underwent MRI during follow-up more than 1 year after surgical intervention were also included. Patients with autoimmune diseases, malignant lymphomas, vascular abnormalities and other systemic and orbital conditions that can bilaterally involve the EOMs were excluded [1,2,3,4,5,6, 16, 17]. Patients with high myopia (spherical power ≥ 6 diopters), which can cause elongation of the EOMs [18], and those who had undergone cataract surgery were also excluded. In addition, we excluded patients who were not cooperative with taking MRI without sedation.
This study was approved by the Institutional Review Board (IRB) of Aichi Medical University Hospital (approval number, 2017-H166) and followed the tenets of the 1964 Declaration of Helsinki. The IRB granted a waiver of informed consent for this study on the basis of the ethical guidelines for medical and health research involving human subjects established by the Japanese Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare. The waiver was granted because the study was a retrospective review and not an interventional study, and because it was difficult to obtain consent from patients who had been treated several years prior to this study. Nevertheless, at the request of the IRB, we published, on the Aichi Medical University Hospital website, an outline of the study, available for public viewing. This public posting also gave patients an opportunity to decline participation, although none of the patients did so. Personal identifiers were removed from the records prior to data analysis.
Data collection
The following data were collected from the medical charts reviewed: age, sex, affected side, past medical history, refractive power, and clinical diagnosis. We also collected data on histopathological results in patients with an orbital tumor.
MRI was performed using a 1.5-Tesla scanner (Magnetom Abant™; Siemens Healthcare, Erlangen, Germany). The head of the patient was stabilized while supine. Instructions were given to look at a central fixation target (light source) to ensure the eyes were fixed in the primary position. Quasi-sagittal (parallel to the optic nerve) and coronal T1-weighted gradient-echo sequences were acquired (T1-repetition time: 500 ms, echo time: 10 ms, field of view: 140 × 140 mm, matrix: 256 × 220, section thickness: 3 mm).
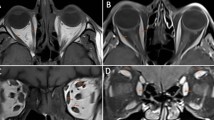
The thickness of the IOM on the side without the orbital lesion was measured on a quasi-sagittal image through the optic nerve and on a coronal image. On the quasi-sagittal plane, the major and minor axes of the maximum cross-section of the IOM were measured (Fig. 1a). The minor axis was perpendicular to the major axis. On the coronal plane, the maximum thickness perpendicular to the course of the IOM was measured (Fig. 1b). All measurements were performed using the digital caliper tool of the image viewing software (ShadeQuest/ViewR; Yokogawa Medical Solutions Corporation, Tokyo, Japan) by one of the authors (YT).
Statistical analysis
Patient age and IOM thickness were expressed as means ± standard deviations. The measurements were compared between sexes and sides using Student’s t-test. The correlation between patient age and IOM thickness was analyzed using Pearson’s correlation coefficient. These statistical analyses were performed using SPSS™ version 22 software (IBM Japan, Tokyo, Japan). A P value of < 0.05 was considered statistically significant.
Results
Seventy-eight patients (36 males and 42 females; 45 right and 33 left; age, 54.2 ± 18.3 years; range: 13–92 years) were included in this study. Patient demographic data is shown in Table 1. None of the patients had high myopia (spherical power ≥ 6 diopters). The measurements and statistical comparisons are summarized in Table 2. Patient age was not significantly different between sexes (P = 0.613) or sides (P = 0.895).
The major and minor axes on the quasi-sagittal plane and the maximum thickness on the coronal plane were 8.00 ± 1.83 mm, 2.98 ± 0.55 mm, 3.and 04 ± 0.55 mm, repectively. These measurements were not significantly different between sexes or sides (P > 0.050). No significant correlation with the major axis (r = 0.064, P = 0.576), minor axis (r = −0.065, P = 0.573) or the maximum thickness on the coronal plane (r = −0.099, P = 0.387) was found in relation to age (Fig. 2).
Discussion
We have described the normative IOM thickness of Japanese through MRI measurements in the quasi-sagittal and coronal planes using a new technique.
No sex- and side-related differences were found between the thickness measurements, which are similar to earlier studies [7, 19]. The relationship between gender and EOM thickness has been described previously, in which males showed significantly larger muscle thickness than females [20,21,22]. However, these studies did not include measurements of the IOM. In our present and previous studies, although the measurement results were also slightly larger in males than females, this difference lacked significance [7].
Age-related changes in EOM size have been previously documented. A previous study reported a subsequent decrease in the thickness of the rectus muscles after 60 years of age, although these differences were not statistically significant [19]. Similarly, the minor axis and maximum thickness on the coronal plane were inversely correlated with age in the present study, but also lacked significance. On the contrary, other studies found that the thickness of the rectus muscles was greater among adults, which was attributed to normal muscle growth with age [19, 23]. It should be noted, however, that there were only two patients under 20 years of age in the current subject pool.
The major and minor axes of the IOM on the quasi-sagittal plane through the optic nerve were both larger (major axis: 8.00 ± 1.83 mm; minor axis 2.98 ± 0.55) compared to our previous study (major axis: 7.23 ± 0.97 mm; minor axis: 2.27 ± 0.49 mm) [7]. This disparity may be explained by the expected tissue shrinkage after formalin fixation [8].
The normal cross-sectional shape of the EOMs is a thin ellipse on imaging studies [20]. In thyroid-associated orbitopathy, lymphocytic infiltration or mucopolysaccharide deposition promote greater elongation along the minor axis compared to the major axis of the EOMs, resulting in a round cross-sectional shape [7, 20]. The minor axis on the sagittal plane and the ratio of the minor and major axes are, therefore, more practical indicators for detecting IOM involvement in thyroid-associated orbitopathy [20]. These dimensions are likewise useful in detecting IOM involvement in other inflammatory disorders such as sarcoidosis [3, 4] and IgG4-ROD [5], as well as neoplastic conditions such as lymphoma [6], since these may also cause tendon-sparing or diffuse enlargement of the IOM [3, 5, 6]. Indeed, IOM enlargement may occur and may occasionally be the only presenting sign in some inflammatory or neoplastic diseases of the orbit.
The morphology and function of the IOM are also important to consider in certain types of strabismus and neuro-ophthalmological diseases affecting this muscle. For example, reduced IOM size was observed among cases of clinically diagnosed IOM palsy, supporting the concept of isolated IOM weakness without involvement of other structures innervated by the oculomotor nerve [11].
The technique we used to evaluate IOM thickness is readily available and easy to perform. The size of the IOM has been previously described through the measurement of its cross-sectional area or volume through high-resolution MRI [10,11,12], but this requires additional tools and expertise. In our technique, identification of the IOM on MRI may be initially difficult because it is small and follows an oblique course [13]. However, the IOM can be identified on the quasi-sagittal plane as it follows a straight path perpendicular to this plane at and medial to the inferior rectus (IR) crossing [24]. Thus, the maximum thickness of the IOM can be measured parallel to the orbital axis at the part passing through the centre of the IR muscle that travels in this plane [11]. More temporal image planes are unreliable to use because of the curved path of the IOM that becomes tangential to the image plane [11]. On the coronal plane, the IOM follows a straight path from its origin on the nasal orbital wall to the region of the IR pulley [24].
In this study, the digital caliper tool of the image viewing software was used to determine the thickness of the IOM, which is relatively straightforward. The software used in this study is part of the picture archiving and communication system module of the hospital. If this is not available, a ruler is a suitable alternative for this purpose since it is more accessible. Similar techniques involving direct measurements of muscle diameter on MRI have been employed by other studies [20, 21]; however, neither included measurements of the IOM and these were done on a different subset of individuals.
This study has some limitations. First, this is a retrospective study. Although the patients were instructed to look at a central fixation target, their position is ideally confirmed by checking the scans during the acquisition of images. However, this was not possible due to the retrospective nature of the study. It should also be recognized that this technique may not be applicable to patients who are not cooperative enough to take an MRI and maintain a straight gaze during the procedure. Second, the study was done on Japanese individuals. Since there are known racial differences in EOM size [14, 15], the findings presented here may not be applicable to other races. Third, we included patients with a unilateral orbital lesion, although taking MRI in normal volunteers is ideal for examining extraocular muscle morphology. The extraocular muscles in the unaffected side are expected to be overactive by Hering’s law. However, as IOM thickness was measured on MRI taken in the primary eye position, the influence of Hering’s law was small. Another limitation is the involvement of a single examiner in the measurement of IOM thickness, which could affect the reliability of this study.
In conclusion, we have presented the normative IOM thickness in Japanese individuals using MRI, which were similar among all ages irrespective of sex and side. The new technique we used is easily applicable, and the results may serve as a guide to detect IOM involvement in inflammatory and neoplastic conditions of the orbit.
References
Kakizaki H (2007) Inflammatory swelling of the inferior oblique muscle in thyroid associated ophthalmopathy. Clin Ophthalmol 1:189–192
Kakizaki H, Zako M, Iwaki M (2007) Thyroid-associated inferior oblique myopathy. Ophthalmology 114:2106
Cornblath WT, Elner V, Rolfe M (1993) Extraocular muscle involvement in sarcoidosis. Ophthalmology 100:501–505
Frank KW, Weiss H (1983) Unusual clinical and histopathological findings in ocular sarcoidosis. Br J Ophthalmol 67:8–16
Sogabe Y, Ohshima K, Azumi A, Takahira M, Kase S, Tsuji H, Yoshikawa H, Nakamura T (2014) Location and frequency of lesions in patients with IgG4-related ophthalmic diseases. Graefes Arch Clin Exp Ophthalmol 252:531–538
Guo PD, Xian JF, Man FY, Liu ZH, Yan F, Zhao J, Wang ZC (2016) Magnetic resonance imaging features of extraocular muscle lymphoma in five cases. Chin Med J 129:2384–2385
Takahashi Y, Kakizaki H, Nakano T, Asamoto K, Iwaki M (2008) Inferior oblique muscle thickness in Asians. Clin Ophthalmol 2:299–302
Boonstra H, Oosterhuis JW, Oosterhuis AM, Fleuren GJ (1983) Cervical tissue shrinkage by formaldehyde fixation, paraffin wax embedding, section cutting and mounting. Virchows Arch A Pathol Anat Histopathol 402:195–201
Bijlsma WR, Mourits MP (2006) Radiologic measurement of extraocular muscle volumes in patients with Graves’ orbitopathy: a review and guideline. Orbit 25:83–91
Nishida Y, Tian S, Isberg B, Tallstedt L, Lennerstrand G (2001) MRI measurements of orbital tissues in dysthyroid ophthalmopathy. Graefes Arch Clin Exp Ophthalmol 239:824–831
Ela-Dalman N, Velez FG, Demer JL, Rosenbaum AL (2008) High-resolution magnetic resonance imaging demonstrates reduced inferior oblique muscle size in isolated inferior oblique palsy. J AAPOS 12:602–607
Kono R, Demer JL (2003) Magnetic resonance imaging of the functional anatomy of the inferior oblique muscle in superior oblique palsy. Ophthalmology 110:1219–1229
Bourlet P, Carrie D, Garcier JM, Dalens H, Chansolme D, Viallet JF, Boyer L (1998) Study of the inferior oblique muscle of the eye by MRI. Surg Radiol Anat 20:119–121
McNutt LC (1979) Echographic measurement of extraocular muscles applied in graves’ orbitopathy. In: Shimizu K, Oosterhuis JA (eds) XXIII Concilium Ophthalmologicum: Kyoto 1978: Acta. Excerpta Medica, Amsterdam, pp 1842–1845
Chandra P, Sudhalkar A, Jalali S, Pesala V, Narayanan R, Sahu C, Chhablani J (2014) Echographic study of extraocular muscle thickness in normal Indian population. Saudi J Ophthalmol 28:281–286
Lacey B, Chang W, Rootman J (1999) Nonthyroid causes of extraocular muscle disease. Surv Ophthalmol 44:187–213
King WM (2011) Binocular coordination of eye movements: Hering’s law of equal innervation or uniocular control? Eur J Neurosci 33:2139–2146
Byrne SF, Gendron EK, Glaser JS, Feuer W, Atta H (1991) Diameter of normal extraocular recti muscle with echography. Am J Ophthalmol 112:706–713
Lee JS, Lim DW, Lee SH, Oum BS, Kim HJ, Lee HJ (2001) Normative measurements of Korean orbital structures revealed by computerized tomography. Acta Ophthalmol Scand 79:197–200
Aydin K, Güven K, Sencer S, Cikim A, Gül N, Minareci O (2003) A new MRI method for the quantitative evaluation of extraocular muscle size in thyroid ophthalmopathy. Neuroradiology 45:184–187
Ozgen A, Aydingöz U (2000) Normative measurements of orbital structures using MRI. J Comput Assist Tomogr 24:493–496
Ozgen A, Ariyurek M (1998) Normative measurements of orbital structures using CT. AJR Am J Roentgenol 170:1093–1096
Saccà S, Polizzi A, Macrì A, Patrone G, Rolando M (2000) Echographic study of extraocular muscle thickness in children and adults. Eye 14:765–769
Demer JL, Oh SY, Clark RA, Poukens V (2003) Evidence for a pulley of the inferior oblique muscle. Invest Ophthalmol Vis Sci 44:3856–3865
Author information
Authors and Affiliations
Contributions
All authors qualify for authorship based on contributions to the conception and design (YT), acquisition of data (YT), literature search (MSS and YT), and analyses and interpretation of data (MSS, HK, and YT). All authors contributed to drafting the article and revising it critically for important intellectual content and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
All authors have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
The Institutional Review Board granted a waiver of informed consent for this study based on the ethical guidelines for medical and health research involving human subjects established by the Japanese Ministry of Education, Culture, Sports, Science, and Technology and by the Ministry of Health, Labor, and Welfare. The waiver was granted because the study was a retrospective chart review, not an interventional study, and because it was difficult to obtain consent from patients who had been treated several years ago. Nevertheless, at the request of the Institutional Review Board, we published an outline of the study, available for public viewing, on the Aichi Medical University website; this also gave patients the opportunity to decline participation in the study. None of the patients declined to participate. Personal identifiers were removed from the records prior to data analysis.
Other contributors
None.
Rights and permissions
About this article
Cite this article
Sabundayo, M.S., Kakizaki, H. & Takahashi, Y. Normative measurements of inferior oblique muscle thickness in Japanese by magnetic resonance imaging using a new technique. Graefes Arch Clin Exp Ophthalmol 256, 839–844 (2018). https://doi.org/10.1007/s00417-017-3871-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3871-y