Abstract
Purpose
To analyze the inter-methods agreement in arteriovenous ratio (AVR) evaluation between spectral-domain optical coherence tomography (SD-OCT) and Dynamic Vessel Analyzer (DVA).
Methods
Healthy volunteers underwent DVA and SD-OCT examination. AVR was measured by SD-OCT using the four external lines of the optic nerve head-centered 7-line cube and by DVA using an automated AVR estimation. The mean AVR was calculated, twice, separately by two independent readers for each tool.
Results
Twenty-two eyes of 11 healthy subjects (five women and six men, mean age 35) were included. AVR analysis by DVA showed high inter-observer agreement between reader 1 and 2, and high intra-observer agreement for both reader 1 and reader 2. With regard to AVR analysis on SD-OCT, we found high inter-observer agreement between reader 1 and 2, and low intra-observer agreement for reader 2 but high intra-observer agreement for reader 1. Overall, the mean AVR measured on SD-OCT turned out to be significantly higher than mean AVR measured through DVA (reader 1, 0.9023 ± 0.06 vs 0.8036 ± 0.08; p < 0.001, and reader 2, 0.9067 ± 0.06 vs 0.8083 ± 0.05; p= 0.003).
Conclusions
No inter-method agreement in AVR could be detected in the present study due to bias in measurements (shift between DVA and SD-OCT). We found significant difference in the two noninvasive methods for AVR measurement, with a tendency for SD-OCT to overestimate retinal vascular caliber in comparison to DVA. This may be useful for achieving greater accuracy in the evaluation of retinal vessel in ocular as well as systemic diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retinal vessel diameter is an important measure for retinal vascular diseases (i.e., retinal arterial and vein occlusion) and for systemic disorders (i.e., diabetes and hypertension) [1–3]. Changes due to vascular abnormalities and those related to age are often subtle and can be overlooked when using visual grading systems. Different methods could be used in order to calculate the diameter of the retinal vessels, such as fundus color photographs, Zeiss retinal vessel analyzer, fluorescein angiograms, and spectral-domain optical coherence tomography scans (SD-OCT) [4–6].
Goldenberg et al. [6] recently proposed a new noninvasive method for blood vessel manual measurement and arteriovenous ratio (AVR) calculation by means of optic nerve head-centered SD-OCT. In particular, two cubes of horizontal scans are placed at the superior and inferior borders of the optic disk to evaluate and measure vessels using image J software, and then calculate AVR [6]. On the other hand, a Dynamic Vessel Analyzer (DVA, Imedos Systems, Ltd, Jena, Germany) allows automated AVR estimation through static analysis, and its software has been validated for retinal vessel assessment [7, 8]. This tool shows great standardization, using a circular grid to measure vessel diameter only in a concentric ring segment one half disc diameter distant from the outer boundaries of the optic nerve head and one half disc diameter in width. AVR is computed by the measurement of central retinal artery equivalent (CRAE) and central retinal vein equivalent (CRVE) diameter, which results from the calculation of individual diameters of their visible branches around the optic nerve head [1].
The aim of this study is to analyze the inter-methods agreement in AVR evaluation between optic nerve head-centered SD-OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) and DVA.
Methods
Study participants
Healthy volunteers >18 years old were enrolled in this study between February 2015 and November 2015 at the Medical Retina and Imaging Unit of the University Vita-Salute, IRCCS Ospedale San Raffaele in Milan. All patients should have normal axial length (normal range: 21-27). Exclusion criteria were uncontrolled systemic hypertension or other systemic diseases potentially influencing the diameter of retinal vessels, and any eye pathology.
All participants underwent DVA and optic nerve head-centered SD-OCT along with a complete ophthalmic evaluation, including assessment of best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, and indirect fundus ophthalmoscopy. Informed consent was obtained from all subjects in agreement with the Declaration of Helsinki for research involving human subjects. Local Ethics Committee approval was obtained for this study.
Measurement of blood vessels with spectral-domain optical coherence tomography
In order to take into consideration exactly the same vessels with two methods, OCT analysis was performed immediately after AVR measurement with DVA.
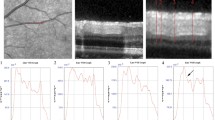
The diameters of all the arteries and veins were measured using the method described by Goldenberg et al. [6]. All subjects underwent optic nerve head-centered SD-OCT with two 7-line cube scans at the superior and inferior borders of optic disc in which the scans were 30° in size and of high resolution, 100 Automatic Real Time (ART) averaging for maximal quality and resolution, and had a 240 μm interscan interval [Fig. 1] Each raster (from 1 to 7) was placed at a known constant distance from the optic disk border (rasters 1–7 were at distances of 0, 240, 480, 720, 960, 1200, and 1440 μm respectively). Only the four most external lines of the 7-line cube were used for the analysis; combining SD-OCT with the infrared image made it possible to identify each artery and vein on the selected raster [Figure 2]. The mean diameter of arteries and veins was manually calculated, twice, separately for each of the rasters by two independent readers (LB and LT) using Image J (ImageJ version 1.47, National Institutes of Health, Bethesda, MD, USA; available at http://imagej.nih.gov/ij/). Vessels that were not clearly distinguishable were not included in the analysis. The mean diameter of arteries and veins was calculated for each raster separately; after that, AVR was obtained.
Two exclusion criteria were added as a result of some technical difficulties that were encountered during the measurements. The first was artery–vein intertwining, and the second was related to cases of the presence of a common trunk where the scan raster passed through it.
Measurement of blood vessels with Dynamic Vessel Analyzer
In each subject, a 50-degree fundus photograph was taken using the FF450 retinal camera (Zeiss AG, Jena, Germany), contained in the Dynamic Vessel Analyzer system. VISUALIS and VesselMap Software (Imedos Systems, Ltd, Jena, Germany) allowed analysis of these photographs. Principles of static vessel analysis have been described earlier [7]. Using an optic disk-centered image, the papilla was marked and the software created an area of one half to one disk diameter from its center to measure all vessels. Arteries and veins were selected manually [Fig. 3].
In each subject, the CRAE and the central retinal vein equivalent CRVE, which relate to the diameter of central retinal artery and vein respectively, were calculated. The mean AVR (CRAE/CRVE) was calculated, twice, separately by two independent readers (LB and LT).
Statistical analysis
Data were described using mean and standard deviation. The difference between AVR measured by DVA and by ImageJ on SD-OCT was analyzed using paired sample t-test. An interclass correlation coefficient (ICC) and its 95% confidence interval (95% CI) was used to evaluate the agreement between the two measurements, considering the effect of reading and reader as a random effect [12]. All test were two-sided and a significance level of 5% was considered. Analyses were performed using SAS V9.2 (SAS Institute, Cary, NC, USA).
Results
Twenty-two eyes of 22 healthy subjects (nine female and 13 male, age 24.7 ± 3 years) were included in the study. Twenty-two eyes of 22 healthy subjects were used in the evaluation of AVR by two methods (DVA and SD-OCT), observed by two different readers and two readings. The mean AVR across readers and readings were calculated. This results in 176 measurements. Mean BCVA was 0.00 LogMAR. Fundus biomicroscopy and SD-OCT scans did not show pathologic findings in all participants.
With OCT a mean AVR of 0.92 (SD 0.06) was found, which was significantly higher than the mean AVR of 0.80 (SD 0.08) found using DVA (p-value <0.0001). These differences were similar across any reading and reader (Table 1): reader 1 first reading (LB_1) presented a mean AVR of 0.90 (SD 0.06) with OCT, and a mean AVR of 0.80 (SD 0.08) with the DVA; and in the second reading (LB_2), the mean AVR of reader 1 was 0.90 (SD 0.06) with the OCT and 0.80 (SD 0.08) with the DVA; the mean AVR for reader 2 first reading (LV_1) was 0.96 (SD 0.06) with the OCT and 0.80 (0.08) with the DVA, and mean AVR at OCT and DVA for second reading of reader 2 (LV_2) was 0.90 (SD 0.06) and 0.80 (SD 0.08) respectively.
An ICC of 0.90 (95% CI 0.88-0.92) was found, showing a very good agreement between the two measures.
Discussion
In this study, we evaluated the inter-methods agreement in AVR evaluation between optic nerve head-centered SD-OCT and DVA. Even if no inter-method agreement in AVR could be detected in the present study due to bias in measurements (shift between DVA and SD-OCT), we found significant difference in the AVR measurement using two noninvasive methods, in which the measurement by SD-OCT, following the technique of Goldenberg et al. [6] seems to overestimate the vascular caliber compared to DVA.
In order to compare these techniques for AVR measurement, we took in consideration only the four outer lines of the 7-line cube at the SD-OCT, instead of all seven lines as done by Goldenberg et al. [6] In particular, the area analyzed by the four outer lines on the SD-OCT corresponds to the one investigated by the DVA, as it allows the measure of vessel diameter only in a concentric ring segment one half disc diameter distant from the outer boundaries of the optic nerve head and one half disc diameter in width. We cannot exclude that the difference in measurements recorded in our study could be ascribed to the smaller area analyzed by DVA as compared to the area originally investigated in the OCT study by Goldenberg et al. [6]; however, it seems unlikely as DVA uses a validated method for AVR measurement [5–11].
The mean AVR (mean across reader and readings) measured on SD-OCT turned out to be significantly higher than mean AVR measured through DVA, the mean AVR measurement by Goldenberg et al. technique [6] showed lower inter-observer and intra-observer agreement. Of note, DVA relies on Visualis and Vesselmap, which are standardized softwares, thoroughly validated and used by different groups for calculation of AVR [5, 7–9]. Given the reliability of the calculation method by semi-automated and validated DVA software [5, 10, 11]), we can state that, based on our results, DVA does not underestimate AVR value; consequently, the method proposed by Goldenberg using SD-OCT, which is a recently developed and non-validated technique, should be considered to overestimating AVR values. Further studies with larger sample are needed in order to confirm our results about overestimation of AVR value using SD-OCT method, and to rule out any potential bias. The comparison between DVA and SD-OCT in the AVR measurement showed a significant difference that should be considered for achieving greater accuracy in evaluation of retinal vessels in ocular as well as systemic diseases. In particular, the lack of agreement of these two techniques is essential for an accurate clinical evaluation. The importance of CRAE, CRVE, and AVR in clinical practice was found for predicting hypertension where generalized arteriolar narrowing as reduction in CRAE was associated with an increased risk in stroke [12, 13]. While in diabetes an increase of CRVE was associated with increased incidence of diabetic retinopathy (DR), progression of DR, including progression to proliferative DR and macular edema, but was unrelated to CRAE. Moreover, retinal vessel parameters have been shown to be of clinical value in ocular diseases such as glaucoma, age-related macular degeneration (AMD) and retinal vein occlusion (RVO). An increased risk of open-angle glaucoma has been associated with a decrease in CRAE, while an increased CRAE was found in early AMD, and a reduced AVR, indicating a general enlargement of the retinal venous network, was found in eyes with RVO [14–17],
The present study has obvious limitations mainly related to the small number of included eyes.
In conclusion, the AVR evaluation between optic nerve head-centered SD-OCT and DVA showed a significant difference in the AVR measurement using two noninvasive methods, in which the measurement by SD-OCT, following the technique of Goldenberg et al. [6], seems to overestimate the vascular caliber compared to DVA. Further studies evaluating larger sample sizes are needed to confirm these preliminary results.
References
Knudston JM, Lee EK, Hubbard LD et al (2003) Revised formulas for summarizing retinal vessel diameter. Curr Eye Res 27:143–149
Klein R, Myers CE, Lee KE, Gangnon R, Klein BE (2012) Changes in retinal vessel diameter and incidence and progression of diabetic retinopathy. Arch Ophthalmol 130:749–755
Wang JJ, Liew G, Klein R et al (2007) Retinal vessel diameter and cardiovascular mortality: pooled data analysis from two older populations. Eur Heart J 28:1984–1992
Seoung-Bock L, Ki Bang U, Chul H (1998) Retinal vessel diameter in normal and primary open-angle glaucoma. Korean J Ophthalmol 12:51–59
Wong TY, Knudtson MD, Klein R et al (2004) Computer-assisted measurement of retinal vessel diameter in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology 111:1183–1190
Goldenberg D, Shahar J, Loewenstein A et al (2013) Diameters of retinal blood vessels in a healthy cohort as measured by spectral domain optical coherence tomography. Retina 33:1888–1894
Garhofer G, Bek T, Boehm AG et al (2010) Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol 88:717–722
Brueckmann A, Seeliger C, Schwefer M et al (2013) The arterio-venous ratio of retinal vessels in the first trimester as a predictor for preeclampsia. Pregnancy Hypertens 3:84–85
Terai N, Haustein M, Siegel A et al (2014) Diameter of retinal vessels in patients with diabetic macular edema is not altered by intravitreal ranibizumab (Lucentis). Retina 34:1466–1472
Heitmar R, Kalitzeos AA, Patel SR et al (2015) Comparison on subjective and objective methods to determine the retinal arterio-venous ratio using fundus photography. J Ophthalm 8:252–257
Jürgens C, Ittermann T, Vötze H, Tost F (2014) Comparison of two non-mydriatic fundus cameras to obtain retinal arterio-venous ratio. Ophthalmic Epidemiol 21:333–338
Shoukri MM (2010) Measures of interobserver agreement and reliability, 2nd edn. CRC Press, Boca Raton
Wong T, Klein R, Sharett A et al (2003) The prevalence and risk factors of microvascular abnormalities in older people: the cardiovascular health study. Ophthalmology 110:658–666
Mitchell P, Wang JJ, Wong TY, Sith W, Klein R, Leeder SR (2005) Retinal microvascular sings and risk of stroke and stroke mortality. Neurology 65:1005–1009
Yang K, Zhan SY, Liang YB et al (2012) Association of dilated retinal arteriolar calibre with early age-related macular degeneration: the Handan Eye Study. Graefes Arch Clin Exp Ophthalmol 250:741–749
Kawasaki R, Wang JJ, Rochtchina E, Lee AJ, Wong TY, Michell P (2013) Retinal vessel calibre is associated with the 10-year incidence of glaucoma: the Blue Mountains Eye Study. Ophthalmology 120:84–90
Corvi F, Querques G, La Spina C, Lattanzio R, Bandello F (2015) Dynamic and static retinal vessel analyses in patients with macular edema secondary to retinal vein occlusion. Retina 35(10):2052–2059
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no proprietary, funding, or conflicts of interest to disclose.
Informed consent was obtained from all subjects in agreement with the Declaration of Helsinki for research involving human subjects.
No animals were involved in the research.
Funding
No funding was received for this research.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Benatti, L., Corvi, F., Tomasso, L. et al. Inter-method agreement in retinal blood vessels diameter analysis between Dynamic Vessel Analyzer and optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 255, 1079–1083 (2017). https://doi.org/10.1007/s00417-017-3602-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3602-4