Abstract
Purpose
The goal of this project was to demonstrate the feasibility of coupling the indirect ophthalmoscope laser delivery system with the 690 nm wavelength diode laser used to perform photodynamic therapy (PDT) in the treatment of retinoblastoma.
Methods
For phase 1, a total of six pigmented rabbits were treated with the indirect laser delivery system. The laser source was provided by the Lumenis Opal 690 nm laser unit, delivered through a 810 nm Indirect ophthalmoscope headpiece and a hand-held 28-diopter indirect lens (1.0 mm spot size). Four rabbits received intravenous verteporfin at doses of 0.43 or 0.86 mg/kg, and two rabbits did not receive verteporfin (controls). A second phase of the study involved eight rabbits using a retinoblastoma xenograft to determine the effect of indirect PDT on subretinal tumors.
Results
For phase 1, a total of 20 laser treatments were performed in the right eyes of six rabbits. Laser power levels ranged between 40 and 150 mW/cm2 and treatment duration ranged between 1 and 3 min. In the four rabbits that received verteporfin, focal retinal scars were noted at 40 mW/cm2 and higher power levels. In the two control rabbits that did not receive verteporfin, thermal burns were confirmed at 75 mW/cm2 and higher power levels. Histopathology showed focal retino-choroidal scars at the site of PDT treatment, without evidence of generalized ocular damage. Using the retinoblastoma xenograft, the indirect PDT system was shown to cause areas of tumor necrosis on histopathology.
Conclusions
The results of this pre-clinical study suggest verteporfin may be activated in the rabbit retina with the indirect delivery system and the 690 nm laser unit (i.e., Indirect PDT). Using verteporfin, treatment effects were observed at 40–50 mW/cm2 in the rabbit retina, while photocoagulation was achieved at 75 mW/cm2 and higher power levels. Fundoscopic and histopathologic examination of treated areas showed circumscribed areas of retinal damage and a lack of generalized ocular toxicity, suggesting that this modality may represent a safe and localized method for treating intraocular retinoblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photodynamic therapy (PDT) was explored as a potential new treatment for ocular diseases during the 1980’s by various investigators [1,2,3,4,5,6,7,8,9,10,11]. At our institution, PDT was extensively investigated as a possible treatment modality for retinoblastoma from 1979 to 1987 [3, 4, 8]. However, there were no FDA-approved photodynamic agents available for ophthalmic use at that time, and prolonged photosensitivity was a major side-effect of treatment. In addition, the generated light used for PDT was delivered through a hand-held fiberoptic cable that did not allow for precise treatment of intraocular lesions [8]. With the FDA approval of a second-generation photosensitizer (verteporfin) in 2002 for macular degeneration, photodynamic therapy became a major clinical modality for several ocular conditions. Because of rapid clearance, photosensitivity was no longer a major clinical issue after treatment. Despite these advances, PDT continues to have a limited role in the treatment of pediatric ocular conditions, such as Coats disease and retinoblastoma. The main reason for this limitation is that ocular laser treatment for young children typically requires delivery through an indirect ophthalmoscope, since patients are in a supine, recumbent position during general anesthesia.
The goal of this project was to demonstrate the feasibility of coupling the indirect ophthalmoscope delivery system with the 690 nm laser used to activate verteporfin (i.e., indirect PDT). Following an intravenous infusion of verteporfin, a 690 nm wavelength laser was directed through the pupil onto a defined area of the rabbit retina using an indirect ophthalmoscope delivery system. Pigmented rabbits were evaluated for acute ocular effects by ophthalmoscopy, Retcam photography and histopathology. This study was designed to define potential ocular side-effects from the use of indirect PDT, as well as to demonstrate the accuracy of this system in creating controlled retinal damage in the rabbit eye. The second phase of the study involved implantation of a retinoblastoma xenograft into the rabbit eye to determine the effect of indirect PDT on subretinal tumors.
Material and methods
Phase 1
A total of six (8–12-week-old) pigmented rabbits were maintained on a normal diet consisting of rabbit chow and water. Verteporfin (Visudyne) was obtained through a donation from the company Valeant Ophthalmics (Bridgewater, NJ, USA). It was supplied as a sterile solution at a concentration of 2 mg/ml, reconstituted with 7 ml of sterile water (with no further dilution); the bottle was protected from light and used within 2 h of reconstitution. The dose of the photodynamic agent used in three rabbits was 0.43 mg/kg, which was extrapolated from the recommended dose in humans of 6 mg/m2. One rabbit received twice this dose at 0.86 mg/kg of verteporfin. Two rabbits were used as controls and did not receive verteporfin or any other type of injection.
The laser source was provided by the Lumenis Opal 690 nm laser unit (Yokneam, Israel) (see Fig. 1). During treatments, the light was delivered through an indirect ophthalmoscope delivery headpiece manufactured by Iridex for the 810 nm laser (Iridex, Mountain View, CA, USA). The size of the laser spot delivered with a 28-diopter biconvex condensing lens held over the cornea with the large spot indirect delivery system manufactured by Iridex is 1300 μm (1.3 mm) [12]. The intensity of light delivery before each laser treatment was measured with a thermopile (model 210; Coherent Radiation, Palo Alto, CA, USA).
An intramuscular injection (IM) was performed for anesthesia prior to all experimental procedures, using a combination of ketamine (30 mg/kg), acepromazine (0.75 mg/kg), and xylazine (5 mg/kg). After the IM injection, the right pupil of the experimental animal was dilated using 10% phenylephrine hydrochloride and 2.5% tropicamide. The animals were then administered a single injection of verteporfin into an ear vein through an intravenous catheter. After pupillary dilation was confirmed, laser treatment was initiated into the right eye of each experimental animal. The timing of laser treatment after verteporfin infusion ranged between 16 and 95 min.
During laser treatment, the cornea was periodically moistened with a 0.9% NaCl solution. Laser treatment was delivered at several power levels into the right eye of the rabbit ocular fundus, ranging between 45 and 190 mW/cm2 on the laser console (40–150 mW/cm2 as measured on the thermopile). The biconvex 28-diopter lens was held in standard fashion over the cornea to obtain a clear focused image of the fundus through the indirect ophthalmoscope headpiece. The location of the delivered laser spots was anatomically below the medullary ray in the rabbit fundus, and above the medullary ray in the ophthalmoscope view. A total of 2–6 laser treatments per eye were delivered in six rabbits with this technique. Fundoscopy was performed with a separate indirect ophthalmoscope after treatment to confirm the presence or absence of a visible laser scar (Table 1). If no retinal burn was appreciated at lower power levels, then the power level and/or treatment duration was increased until ophthalmoscopy confirmed a positive treatment effect. Reversal of anesthesia was accomplished with yohimbine IM injections, 1–2 mg per rabbit.
One week after laser treatment, animals were sedated and the right pupil dilated using the identical protocol described above. Fundus photography was performed using the Retcam unit. Immediately after Retcam photography, the rabbits were euthanized using an intramuscular injection of sodium pentobarbital (120 mg/kg). The size of the visible laser scars on the Retcam photograph was measured using a digital caliper system, using the optic disc as a reference diameter (1 mm). Using this method, the diameters for the largest retinal scars in the Retcam photographs for all six rabbits were measured and reported in Table 2.
Phase 2
The second phase of the study utilized eight right eyes of eight additional pigmented rabbits. All rabbits were immunosuppressed with daily intramuscular injections of cyclosporine A (CsA; Sandimmune 50 mg/ml; Novartis Pharmaceuticals, Cambridge, MA, USA). The dosing schedule was 15 mg/kg per day for the first week and 9–10 mg/kg per day for the last 8 weeks of the experiment. CsA doses were adjusted weekly according to each animal’s body weight. During the duration of the experiment, the rabbits were monitored daily for signs of CsA toxicity such as gingival hypertrophy, drooling, diarrhea, and weight loss.
The human retinoblastoma WERI-Rb cells (ATCC HTB-169;American Type Culture Collection, Manassas, VA, USA) were cultured in RPMI 1640 medium with 2 mM L-glutamine adjusted to contain 1.5 g/l sodium bicarbonate, 4.5 g/l glucose, 10 mM HEPES, and 1.0 mM sodium pyruvate, and 10% fetal bovine serum. The cells were grown in suspension at a concentration of 105–106 cells/ml. For sedation during the implantation procedure, an intramuscular injection of a combination of ketamine (100 mg/kg), xylazine (100 mg/kg), and acepromazine (10 mg/kg) were used to anesthetize the animals prior to all experimental procedures. One week after the start of the CsA injections, the rabbits received a subretinal injection of 100 ul of cultured WERI retinoblastoma cells (1.5 × 106 cells). The implantation procedure was performed under indirect ophthalmoscopy. A 1 cm3 syringe was used to draw up 0.1cm3 of the cultured WERI retinoblastoma cell solution using a 27G needle. A sharp 30G needle was then placed on the syringe and the air bubbles expressed to titrate a 0.1 ml volume of injection. The 30G needle was inserted 2 mm from the limbus temporally. Under indirect ophthalmoscopy guidance, the needle was used to perforate the retinam, and then a subretinal pocket of fluid was created. The needle was then removed and the eye examined for any signs of intraretinal and/or intravitreal hemorrhage.
At 7 weeks after intraocular tumor inoculation, assessment of tumor growth was performed and indirect PDT performed. Using indirect ophthalmoscopy, tumor growth was graded as follows: 1) no growth, 2) minimal growth, or 3) definite growth. The eyes graded as “definite growth” were treated with indirect PDT (similar protocol as Phase 1). The rabbits were euthanized 1 week after indirect PDT (8 weeks after start of experiment), using an intravenous overdose of sodium pentobarbital (120 mg/kg). The right eyes of the eight rabbits were surgically enucleated and fixed in half-strength Karnovsky’s fixative (2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 m sodium phosphate buffer) for histopathologic analysis.
Histopathologic study
The enucleated eyes of each rabbit were immediately fixed in formalin. After fixation the eyes were macroscopically examined, and measurements of the globe, cornea, and optic nerve were obtained. After transillumination, all eyes were opened horizontally with a blade, removing the superior calotte first. Measurements of grossly visible retinal scars were taken. The specimens were routinely processed for paraffin embedding and sectioning. Step sections were stained with hematoxylin and eosin (H&E), and others in between were left unstained for possible immunohistochemistry. All of the findings were analyzed by an ocular pathologist.
Immunohistochemistry using the fully automated system Benchmark Ultra from Ventana (Ventana Medical Systems, Tucson, AZ, USA), anti-glial fibrillary acidic protein (GFAP) [rabbit polyclonal antibody to cow GFAP (Dako, Carpinteria, CA, USA), diluted 1:40] was performed in all cases to examine glial activation and proliferation (retinal scarring). Pancytokeratin [OSCAR cocktail pre-diluted (Covance Research Products, Berkeley, CA, USA)] was used in some cases where marked chorioretinal scarring or epiretinal cellularity was found to exclude RPE proliferation. In all cases, 3, 3′-Diaminobenzidine (DAB) brown chromogen was used for labeling.
Results
Phase 1
In the first phase of the study, a total of 20 laser treatments were performed in six right eyes of six rabbits (Table 1). The weights of the rabbits ranged between 3.59–3.99 kg, and the dose of verteporfin was .43 mg/kg for three rabbits. One rabbit received an additional bolus of .43 mg/kg, 75 min after the first infusion, for a total of .86 mg/kg (rabbit 2). For the four rabbits, the total dose of verteporfin injection ranged between 1.54 mg to 3.43 mg, and the total volume of the injection was less than 2 ml per rabbit. Two rabbits did not receive verteporfin and represented controls. Laser power levels from the 690 nm laser console ranged between 45 and 190 mW/cm2. Actual laser power measured on the thermopile ranged between 40 and 150 mW/cm2. Treatment duration ranged between 1 and 3 min (Table 1).
In the two control rabbits that did not receive verteporfin, thermal burns in the retina were confirmed with indirect ophthalmoscopy at 75 mW/cm2 with a 1–3 min duration. A faint retinal burn was noted at 1 min and a more intense burn was noted at 3 min. At lower power levels (40–50 mW/cm2) no retinal burn was visualized by ophthalmoscopy in the control rabbit eyes, even at 50 mW/cm2 at 3 min. In the four rabbits that received verteporfin, retinal burns were confirmed at 75 mW/cm2 at 1 min, as well as all higher power levels and longer durations. The one exception was that no retinal burn was seen at 75 mW/cm2 with a 2 min duration in the first rabbit treated (rabbit 3). However, this was the first laser treatment delivered and the focus of the laser energy on the retina through the 28-diopter condensing lens may have been inadequate, possibly due to poor focus and/or inexperience with this system. This first treatment (rabbit 3) was also initiated at 16 min after verteporfin infusion, which is the earliest in the series. In three of the four rabbits receiving verteporfin, retinal burns were created at the first and lowest power level used to treat the eye (Table 1).
At 40 mW/cm2 and the standard verteporfin dose, no retinal burns were seen at 1 or 2 min, but a faint retinal burn was noted by ophthalmoscopy at 3 min. At 50 mW/cm2 and the standard verteporfin dose, no burn was seen at 1 or 2 min but was seen at 3 min. It appeared that at these lower power levels and at standard verteporfin doses, at least a 3-min duration of laser treatment was required to create a visible retinal burn. At the higher verteporfin dose of .86 mg.kg (rabbit 2), fundoscopy showed retinal burns at 50 mW at 1,2, and 3 min. Based on our results, retinal thermal injury (without verteporfin) with the indirect PDT system can be expected at 75 mW/cm2 at 1 min. At 40–50 mW/cm2, at standard doses of verteporfin, laser duration has to be at 3 min to create a retinal scar. However at the higher dose of verteporfin (.86 mg/kg), a retinal burn can be produced at 50 mW/cm2 at 1 min. Digital measurements of the size of the retinal scars on the Retcam images are summarized in Table 2. Using the optic disc reference size of 1 mm, the average horizontal and vertical diameters of the scars for the four rabbits treated with verteporfin were 1.5 mm and 1.03 mm respectively. Without verteporfin, the average horizontal and vertical diameters were 0.93 mm and 1.13 mm respectively. The maximum size of the scars for both groups was 1.63 mm (horizontal) and 2.43 mm (vertical) (see Fig. 2).
a Retcam fundus photograph showing three laser burns created with Indirect photodynamic therapy (with verteporfin) at 50 mW/cm2 and 1 min, 2 min, and 3 min durations (rabbit 2 in Table 1). b Retcam fundus photograph showing 690 nm diode laser burns in the rabbit retina created with the indirect delivery system (no verteporfin, rabbit 6 in Table 1). The superior retinal burn was created with 75 mW/cm2 at 3 min and the inferior burn was created with 150 mW/cm2 at 3 min
Histopathologic findings (phase 1)
The results of the histopathologic evaluation are summarized in Table 3. In addition to the analysis of the six treated eyes, the left untreated eye of rabbit 1 was also processed in identical fashion to serve as a baseline for normal anatomy. In none of the treated eyes was there evidence of damage to the cornea or lens. Findings associated with PDT were limited to the retina, retinal pigment epithelium (RPE), choroid, and vitreous. Necrosis was not seen in any of the specimens. The size of the retinal scars on gross examination ranged between 1.3–1.8 mm, and by light microscopy 1.2–1.8 mm.
With the standard dose of verteporfin and power levels of 40–50 mW/cm2 (rabbit 1), there were well-defined chorioretinal scars with pigment migration on H&E stain. The pigment migration was due to both pigment dispersion and proliferation and migration of the RPE. In this particular specimen, karyorrhexis with gliosis was also noted in the area of retinal scarring. Overlying the scar there was mild vitreous inflammation (1–2+) with lymphocytes and macrophages in the vitreous. Glial fibrillary acidic protein stain (GFAP) confirmed subretinal gliosis with proliferation of astrocytes. In the region of the retinal scarring, some focal choroidal fibrosis was also noted. At twice the normal dose of verteporfin and at 50 mW/cm2 (rabbit 2), there was again a circumscribed and well-defined retinal scar extending from the internal limiting membrane to the RPE, demonstrated both on H&E and GFAP. Several of the eyes including rabbit 2 demonstrated an artifactious retinal detachment after processing. At standard doses of verteporfin and higher power levels of 75–150 mW/cm2 (rabbits 3,4), there was full-thickness degeneration of the retina within a focal retino-choroidal scar, with RPE migration into the retina. One of the eyes treated at higher power demonstrated a deeper choroidal-scleral scar (rabbit 4). In the other eye (rabbit 3, Fig. 5) treated at the higher power levels, there was a large chorioretinal scar with a localized serous retinal detachment adjacent to the scar, as well as localized choroidal hemorrhage and vessel thrombosis. In the control rabbit eyes (rabbits 5 and 6), treated at power levels of 40–150 mW/cm2 without verteporfin, there was a chorio-retinal scar with marked RPE proliferation and migration into the sensory retina, as well as hemorrhage and focal fibrosis in the choroid. There was mild response of the glial cells mostly at the periphery of the lesion on GFAP (see Figs. 3, 4, and 5).
Histopathology of a rabbit eye treated with Indirect PDT using verteporfin at 50 mW/cm2 (rabbit 2 in Table 1 and Fig. 2a): a gross section photograph showing the retinal scar (arrow) b Hematoxylin and eosin stain at low power showing central retinal-choroidal scar and the adjacent untreated retina. c GFAP stain at high power showing Muller cells and glial astrocytes forming the scar
Histopathology of a rabbit eye treated with the Indirect PDT system but without verteporfin at 75–150 mW/cm2 (control, rabbit 6 in Table 1). a Gross section photograph showing the retinal scar. b Hematoxylin and eosin stain at low power showing central retinal-choroidal scar and an adjacent artifactious retinal detachment after processing. c Glial fibrillary acidic protein (GFAP) stain at high power demonstrating extensive gliosis within the scar
Histopathology of a rabbit eye treated with the Indirect PDT system using verteporfin at 75–150 mW/cm2 (rabbit 3 in Table 1). a Hematoxylin and eosin stain at low power showing an extensive chorioretinal scar with serous retinal detachment and choroidal hemorrhage. b High power image of the same scar showing localized choroidal hemorrhage with vessel thrombosis. c low power GFAP stain showing glial proliferation. d High power GFAP stain showing glial and retinal pigment epithelial proliferation
Phase 2
In the second phase of the study, seven of the eight rabbits survived the entire duration of the study. One rabbit was euthanized after 3 weeks of CsA injections due to worsening pneumonia (rabbit 14). No rabbits developed external signs of CsA toxicity such as gingival hypertrophy, drooling, diarrhea, or significant weight loss. Using indirect ophthalmoscopy, definite growth of subretinal tumors was confirmed in five of the seven surviving rabbits (Fig. 6). The five rabbits with definite growth were treated with Indirect PDT using the higher dose of verteporfin (0.86 mg/m2). The indirect PDT treatment was delivered to the area of greatest subretinal tumor growth, as judged on indirect ophthalmoscopy and Retcam photography. The specific parameters used to treat the five rabbits in the second phase of the study with Indirect PDT are listed in Table 2.
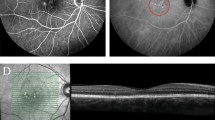
Retcam fundus photograph showing a subretinal retinoblastoma xenograft in a rabbit eye (rabbit 10 in Table 4). a Before indirect PDT b After indirect PDT
Histopathologic findings (phase 2)
Xenograft tumor growth was noted in seven of the eight rabbits in the second phase of the study, and the results are summarized in Table 4. The first rabbit eye injected did not demonstrate any tumor growth, and this was attributed to poor technique. Vitreous tumor growth was noted in six rabbits, while retinal, subretinal, and choroidal tumor growth was noted in all five rabbits treated with PDT. Extrascleral tumor growth was observed in five eyes (including a rabbit not treated with PDT), and appeared to be related to growth through the needle tract during the injection process (Fig. 7d). In the five eyes treated with PDT, areas of tumor necrosis in the subretinal tumor location was confirmed in 4/5 rabbits (Fig. 7a), ranging in power from 50 mW/m2–80 mW/m2. There was no tumor necrosis observed in the eye treated with 40 mW/m2. There was no evidence of damage to the lens or cornea in any of the eyes implanted with the RB xenograft and treated with indirect PDT.
Histopathology of the same eye in Fig. 5 (rabbit 10 in Table 4): hematoxylin and eosin stain at a low power and b high power, showing a subretinal tumor nodule (arrowhead) and focal area of tumor necrosis following Indirect PDT (arrow). c Viable vitreous seeds created in this xenograft retinoblastoma model. d Needle tract (arrow) from the xenograft procedure with a focus of extraocular tumor in a rabbit not treated with PDT (rabbit 14, Table 4)
Discussion
Given the intrinsic properties of photodynamic therapy, there is potential for PDT to become an important new modality for the treatment of intraocular retinoblastoma. PDT exhibits preferential tumor localization, as photosensitizers have been shown to be retained within the tumor at higher concentrations than in some normal tissues [2, 13]. Unlike other intraocular neoplasms, the lack of inherent pigmentation with retinoblastoma may allow for deep penetration of the light into the tumor [1]. The treatment effect induced by PDT is believed to result from the formation of singlet oxygen [1], leading to direct cytotoxicity, induction of apoptosis, or obliteration of blood vessels [1, 2]. Direct vascular damage leading to tumor necrosis is thought to be a particularly important mechanism for the treatment of retinoblastoma, given the importance of the vascular supply in its develoment [3]. With the arrival of second-generation photosensitizers (e.g., verteporfin), side-effects such as prolonged clearance and photosensitivity have been significantly reduced [1]. However, the lack of an indirect delivery method for the 690 nm wavelength laser has greatly limited its use in the pediatric population. Curiously, the indirect laser delivery system is commonly employed for retinoblastoma with the 532 nm (argon) and 810 nm (diode) lasers [14,15,16,17], but has never been used for PDT. The main advantage of the indirect delivery system is the ability to precisely treat retinal lesions in patients under general anesthesia.
The rabbit model used in this study was designed to demonstrate the feasibility of performing ocular PDT through an indirect laser delivery system. We coupled the 690 nm laser console used to activate verteporfin with an indirect ophthalmoscope delivery system initially designed for the 810 nm diode laser. Power delivery of the 690 nm wavelength through the fiber-optic system of the indirect ophthalmoscope was shown to be predictable and linear in the laboratory. In this animal model, accurate and focal laser scars were created both with and without verteporfin, indicating controlled delivery of laser energy to the rabbit retina. After verteporfin infusion, retinal scars were created at lower power levels than required for photocoagulation without verteporfin. In phase 1, the indirect PDT system appears to have been successful in activating verteporfin within the rabbit retina at 40-50 mW/cm2 and duration times of 1–3 min. Without verteporfin, retino-choroidal burns were created at higher power levels (75–150 mW/cm2), but not at 40–50 mW/cm2 (even after 3 min). Since this pilot study only had two control rabbits, it was difficult to prove conclusively that a thermal effect did not occur at these lower power levels, with a treatment effect not related to the activation of verteporfin. Photocoagulation can cause regression of retinoblastoma in the absence of PDT [15, 16]; therefore, any thermal effect created by the indirect PDT system is not considered a disadvantage for treating retinoblastoma. Traditional photocogulation is used routinely for small retinoblastoma tumors of 3 mm or less in diameter [18], while any tumor larger than 3 mm is typically treated with a combination of systemic chemotherapy and photocoagulation [19]. The photodynamic effect created by verteporfin may offer a benefit over traditional laser treatment given the potential for a deeper treatment effect within the tumor (with PDT), which may translate into the ability to treat some patients without chemotherapy. However, future clinical investigations of PDT for retinoblastoma are needed before determining whether there is any benefit of this potential new modality over traditional laser photocoagulation.
For this animal study we chose a lower light radiance or fluence rate (40–150 mW/cm2) than is currently used with the slit-lamp delivery system in adult patients (600 mW/cm2). The lower power level was chosen mainly because of the smaller spot size used for indirect PDT vs traditional slit-lamp PDT (1.4 mm vs 3.0 mm). A number of recent studies have also suggested that tumor responsiveness following PDT may be enhanced when light is delivered at lower irradiances [20,21,22]. The improved effect on tumors appears to be due in part to a decrease in oxygen depletion at lower light irradiances. Lowering the irradiance from 600 mw/cm2 and 83 s to 40–50 mW/cm2 over 1–3 min appeared to be appropriate, given that this power range was able to create retinal scars in our study. We also selected a treatment duration time of 1–3 min, which encompasses the duration of the established slit-lamp delivery system (83 s). The standard laser treatment time for ocular PDT (with verteporfin) in adults is 15 min after intravenous administration, and the systemic half-life in adults has been estimated to be 2–5 h [23]. We chose a time period of 15–35 min between verteporfin infusion and initiation of treatment with the 690 nm laser, as a minimum time period is needed to allow the drug to distribute into the ocular tissues but general anesthesia times for children cannot be extended indefinitely. We acknowledge that our study had a limited number of animals and that the duration of time between infusion and laser treatments varied significantly. In addition, the dose escalation design of the study makes it difficult to be certain whether subsequent treatments are successful because of increasing power levels, or due to the added experience of the investigator.
On histopathology, there was no evidence of generalized ocular toxicity outside of the treated zones in the retina. The area of retinal damage was limited to the treatment field following Indirect PDT in five of the six eyes. In one globe, a localized retinal detachment and choroidal hemorrhage were noted around the treated area that received verteporfin and the highest power level (150 mW/cm2). The eyes that received 50 mW/cm2 or less power (with verteporfin) demonstrated circumscribed areas of retinal scarring without collateral injury. The lack of generalized ocular toxicity following PDT was previously demonstrated in a similar rabbit study performed by Gomer and colleagues [13]. When the rabbits were enucleated 14 days after treatment, histopathologic evaluation showed that the area of ocular damage was limited to the treatment field in the retina [13]. The effects on the retina were permanent but not progressive, and similarly to the current study, there was no evidence of opacities involving the cornea or vitreous [12]. Since the photosensitizing agent is not concentrated in the lens or cornea, indirect PDT should not cause cataracts or corneal damage in young children [13]. Overall, common features in all of the treated eyes were proliferation of the RPE and mild vitreous inflammation in the region of the chorioretinal scars, as well as subretinal gliosis and proliferation of astrocytes. Those eyes treated at higher power levels demonstrated deeper levels of damage into the choroid causing fibrosis and hemorrhage.
Thousands of patients with solid tumors in various anatomic locations have been successfully treated with PDT [2]. Photodynamic treatment of transplanted ocular melanoma cells in the anterior chamber of rabbit eyes has been described previously [9, 10, 13]. Other studies have demonstrated that the use of PDT on ocular tumors does not induce tumor metastasis or new mutations [3, 24]. There are numerous case reports of ocular tumors being successfully treated with ocular PDT performed with the standard slit-lamp delivery system and the 690 nm laser [25,26,27]. Verteporfin has also been used in a limited number of cases in young children (as young as 6 years old) with no significant ocular or systemic side-effects [28,29,30]. However, retinoblastoma affects a very young population (1–5 years of age), and therefore caution is warranted, with the possible use of a photodynamic agent in this age group. In addition, there are potential unknown ocular or systemic toxicities of verteporfin in children who have received chemotherapy. For phase 1 of this study, we chose a lower dose of verteporfin (0.43 mg/kg) than has been previously used in other rabbit studies evaluating the effects of PDT [31, 32]. This dose was chosen because it correlates with the FDA-approved human dose of 6 mg/m2, and the goal of this animal study was to provide pre-clinical data for a future human pilot trial for children 1–5 years of age. The only major clinical side-effect observed after PDT in humans has been prolonged skin sensitivity, which has been largely mitigated with the introduction of verteporfin. However, we acknowledge that Indirect PDT would involve more ocular and systemic risk than traditional laser photocoagulation.
Our previous clinical work on ocular PDT in 1987 utilized a 630 nm rhodamine dye laser, with light being emitted through an optical fiber held 5 mm from the cornea [8]. This system was able to activate the photosensitizer in the eye, but was cumbersome to use and the clinical results were limited. Discrete retinoblastoma lesions treated with PDT had remarkable initial responses to treatment [8]. However, regrowth was noted within 3–4 months in the majority of cases. The indirect PDT system may lead to better long-term tumor control because of the targeted treatment of the tumors through the indirect viewing system. An indirect ophthalmoscope designed specifically for the 690 nm laser is not widely available for clinical use in the current marketplace. However, it is hoped that with further investigation of this new modality, laser companies will develop and introduce an indirect delivery system for the 690 nm laser unit. Company data for the large-spot indirect delivery system for the 810 nm laser is 1400 μm in the ocular fundus (1.4 mm) [12]. Measurements performed on Retcam photographs from our study showed that the indirect system using the 690 nm laser created retinal scars mainly in this size range, although some larger scars were also observed, possibly due to difficulty in focusing the laser energy on the retina. The ability to create scars larger than 1 mm is not considered a disadvantage in treating retinoblastoma tumors since lesions treated with indirect PDT are expected to be larger than 3 mm in diameter. One drawback of the indirect PDT system used in this study is that there is no ocular protection for the person performing the treatment, since the indirect delivery system has filters for the 810 nm laser but not the 690 nm wavelength. An indirect ophthalmoscope with a 690 nm filter and an aiming beam with a different wavelength would solve this issue.
The xenograft phase of the study was important in establishing a rabbit model for PDT, both for the current study and future investigations. Similar to a previously published protocol [33], we demonstrated that a viable xenograft model in a rabbit could be achieved with a commercially-available retinoblastoma cell line and daily immunosuppression with intramuscular cyclosporine. Histopathologic evidence suggested that subretinal, choroidal, and even extrascleral tumor foci can be created using a needle and indirect ophthalmoscopic guidance. The extrascleral tumor growth was believed to be related to the needle injection technique of the xenograft procedure since it was noted in a rabbit not treated with PDT, but caution is warranted with any new modality in ocular oncology. Future studies are planned using an operating microscope viewing system to implant the tumors more focally in the subretinal space (Gloss). Additionally, vitreous tumor growth was exuberant in our xenograft model by 7 weeks, and partially obscured the fundus view when performing indirect PDT. With the needle injection technique, the ideal time to perform indirect PDT appeared to be at 4 weeks after xenograft implantation when subretinal tumor growth was confirmed, rather than waiting 7–8 weeks when vitreous seeding began to obscure the fundus view. Areas of tumor necrosis was demonstrated on histopathology following Indirect PDT on areas of subretinal xenograft tumor growth, with power levels ranging between 50 and 80 mW/m2. The exact power levels used to achieve tumor regression in children with retinoblastoma will need to be defined with future studies. It appears that 50 mW/cm2 was the ideal power level for Indirect PDT when treating the rabbit retina, as higher levels can result in photocoagulation and lower power levels may not achieve tumor necrosis. However, it should be noted that even at 80 mW/cm2, we did not have any evidence of damage to the lens, cornea or choroid in the xenograft model.
In conclusion, the results of this preclinical study suggest that targeted, circumscribed areas of retinal damage can be created by the indirect PDT system. The indirect viewing system allows targeted delivery of the laser energy to a specific location in the fundus, using direct visualization. Given the optical characteristics of the eye and the relatively small size of the intraocular tumors, indirect PDT could become an important modality for the treatment of retinoblastoma, although it remains to be seen whether long-term regression can be achieved. There is enough encouraging evidence in this pre-clinical model to support further investigation of indirect PDT as a potential new modality to treat patients with intraocular retinoblastoma.
References
Fisher AM, Murphree AL, Gomer CJ (1995) Clinical and preclinical photodynamic therapy. Lasers Surg Med 17:2–31
Gomer CJ, Ferrario A, Hayashi N, Rucker N, Szirth BC, Murphree AL (1988) Molecular, cellular, and tissue responses following photodynamic therapy. Lasers Surg Med 8:450–463
Gomer CJ, Ferrario A, Murphree AL (1987) The effect of localized porphyrin photodynamic therapy on the induction of tumour metastasis. Br J Cancer 56:27–32
Gomer CJ, Hayashi N, Murphree AL (1987) The influence of sodium pentobarbital anesthesia on in vivo photodynamic therapy. Photochem Photobiol 46:843–846
Gomer CJ, Rucker N, Razum NJ, Murphree AL (1985) In vitro and in vivo light dose rate effects related to hematoporphyrin derivative photodynamic therapy. Cancer Res 45:1973–1977
Gomer CJ, Rucker N, Ferrario A, Murphree AL (1986) Expression of potentially lethal damage in Chinese hamster cells exposed to hematoporphyrin derivative photodynamic therapy. Cancer Res 46:3348–3352
Gomer CJ, Rucker N, Murphree AL (1988) Transformation and mutagenic potential of porphyrin photodynamic therapy in mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med 53:651–659
Murphree AL, Cote M, Gomer CJ (1987) The evolution of photodynamic therapy techniques in the treatment of intraocular tumors. Photochem Photobiol 46:919–923
Liu LH, Ni C (1983) Hematoporphyrin phototherapy for experimental intraocular malignant melanoma. Arch Ophthalmol 101:901–903
Sery TW, Dougherty TJ (1984) Photoradiation of rabbit ocular malignant melanoma sensitized with hematoporphyrin derivative. Curr Eye Res 3:519–528
Ohnishi Y, Yamana Y, Minei M (1986) Photoradiation therapy using argon laser and a hematoporphyrin derivative for retinoblastoma--a preliminary report. Jpn J Ophthalmol 30:409–419
Iridex (2014) Laser Indirect Ophthalmoscope (LIO) by Iridex. http://www.iridex.com/Portals/0/pdf/LT0011_LIO.pdf. Accessed 15 Jun 2015
Gomer CJ, Jester JV, Razum NJ, Szirth BC, Murphree AL (1985) Photodynamic therapy of intraocular tumors: examination of hematoporphyrin derivative distribution and long-term damage in rabbit ocular tissue. Cancer Res 45:3718–3725
Schefler AC, Cicciarelli N, Feuer W, Toledano S, Murray TG (2007) Macular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology 114:162–169
Shields CL, Shields JA, Kiratli H, De Potter PV (1995) Treatment of retinoblastoma with indirect ophthalmoscope laser photocoagulation. J Pediatr Ophthalmol Strabismus 32:317–322
Masuyama Y, Fukuzaki M, Kodama Y, Baba Y, Sawada A (1984) Treatment of retinoblastoma with argon laser photocoagulation. J Pediatr Ophthalmol Strabismus 21:169–171
Augsburger JJ, Faulkner CB (1992) Indirect ophthalmoscope argon laser treatment of retinoblastoma. Ophthalmic Surg 23:591–593
Abramson DH, Schefler AC (2004) Transpupillary thermotherapy as initial treatment for small intraocular retinoblastoma: technique and predictors of success. Ophthalmology 111:984–991
Zhu D, Berry JL, Ediriwickrema L, Wong K, Lee TC, Murphree AL, Kim JW, Jubran R (2015) Long-term outcomes of group B eyes in patients with retinoblastoma treated with short-course chemoreduction: experience from Children’s hospital Los Angeles/University of Southern California. Ocul Oncol Pathol 2(2):105–111
Seshadri M, Belinier DA, Vaughan LA, Spernyak JA, Mazurchuk R, Foster TH, Henderson BW (2008) Light delivery over extended time periods enhances the effectiveness of photodynamic therapy. Clin Cancer Res 14:2796–2805
Henderson BW et al (2000) Photofrin photodynamic therapy can significantly deplete or preserve oxygenation in human basal cell carcinomas during treatment, depending on fluence rate. Cancer Res 60:525–529
Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, Cheney RT, Morgan J (2004) Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res 64:2120–2126
Richter AM, Cerruti-Sola S, Sternberg ED, Dolphin D, Levy JG (1990) Biodistribution of tritiated benzoporphyrin derivative (3H-BPD-MA), a new potent photosensitizer, in normal and tumor-bearing mice. J Photochem Photobiol B 5(2):231–244
Gomer CJ, Rucker N, Banerjee A, Benedict WF (1983) Comparison of mutagenicity and induction of sister chromatid exchange in Chinese hamster cells exposed to hematoporphyrin derivative photoradiation, ionizing radiation, or ultraviolet radiation. Cancer Res 43:2622–2627
Cerman E, Cekic O (2015) Clinical use of photodynamic therapy in ocular tumors. Surv Ophthalmol 60:557–574
Kaliki S, Shields CL, Al-Dahmash SA, Mashayekhi A, Shields JA (2012) Photodynamic therapy for choroidal metastasis in 8 cases. Ophthalmology 119:1218–1222
Hussain RN, Jmor F, Damato B, Heimann H (2015) Verteporfin photodynamic therapy for the treatment of retinal vasoproliferative tumors. Ophthalmology 122:2361–2363
Giansanti F, Virgili G, Varano M et al (2005) Photodynamic therapy for choroidal neovascularization in pediatric patients. Retina 25:590–596
Varano M, Iacono P, Giorno P, Chiaravalloti A, Parravano M (2014) Photodynamic therapy in subfoveal and juxtafoveal myopic choroidal neovascularization: a 10-year retrospective analysis. Ophthalmologica 231:204–210
Yildirim C, Cetin EN, Yayla K, Avunduk AM, Yaylali V (2011) Photodynamic therapy for unilateral idiopathic peripapillary choroidal neovascularization in a child. Int Ophthalmol 31:333–335
Chuang L, Hwang Y, Wang N et al (2014) The chorioretinal damage caused by different half parameters of photodynamic therapy in rabbits. J Ocul Pharmacol Ther 30(8):642–649
Framme C, Flucke B, Birngruber R (2004) Comparison of reduced and standard light application in photodynamic therapy of the eye in two rabbit models. Graefes Arch Clin Exp Ophthalmol 244(7):773–781
Kang SJ, Grossniklaus HE (2011) Rabbit model of retinoblastoma. J Biomed Biotechnol 2011:1–5
Funding
Research to Prevent Blindness and the Las Madrinas Endowment in Experimental Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Animal experiments
Ethical approval: “All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.”
University of California, Los Angeles: “All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.”
Rights and permissions
About this article
Cite this article
Kim, J.W., Jacobsen, B., Zolfaghari, E. et al. Rabbit model of ocular indirect photodynamic therapy using a retinoblastoma xenograft. Graefes Arch Clin Exp Ophthalmol 255, 2363–2373 (2017). https://doi.org/10.1007/s00417-017-3805-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3805-8