Abstract
Purpose
To investigate whether single-nucleotide polymorphisms (SNPs) known to be strongly associated with the development of age-related macular degeneration (AMD) have an influence on recurrence rate of choroidal neovascularization (CNV) activity during 4-year ranibizumab treatment for exudative AMD.
Methods
This prospective study included 103 treatment-naïve patients (103 eyes) that received initially a loading dose of 3 monthly ranibizumab injections and thereafter, were treated according to an as-needed regimen for a 4-year follow-up period. Baseline values, visual outcome, and recurrence rate were examined. CFH Y402H and ARMS2 A69S polymorphisms were determined and their association with lesion recurrence and visual outcome was analyzed using a one-way analysis of variance (ANOVA) with post hoc comparison tested by Fisher’s LSD method. Multivariate linear regression analysis was then used to identify factors associated with recurrence rate.
Results
The cumulative total mean number of ranibizumab injections at the end of each year of the follow-up was 5.3 ± 1.8, 9.2 ± 2.9, 12.6 ± 4.6, and 15.7 ± 6.1. There was great inter-patient variability. Nineteen eyes (18.5%) did not experience recurrence during the first year, and five (4.8%) still displayed inactive CNV after 4 years of follow-up. No significant association was found between the number of injections and mean best corrected visual acuity (BCVA) change or final BCVA at the end of the study period. Genotypes had no influence on baseline characteristics or visual outcome but a significant association was found between the A69S polymorphism and the number of injections needed by the patients. Homozygous for the T risk allele required more retreatments over the 48-month follow-up.
Conclusions
The ARMS2 A69S polymorphism was associated with CNV recurrence rate in our patient cohort. Prediction of a greater risk of recurrence could help to design more appropriate follow-up treatment strategies for patients with neovascular AMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the last decade, anti-vascular endothelial growth factor (anti-VEGF) therapy has become the gold standard treatment for exudative age related macular degeneration (AMD) based on the results of the MARINA and ANCHOR trials [1, 2]. Effectiveness of this monthly dosing antiangiogenic therapy was evaluated in terms of visual acuity (VA) improvement before optical coherence tomography (OCT) became widespread. The PrONTO study [3] pioneered retreatment based on OCT findings indicative of lesion reactivation. Since then, different OCT-guided variable-dosing regimens have been studied in an attempt to enhance visual outcome while decreasing frequency of injections. Pro re nata (PRN) and “treat and extend” (TEX) regimens have proven to be effective in the mid-term when properly administered and are the most commonly used treatment protocols in clinical practice [4, 5]. Reduction in the number of injections to those strictly necessary is important not only to minimize treatment burden, but also because there is increasing evidence that more frequent dosing of anti-VEGF agents and long-term antiangiogenic therapy are associated with an increased risk of geographic atrophy (GA) and a greater rate of pre-existing GA progression [6,7,8,9,10].
The major challenge associated with anti-VEGF treatment in exudative AMD is the heterogeneity in individual response with respect to visual outcome and also to the need for retreatment due to recurrence of neovascular activity over time [11]. So far, several studies have reported prognostic factors associated with visual outcomes but predictors for recurrence after the initial resolution of exudative changes with anti-VEGF therapy are largely unknown. There is an unquestionable interest in identifying those factors playing a role in a higher individual demand for anti-VEGF agents, not only for patients, clinicians, and sanitary systems planning, but also to guide new therapeutic approaches. Genetic factors are known to be associated with increased risk of developing AMD [12] and may also influence individual anti-VEGF response. Previous studies have looked into effectiveness of anti-VEGF agents in terms of visual outcome or tomographic improvement searching for a possible relationship with genetic variants with controversial results [reviewed in 11–15]. In most of these studies, follow-up periods were limited to 12 months. Since many patients require prolonged or even permanent follow-up because of periodic recurrences, we hypothesized that longer endpoints could be necessary to detect influence of genotypes on the need of retreatments over time. The purpose of this study was to investigate the influence of the two major AMD-susceptibility SNPs in the CFH (Y402H) and ARMS2 (A69S) genes on the number of intravitreal injections required during 4 years of follow-up by naïve patients treated with ranibizumab for exudative AMD in a PRN regimen.
Materials and methods
The study included 103 eyes of 103 non-consecutive treatment-naïve patients recruited between those diagnosed with neovascular AMD and treated at the Ophthalmology Service of the San Carlos Clinical Hospital (Madrid, Spain) who started ranibizumab treatment between May 2010 and April 2011 allowing for at least 4 years of potential follow-up since therapy initiation. The study was conducted in accordance with the tenets of the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of the San Carlos Clinical Hospital. All patients had active choroidal neovascularization (CNV) attributable to AMD confirmed by fluorescein angiography (FA), indocyanine green angiography (ICGA) if indicated, and OCT. Best-corrected visual acuity (BCVA) was determined using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. They received three consecutive, monthly loading intravitreal injections of 0.5 mg ranibizumab (Lucentis, Novartis Pharma AG, Basilea, Switzerland), followed by an as-needed regimen of retreatment with monthly monitoring. Eligibility criteria were: treatment-naïve patients and resolution of retinal exudative changes 1 month after the loading dose. Exclusion criteria were: myopic eyes (3 D spherical equivalent or axial length above 26 mm), any condition different from AMD that could lead to macular edema or CNV, active malignancies, and any previous ophthalmic surgery (except for phacoemulsification if done at least 6 months before the study). No limits on visual acuity were set as either inclusion or exclusion criteria. In cases of bilateral AMD, only the best eye was included in the study.
Monthly evaluation during the follow-up included BCVA measurement, fundus examination and OCT. FA and ICGA were not performed routinely and were reserved to cases with unusual treatment responses or unexpected morphologic changes observed on OCT. Retreatment was applied if intraretinal and/or subretinal fluid on OCT, or new retinal hemorrhage were present. Stable retinal pigment epithelial detachment was not included as a retreatment criterion. Besides routine monthly follow-up, patients were asked to visit our hospital immediately in case of visual loss. Written informed consent for genotyping was obtained from 103 patients who completed the 4-year follow-up and met the specified criteria to be included in the study during the whole period. Genomic DNA was prepared from peripheral blood samples using the EZ1 DNA Blood 350 μl Kit (Quiagen, Germantown, MA, USA) according to the manufacturer’s instructions. Genotyping was performed by polymerase chain reaction (PCR) using gene specific primers as previously described [16, 17], followed by digestion with NlaIII or Fnu4HI enzymes (New England Biolabs, Beverly, MA, USA) and subsequent restriction-fragment length polymorphism (RFLP) analysis. Direct DNA sequencing was carried out on an ABI 310 PRISM sequencer using Genescan and Genotyper software (Applied Biosystems, Foster City, CA, USA).
Statistical analysis was performed using Centurion XVI Statgraphics (StatPoint Technologies, Inc. Warrenton, VA, USA). Quantitative variables are presented as means and standard deviation (SD). Qualitative variables are expressed as a percentage of the study population or the genotype subpopulation. The chi-square test was used to analyze qualitative data. BCVA at each point was compared with baseline BCVA with paired t-test. The association of baseline characteristics to number of injections needed was first evaluated using univariate analysis. For quantitative variables, Pearson correlation test was used. Comparison between groups was performed using a one-way analysis of variance (ANOVA) with post hoc comparisons tested by Fisher’s LSD method. The baseline characteristics with a P value <0.05 in the univariate analysis were included in a multivariate linear regression analysis by stepwise backward selection. Dummy variable coding was employed for genotype. Statistical significance was defined at P < 0.05.
Results
One hundred and three patients were finally included in the analysis. All of them were Caucasian, and 60.2% were women. At presentation, all patients had active subfoveal (80.9%) or juxtafoveal (19.1%) CNV attributable to AMD. Mean age was 80.3 years (SD 7.05), and mean BCVA was 58.8 EDTRS letters (ranging from 17 to 90 letters; SD 14.8). FA classified the lesions as: occult (56.3%), minimally classic (3.9%), classic and predominantly classic (16.5%), retinal angiomatous proliferation (RAP, 19.4%), and polypoidal choroidal vasculopathy (PCV, 3.9%). Sixty patients presented bilateral disease.
Figure 1 shows the change in BCVA during the follow-up period. After loading dose, the mean BCVA increased +7.8 ETDRS letters (SD 11.5) from baseline value (P < 0.005). Vision improvement was inversely and significantly related to initial VA (P < 0.01). Patients with better initial vision (>70 letters) improved a mean of 1.76 letters, patients with baseline vision between 69 and 50 letters improved 6.8 letters, and patients starting treatment with vision below 50 letters gained 15.6 letters. Central retinal thickness decreased from a mean value of 412.3 μm (SD 213.5) at baseline to 287.2 μm (SD 120.6) (P < 0.001) after the loading dose. Vision improvement was maintained until month 24, but the gain was not sustained over the entire follow-up. By month 36, the mean BCVA was still above baseline values although the difference did not reach statistical significance: 63.0 (SD 14.7) vs. 58.8 (SD 14.8) ETDRS letters (P = 0.076). By month 48 it declined back to initial values (59.4, SD 19.4; P = 0.81). At the end of the study period, BCVA remained stable from baseline in 34 patients (33%), improved up to 15 letters in 21 (20.4%), and to 15 or more letters in 19 (18.4%). Fifteen eyes (14.5%) lost ≤15 letters and 14 (13.6%) lost >15 letters.
Mean best-corrected visual acuity (BCVA) at baseline and at each follow-up visit after initial treatment with intravitreal ranibizumab. Visual acuity improvement achieved at 3 months was maintained by month 24, but after 4 years, BCVA returned to baseline values. Vertical lines are standard deviation (SD) of the means. * Significantly different form baseline, P < 0.005
The mean total number of ranibizumab injections during the 48-month follow-up was 15.7 (SD 6.1). By month 12, 24, and 36, patients had received a mean number of injections of 5.3 (SD 1.8), 9.2 (SD 2.9), and 12.6 (SD 4.6), respectively. Most common reasons for retreatment were intra- and subretinal fluid. Inter-individual differences in the total number of injections per eye during the study period were remarkable (range: three to 34 injections). Nineteen eyes (18.5%) did not experience recurrence during the first year after resolution of initial symptoms of CNV with the loading dose. This number was reduced at longer follow-up times: 11 (10.6%), six (5.8%), and five (4.8%) of the total eyes, still displayed inactive CNV after 2, 3, and 4 years, respectively. In contrast, 29 patients (28.1%) required a total of 20 or more retreatments. No significant association was found between the number of injections and final BCVA (P = 0.41) or BCVA change (P = 0.26).
Table 1 shows the distribution of demographic and clinical phenotype data for the AMD patients according to CFH Y402H genotype. The high-risk CC genotype was seen in 26 patients (25.2%) and the overall frequency of the C allele in AMD patients was 51.9%. No statistically significant differences were found among Y402H genotypes in mean age at presentation, gender, rate of bilateral disease, subtype of AMD, and baseline or final BCVA. Regarding follow-up, the number of injections and functional outcome were similar in all groups. The presence of one or two copies of the risk allele had no influence on the number of injections.
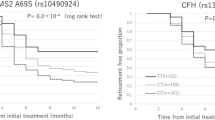
Table 2 displays the same data according to ARMS2 A69S genotype. The high-risk TT genotype was seen in 20 patients (19.6%) and the overall frequency of the T allele in AMD patients was 42.6%. Age, gender, rate of bilateral disease, subtype of AMD, and baseline BCVA were similar in the three groups. During the follow-up, there was a wide variability of letters gained within groups and no significant differences between them were found at the end of the study period. Mean number of injections for patients in GG and GT groups was almost the same not only by month 12, but also at each of the years during the follow-up. Homozygous for the risk allele showed no significant differences with the other groups within the first year but needed more injections afterwards, with increasing difference every year of follow-up (Fig. 2). By the fourth year, there was a mean difference of 4.1 injections between the TT group and the other two groups (P = 0.05; P = 0.03 if TT is compared with grouped GG + GT). Considering that the first three injections are mandatory for all patients (loading dose), this difference means indeed 37% more injections in the TT group.
Mean number of injections during the 4-year follow-up period according to ARMS2 A69S genotype. By month 12, there was no difference between the three genotypes. Afterwards, heterozygous (GT) and non-risk homozygous (GG) followed the same trend, whereas risk homozygous (TT) diverged with increasing differences with the other genotypes over time of follow-up.(▼) TT genotype; (●) GG genotype; (○) GT genotype
Older age, gender, baseline BCVA, CNV type, neovascularization location relative to the center of the fovea, and genotype for A69S and Y402H polymorphisms were analyzed as possible risk factors for recurrence. In univariate analyses, age (P = 0.03), baseline BCVA (P = 0.05), and A69S TT genotype (P = 0.03) showed significant association with the number of retreatments. Multivariate linear regression analysis revealed that only A69S polymorphism was significantly associated (P < 0.01) with recurrence rate.
Discussion
To our knowledge, no other pharmacogenetic studies have previously analyzed the influence of genetic factors on the response of neovascular AMD to long-term ranibizumab treatment. Our results revealed an association between the ARMS2A69S polymorphism and number of ranibizumab injections administered during 4 years of follow-up according to an as-needed regimen. Homozygous for the risk allele needed more injections than heterozygous and non-risk genotype patients over the whole study period. Previous studies [18,19,20,21,22], some of which as the CATT trial included a large cohort of patients [20], failed to demonstrate an association of ARMS2 A69S with the number of anti-VEGF injections. However, the follow-up period in these studies never exceeded 2 years and was limited to 12 months in some of them [18, 20]. As shown in this work, no significant association would be found at these relatively short-term end points because a longer period may be required to detect the influence of the risk allele on recurrence rate.
The A69S polymorphism, located in the exon 1 of the ARMS2 gene, represents the AMD risk haplotype at the ARMS2/HTRA1 locus and is recognized as one of the major susceptibility SNPs for exudative AMD in various ethnic groups [23]. Our results show that the high-risk TT genotype was also associated with clinical recurrences. The biological function of the ARMS2 gene remains unclear and the localization of the encoded protein in the retina is controversial [12]. A recent study [24] describes that monocytes and microglia cells of human retina express ARMS2 protein, which is secreted by a non-classical pathway and attaches to late apoptotic and necrotic cells, recruiting properdin and enhancing C3b opsonization by complement activation, thus augmenting phagocytosis. The high-risk homozygous TT genotype was proved to show ARMS2 protein deficiency so that clearance of apoptotic cells could be impaired. Irrespective of the exact mechanism, it is reasonable to assume that this variant could influence CNV development, as well as CNV reactivation through pathways common to both processes.
Our study found no significant factor associated with recurrence other than the ARMS2 A69S polymorphism. Previous studies have also reported a great inter-individual variability in the number of ranibizumab injections needed by AMD patients [3, 20, 25]. Angiographic lesion type, lesion size or lesion location, baseline visual acuity, or BCVA improvement were not associated with the number of retreatments required by patients, as found in the present work. Good functional response in loading phase does not necessarily mean that frequency of retreatment will be low in the follow-up. Recently it has been reported that older age was a risk factor for recurrence after initial ranibizumab treatment within a 12-month period although the statistical significance disappeared at 2 years [22]. We did not detect significant association of age with recurrence rate but the evaluated period was longer (4 years) and our cohort was older (mean age 80.3 vs. 74.3 years). Therefore, the significance of age as a predictive factor is unclear.
Individual requirements for retreatment are likely to be influenced by other factors in addition to genetic background. Pharmacokinetic studies have shown that ranibizumab injection leads to a complete suppression of VEGF during a mean period of 41 days with inter-individual suppression time ranging from 28 to 67 days, after which VEGF levels returned again to the same individual baseline values [26]. However CNV recurrence occurs in many patients at intervals longer than the upper limit of the effective suppression time. In fact, in our cohort, 18.5% of the total eyes did not require retreatments during the first year of follow-up, and this percentage ranged between 17.5% and 40% in other studies that used an as-needed regimen [3, 22, 25]. Thus, patients receiving fewer than five injections per year have longer quiescent periods than maximum VEGF suppression time suggesting that restoration of basal VEGF levels is a necessary, but not sufficient requisite to reactivate CNV. Stabilization of endothelial barrier function in the vessels of the CNV would prevent exudative signs even in the presence of VEGF. In support of this idea, Ichiyama et al. [27] have recently reported that OCT angiography revealed blood flows in CNV membranes of eyes without clinical signs of exudative activity during 6 months after anti-VEGF treatment.
Regarding visual outcomes, neither ARMS2 A69S nor CFH Y402H polymorphisms were associated with BCVA improvement or visual acuity at the end of the follow-up period. The pharmacogenetic relevance of these SNPs on treatment response in neovascular AMD has been investigated in numerous studies with contradictory evidence. Some studies reported worse response to anti-angiogenic treatment in patients with homozygous risk genotype for Y402H (CC) or A69S (TT), whereas others did not find any significant association between different genotypes and visual outcomes [11,12,13,14,15]. The reasons for these discrepancies are unclear. In the multicenter randomized clinical trial CATT, the lack of association was found regardless of whether patients received monthly or PRN dosing [20].
The efficacy of ranibizumab in maintaining or improving BCVA lasting up to 24 months has been demonstrated in numerous studies, but the evidence on long-term outcomes remains limited. Functional results at two years in our study (+6.8 letters with respect to baseline BCVA) are comparable to those of the PRN corresponding arms of randomized trials as HARBOR (+7.9) [28] and CATT (+6.7) [29], and also to MARINA study (+6.6) with monthly injections [1]. At 4 years, 72% of the eyes could keep a stable or improved BCVA compared to baseline. However, mean BCVA, all group considered, returned to baseline values as found in other recent studies after 4–5 years of anti-VEGF flexible therapy [8, 30,31,32]. A further decline of visual acuity appears to occur after longer follow-up periods [31, 33]. Low monitoring frequency and/or under-treatment have been suggested as potential causes why early visual gains are not maintained over long follow-up periods [31, 33]. In the present study, monthly monitoring was performed, but even in these conditions 61% of the eyes did not preserve the visual improvement achieved after the loading dose. The PRN regimen is a reactive treatment method in which anti-VEGF injections are administered when CNV is already active. Hence, delay in treating recurrent exudation could cause damage to the retina. TEX and continuous fixed-interval dosing are instead proactive regimens that intend to anticipate injections to possible recurrences and exclude under-treatment risk. However, administration of maintenance injections regardless of disease activity can be considered overtreatment for those patients who do not show exudative changes after the loading dose for periods longer than the scheduled intervals for retreatment. More frequent dosing of anti-VEGF agents has been associated with an increased risk of geographic atrophy [6], and there is increasing evidence that a large number of patients show macular atrophy after long-term antiangiogenic therapy [7,8,9, 31,32,33]. Hence, it would be important not to undertreat, but also not to over treat in a chronic disease as neovascular AMD. The identification of the A69S polymorphism as a factor associated to recurrence may help to design more appropriate strategies for treatment and follow-up planning.
The long follow-up and recruitment criteria of this study, mainly focused on ranibizumab requirements rather than vision gains by consecutive patients, allowed us to find a difference in need for retreatment based on genotype for ARMS2 A69S polymorphism. The major limitation is the relatively small sample size. Homogeneity of retreatment criteria by same staff members in a single center is a strength of this study. In addition, anti-VEGF treatment is covered by the National Health Insurance in Spain and patients have unrestricted access to treatment. Therefore, our study is not biased by limitations in the access of patients to long-term therapy.
In conclusion, this 4-year study shows that there is a relationship between genotype for the ARMS2 A69S polymorphism and the number of ranibizumab injections required over the entire follow-up period. Visual improvement and final visual acuity are independent of the number of injections.
References
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, ANCHOR Study Group (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116(57–65):e5
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W et al (2009) A variable-dosing regimen with intravitreal ranibizumab for neovascular agerelated macular degeneration: year 2 of the PrONTO study. Am J Ophthalmol 148:43–58.e1
Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R et al (2014) Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol 98:1144–1167
Stewart MW (2015) Individualized treatment of neovascular age-related macular degeneration: what are patients gaining? Or losing? J Clin Med 4:1079–1101
Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA et al (2014) Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 121:150–161
Bhisitkul RB, Mendes TS, Rofagha S, Enanoria W, Boyer DS, Sadda SR, Zhang K (2015) Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol 159:915–924
Maguire MG, Martin DF, Ying GS, Jaffe GJ, Daniel E, Grunwald JE et al (2016) Five-year outcomes with anti-vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology 123:1751–1761
Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ et al (2017) Incidence and growth of geographic atrophy during 5 years of comparison of age-related macular degeneration treatments trials. Ophthalmology 124:97–104
Gemenetzi M, Lotery AJ, Patel PJ (2017) Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye (Lond) 31:1–9
Finger RP, Wickremasinghe SS, Baird PN, Guymer RH (2014) Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol 59:1–18
Lambert NG, Singh MK, ElShelmani H, Mansergh FC, Wride MA, Padilla M et al (2016) Risk factors and biomarkers of age-related macular degeneration. Prog Retin Eye Res 54:64–102
Dedania VS, Grob S, Zhang K, Bakri SJ (2015) Pharmacogenomics of response to anti-VEGF therapy in exudative age-related macular degeneration. Retina 35:381–391
Hu Z, Xie P, Ding Y, Yuan D, Liu Q (2015) Association between variants A69S in ARMS2 gene and response to treatment of exudative AMD: a meta-analysis. Br J Ophthalmol 99:593–598
Hong N, Shen Y, Yu CY, Wang SQ, Tong J (2016) Association of the polymorphism Y402H in the CFH gene with response to anti-VEGF treatment in age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol 94:334–345
Brantley MA Jr, Fang AM, King JM, Tewari A, Kymes SM, Shiels A (2007) Association of complement factor H and LOC387715 genotypes with response of exudative age-related macular degeneration to intravitreal bevacizumab. Ophthalmology 114:2168–2173
Pulido JS, McConnell JP, Lennon RJ, Bryant SC, Peterson LM, Berger PB, Somers V, Highsmith WE (2007) Relationship between age-related macular degeneration-associated variants of complement factor H and LOC387715 with coronary artery disease. Mayo Clin Proc 82:301–307
Teper SJ, Nowinska A, Pilat J, Palucha A, Wylegala E (2010) Involvement of genetic factors in the response to a variable-dosing ranibizumab treatment regimen for age-related macular degeneration. Mol Vis 16:2598–2604
Orlin A, Hadley D, Chang W, Ho AC, Brown G, Kaiser RS, Regillo CD et al (2012) Association between high-risk disease loci and response to anti-vascular endothelial growth factor treatment for wet age-related macular degeneration. Retina 32:4–9
Hagstrom SA, Ying GS, Pauer GJ, Sturgill-Short GM, Huang J, Callanan DG et al (2013) Pharmacogenetics for genes associated with age-related macular degeneration in the comparison of AMD treatments trials (CATT). Ophthalmology 120:593–599
Park UC, Shin JY, McCarthy LC, Kim SJ, Park JH, Chung H, Yu HG (2014) Pharmacogenetic associations with long-term response to anti-vascular endothelial growth factor treatment in neovascular AMD patients. Mol Vis 20:1680–1694
Kuroda Y, Yamashiro K, Miyake M, Yoshikawa M, Nakanishi H, Oishi A et al (2015) Factors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment: a retrospective cohort study. Ophthalmology 122:2303–2310
Tong Y, Liao J, Zhang Y, Zhou J, Zhang H, Mao M (2010) LOC387715/HTRA1 gene polymorphisms and susceptibility to age-related macular degeneration: a HuGE review and meta-analysis. Mol Vis 16:1958–1981
Micklisch S, Lin Y, Jacob S, Karlstetter M, Dannhausen K, Dasari P et al (2017) Age-related macular degeneration associated polymorphism rs10490924 in deficiency of a complement activator. J Neuroinflammation 14:4
Arias L, Roman I, Masuet-Aumatell C, Rubio MJ, Caminal JM, Catala J, Pujol O (2011) One-year results of a flexible regimen with ranibizumab therapy in macular degeneration: relationship with the number of injections. Retina 31:1261–1267
Saunders DJ, Muether PS, Fauser S (2015) A model of the ocular pharmacokinetics involved in the therapy of neovascular age-related macular degeneration with ranibizumab. Br J Ophthalmol 99:1554–1559
Ichiyama Y, Sawada T, Ito Y, Kakinoki M, Ohji M (2017) Optical coherence tomography angiography reveals blood flow in choroidal neovascular membrane in remission phase of neovascular age-related macular degeneration. Retina 37:724–730
Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z, Rubio RG, Lai P, HARBOR Study Group (2014) Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 121:2181–2192
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE et al (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119:1388–1398
Rasmussen A, Bloch SB, Fuchs J, Hansen LH, Larsen M, Lacour M, Lund-Andersen H, Sander B (2013) A 4-year longitudinal study of 555 patients treated with ranibizumab for neovascular age-related macular degeneration. Ophthalmology 120:2630–2636
Gillies MC, Campain A, Barthelmes D, Simpson JM, Arnold JJ, Guymer RH et al (2015) Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology 122:1837–1845
Arevalo JF, Lasave AF, Wu L, Acón D, Berrocal MH, Díaz-Llopis M et al (2016) Intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration: 5-year results of the pan-American collaborative retina study group. Retina 36:859–867
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group (2013) Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 120:2292–2299
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Valverde-Megías, A., Veganzones-de-Castro, S., Donate-López, J. et al. ARMS2 A69S polymorphism is associated with the number of ranibizumab injections needed for exudative age-related macular degeneration in a pro re nata regimen during 4 years of follow-up. Graefes Arch Clin Exp Ophthalmol 255, 2091–2098 (2017). https://doi.org/10.1007/s00417-017-3748-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3748-0