Abstract
Purpose
The aim was to investigate the involvement of intrinsically photosensitive retinal ganglion cells (ipRGCs) in patients with manifest glaucoma and ocular hypertension (OH) using specific parameters of the pupil light reflex to chromatic stimuli.
Methods
Twenty-five patients with manifest glaucoma, 16 patients with OH and 16 healthy control subjects were stimulated with 28 lx red (605 nm) or blue (420 nm) light with a duration of either 1 s or 4 s. The consensual pupil light reaction was recorded by means of infrared pupillometry. The maximal relative amplitude (MRA), the post-illumination pupil response PIPRblue-red, and the slope of the response during exposure to the 4 s red stimulus (SORRS) were calculated and compared using ANOVA and Tukey-Kramer post-hoc tests. Correlations between pupil parameters and visual field defects were analyzed using Pearson correlation coefficient r.
Results
PIPRblue-red was reduced in glaucoma patients compared to normals (p < 0.001) and OH (p < 0.01). There was no significant difference between OH and normals. Glaucoma patients showed additionally reduced MRA for red and blue light (p < 0.05) and a pupillary escape during exposure to red light (increased SORRS, p < 0.0005). This pupillary escape could also be seen in single subjects with OH. Significant correlations between pupil parameters and visual field defects were detected.
Conclusions
The reduced PIPRblue-red indicates a characteristic impairment of the melanopsin-driven pathway of ipRGCs in glaucoma patients, whereas the reduced MRA and increased SORRS suggest a disturbed synaptic function and altered interaction between outer photoreceptors, RGCs, and ipRGCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is a heterogeneous group of diseases characterized by a progressive optic neuropathy with a decline of retinal ganglion cells leading to visual field defects or even blindness. Due to demographic changes, it is becoming increasingly prevalent and socioeconomically relevant. Early diagnoses as well as reliable follow-ups are desirable to prevent irreversible damage to the optic disc. Higher intraocular pressure (IOP), greater cup-disc-ratio, thinner central corneal measuremen,t and older age are reported to be the most important risk factors for a progression of glaucoma or ocular hypertension [1]. Ocular hypertension (OH) is defined as an elevated IOP >21 mmHg without any morphological damage to the optic disc. Although these patients are considered “healthy,” it is known that OH can convert into glaucoma. The Ocular Hypertension Treatment Study revealed a cumulative risk of 9.5 % for the development of glaucoma within 60 months [2]. A clear prediction of the individual risk is not possible at the moment, and the attending physician must decide whether to initiate or withhold an IOP-lowering therapy in these patients.
Our group demonstrated in a recent study that pupil campimetry is able to show a significant reduction in pupil contraction amplitude in glaucoma patients compared to normals - nevertheless the sensitivity and specificity were not suitable for use as a screening method [3]. However, since the recent discovery of melanopsin-containing retinal ganglion cells and their involvement in the pupillary response, colour pupillography has gained increasingly in importance. This small subpopulation of retinal ganglion cells is intrinsically photosensitive (intrinsically photosensitive retinal ganglion cells = ipRGCs) due to the expression of the photopigment melanopsin [4], in addition to receiving input from the rod and cone phototransduction pathway [5]. The information of both the intrinsic and synaptic pathways, is integrated and transmitted to numerous brain regions involved in both non-image and image-forming vision. So as well as being involved in the circadian photoentrainment via the suprachiasmatic nucleus (SCN), ipRGCs also transmit information to the olivary pretectal nucleus (OPN) and consequently contribute to the pupil light reflex [5–10]. They are thought to determine the pronounced post-illumination pupil response (PIPR) following a bright blue light stimulus [5, 9] with a maximum of spectral sensitivity at around 480 nm [5, 8, 11, 12]. Moreover, there is convincing evidence that ipRGCs take part in the sleep/wake state, neuroendocrine systems, anxiety, light aversion, and even contribute to the thalamo-cortical visual system [10, 13]. Contrary to previous thinking, the ipRGC population consists of several subtypes with distinct responses to light. Controlling pupil constriction seems to be predominantly guided by the subtype M1 [10]. Nevertheless it remains unclear to what extent the subtypes of retinal ganglion cells and ipRGCs are involved and impaired in glaucoma. It has been proposed that ipRGCs are highly resistant to injury-induced damage as in vitro rodent retinas did not show a significant ipRGC cell loss after chronic ocular hypertension over 12 weeks [14]. On the other hand, Kankipati et al. [15] demonstrated a significant reduction in the ipRGC-driven PIPR in glaucoma patients (n = 16) and the Feigl group [16] confirmed a reduced PIPR in moderate and severe glaucoma patients but not at an early stage of the disease (n = 25).
In this project we analyzed pupil responses to specific chromatic light stimuli in two different patient groups (manifest glaucoma and ocular hypertension) and compared the results to those of an age-matched healthy control group. In the glaucoma group, our aim was to investigate the involvement of the melanopsin-driven pathway of ipRGCs and to identify additional parameters above PIPR of the pupillary light reflex being impaired in glaucoma to learn more about the interactions of the different receptors (ipRGCs, RGCs, rods/cones) and to strengthen the role of pupillography in glaucoma diagnostics.
To the best of our knowledge, no one has examined the function of ipRGCs in patients with ocular hypertension. Therefore, we additionally examined this patient group exploratively to find out whether any difference between the OH group and normal subjects can be seen.
Methods
Participants
Twenty-seven patients with manifest glaucoma, 16 patients with ocular hypertension and 16 age-matched healthy subjects were examined. Patients with glaucoma and ocular hypertension were recruited from the Glaucoma Clinic and the data base of the University Eye Hospital Tübingen; the healthy control group was recruited from employees and acquaintances of the University. All subjects underwent an ophthalmological examination including visual acuity, swinging flashlight test, slit lamp examination, IOP measurement and fundus ophthalmoscopy; glaucoma and ocular hypertension patients were additionally tested with standard 30° static perimetry (Octopus perimeter, except for one patient examined with Oculus Twinfield). Included in the glaucoma group were 27 patients with manifest glaucoma according to the Guidelines of the European Glaucoma Society (open-angle glaucoma, pigment dispersion glaucoma or normal-pressure glaucoma) showing characteristic glaucomatous optic disc changes (cup-disc-ratio 0.6–1.0, neuroretinal rim thinning, asymmetries; see Table 1) in the funduscopy and glaucomatous visual field defects of different severity. Thus, our glaucoma group represented a typical cohort of an advanced stage of the disease, and all were treated with IOP-lowering eyedrops. Two of them were excluded due to previous glaucoma surgery on the measured eye to avoid any effect caused by surgery on their pupil reactions. For the analysis, therefore, the glaucoma study group consisted of 25 glaucoma patients (seven males, 18 females; age: 61.8 ± 12.9 years). Ocular hypertension was defined by an IOP > 21 mmHg (before tension-lowering therapy) without any glaucomatous damage to the optic disc in the funduscopy (intact neuroretinal rim, no peripapillary hemorrhages, no pallor) and a normal visual field (six males, ten females; age: 57.9 ± 15.4). The control group consisted of 16 healthy subjects with normal ophthalmological findings (five males, 11 females; age: 57.8 ± 13.1).
All visual fields were precisely analyzed and scored on the basis of the monocular Esterman grid [17] according to the extent of the scotoma (adjusted percentage of scotoma of the 30° visual field, increasing score means increasing visual field defects). Then the glaucoma patients were categorized into three subgroups (called “defect groups”): scotoma score >65 % = defect group 4, scotoma score 30–64 % = defect group 3, and scotoma score 1–29 % = defect group 2. All subjects of the ocular hypertension group (score 0 %) were named defect group 1 and all healthy subjects defect group 0.
Table 1 shows the patients characteristics of the glaucoma group in detail.
Written informed consent was obtained from all individual participants included in the study. The study was approved by the local institutional ethics committee and followed the tenets of the Declaration of Helsinki.
Colour pupillography
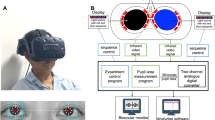
One eye was stimulated under mesopic conditions with 28 lx bright light, either red (605 nm ± 20 nm) or blue (420 nm ± 20 nm), provided by a mini-Ganzfeld colour LED stimulator (CH Electronics) (see Fig. 1). The consensual pupil light reaction of the fellow eye was recorded by means of infrared pupillometry (Compact Integrated Pupillograph CiP by AMTech Germany) over a period of 16 s including a pre-stimulus time of 5 s. Stimulus duration was either 1 s or 4 s. The interstimulus interval was at least 5 s providing that the approximate baseline value was reached before starting a new measurement. Changes in pupil diameter were recorded with a spatial resolution of 0.05 mm and a temporal resolution of 250 Hz (4 ms). The subjects were asked to avoid blinking and eye movements during the measurements if possible. For each stimulus condition four good quality pupillograms with few artefacts were saved and averaged. Measurements were mainly performed in the mornings.
Statistical analysis
All pupillograms were analyzed off-line. Blink artefacts were removed manually with the support of the JMP statistical software used. Examined parameters were baseline pupil diameter (mm), maximal relative amplitude MRA (%), the PIPRblue-red (AUC), and the slope of the fitted line to the response during exposure to the 4 s red stimulus (SORRS). PIPRblue-red was considered as the target variable.
Baseline pupil diameter was determined by the median pupil diameter during the pre-stimulus time of 5 s. The absolute pupil diameter at any given time was converted into the relative amplitude by dividing by the baseline pupil diameter:
The post-illumination pupil response (PIPR) was calculated by adding the relative amplitudes from time = t0 to time = t1 and dividing the sum by 250 (due to the temporal resolution of 4 ms): \( {\sum}_{t0}^{t1} relative\; amplitude*\;\frac{1}{250} \). This value approximately represented the area over the pupillogram, which is statistically equal to the Area under the Curve (AUCt0-t1s). We calculated three AUCs for the 1 s stimulus condition and two AUCs for the 4 s stimulus condition:
For the 4 s red stimulus the dynamics of the pupillographic curve during the stimulus were additionally analyzed for each subject, as shown in Fig. 2: Within an interval from 5 to 10 s, a line was fitted between the first maximum after stimulus onset (latest at time 6.5 s) and the end of the plateau, which is characterized as a predominant redilation after stimulus offset (from 9 to 10 s). Then the corresponding slope was calculated for each subject, and the data analyzed for the three groups.
Statistical analysis was done using the JMP 11.0 statistical software (SAS Institute, Cary, NC, U.S.A.). Differences in glaucoma patients, patients with OH, and healthy controls were tested using one-way analysis of variance (ANOVA). Assumptions for ANOVA were checked and assumed. Post-hoc comparisons were performed using the Tukey-Kramer procedure. To adjust for multiple testing, a p value < 0.017 (for the 1 s stimulus PIPR), p < 0.025 (for the 4 s stimulus PIPR), and p < 0.05 (for MRA and SORRS), respectively, were considered significant.
Additionally, for the 4 s stimulus condition, correlations between the pupil parameters (MRA, PIPR, SORRS) and the visual field defects were analyzed using Pearson correlation coefficient r.
As the right and left eye from an individual cannot be assumed to be independent, we used only one eye from each subject for the statistical evaluation. In the glaucoma group, if both eyes fulfilled the inclusion criteria, we examined the worse one. In the ocular hypertension and healthy control group we randomized the subjects equally to left and right eye.
Results
All subjects were examined by means of full-field colour pupillography under mesopic conditions. In the first trial the stimulus duration was 1 s, in the second trial, 4 s.
A pre-stimulus time of 5 s served as baseline, and the relative amplitudes (see Methods) were used for further analysis. Figure 3 shows the mean relative pupillographic curves obtained from all subjects for red and blue stimuli for either glaucoma patients vs. normals (Fig. 3 a + c) or ocular hypertension patients vs. normals (Fig. 3 b + d).
Relative amplitude (%) versus time (s) for each subject group; stimulation with either red (605 nm) or blue (420 nm) 28 lx light, stimulus length was either 1 s (a + b) or 4 s (c + d). a + c: dotted line = normals (n = 16); solid line = glaucoma (n = 25); b + d: dotted line = normals (n = 16); solid line = ocular hypertension (n = 16)
Maximal relative amplitude (MRA) (%)
We found significantly reduced maximal amplitudes in glaucoma patients, especially when stimulating with blue for both stimulus conditions, but also when stimulating with red (p < 0.05). This can be seen in Table 2.
PIPRblue-red
Using the 1 s stimulus condition, we found a highly significant reduction of the PIPRblue-red (AUC) in glaucoma compared to normals (p < 0.001) and OH (p < 0.01) for all three AUC calculations as shown in Table 3. There was no significant difference between OH patients and normals.
Using the 4 s stimulus condition, a reduced PIPRblue-red could only be found if the AUC was calculated from 11 to 16 s, instead of directly after stimulus offset (Table 4). This might be due to the additionally observed differences in the pupillary dynamics during the exposure to the 4 s red stimulus between the three groups.
Slope (SORRS)
To compare these different pupillary dynamics during stimulation with a 4 s red light, the individual slopes of the fitted lines to the pupillographic curves were calculated and statistically analyzed. Many glaucoma patients showed a pupillary escape behavior, where the initial pupil contraction is not maintained, and it slowly redilates during light exposure, as can be seen in Fig. 4. The mean of the slopes is significantly steeper compared to normals (glaucoma 2.40 ± 1.81; normals 0.04 ± 1.54, p < 0.0005, Table 5). The mean of the OH group (1.26 ± 2.00) also showed a tendency towards steeper slopes than normals, but this was not statistically significant. However, from the distribution of the slopes, shown in Fig. 5, it can be seen that there are two subjects with OH that stand out, with elevated slope values up to a maximum of 5.21, whereas the maximum of all slopes of the normal group was not higher than 1.82. On the other hand, the minimum was −1.51 in the manifest glaucoma group, −1.75 in the OH patients and −2.53 in normals.
Correlations between pupil parameters and visual field defects
For the 4 s stimulus condition, Pearson correlation coefficient r was calculated to assess the correlation between pupil parameters and visual field defects. When all subjects were analyzed, the extent of the visual field defects was positively correlated to the SORRS (r = 0.48; 95 % confidence interval CI = 0.25,0.66; p < 0.001) and negatively correlated to the MRAs for red (r = −0.45; CI = −0.64,−0.22; p < 0.001) and blue stimulation (r = −0.67; CI = −0.79,−0.5; p < 0.001) and to the PIPRblue-red 11–16 s (r = −0.45; CI = −0.64,−0.21; p < 0.001). To distinguish normals and OH easily from glaucoma patients, all subjects were categorized into five “defect groups” 0–4 (0 = normals, 1 = OH, 2 = glaucoma, scotoma score 1–29 %, 3 = glaucoma, scotoma score 30–64 %, 4 = glaucoma, and scotoma score >65 %). Figure 6a-c visualizes in the form of box-plots the changes of the specific pupillary parameters in relation to the extent of visual field defects.
Box plots of specific parameters of the pupillary light reflex (a-c) in relationship to the visual field defects categorizing the sample on the basis of the monocular Esterman grid into five defect groups (0 = normals, 1 = ocular hypertension, scotoma score 0 %, 2 = glaucoma, scotoma score 1–29 %, 3 = glaucoma, scotoma score 30–64 %, 4 = glaucoma, scotoma score >65 %) a: slope of the response during exposure to the 4 s red stimulus (SORRS) b: maximal relative amplitude (MRA) for blue (left) and red (right) stimulation c: post-illumination pupil response PIPRblue-red
Discussion
One important outcome of the present study was to demonstrate a significantly reduced PIPR in advanced glaucoma patients, indicating a characteristic affection of the melanopsin-driven intrinsic response of ipRGCs in glaucomatous damage and supporting the work of Kankipati [15], Feigl [16] and Nissen [18]. Furthermore, we demonstrated a significant pupillary escape for the glaucoma group compared to normals by calculating the slope between the first maximum of contraction after stimulus onset and the predominant redilation after stimulus offset of a 4 s red stimulus (p < 0.0005) – a, to the best of our knowledge, so far unknown phenomenon in glaucoma patients. Interestingly, the mean of the ocular hypertension group lies between the normal and glaucoma groups, and the distribution overlaps both of them. An explanation for this pupillary escape behavior remains currently unclear, although it seems to indicate an impairment of the synaptic pathway via rods and cones to both, RGCs and ipRGCs, as well as a disturbed interaction between them. The slope seems to be a promising parameter to distinguish between advanced glaucoma patients and normals - although not every single glaucoma patient showed this phenomenon. Nevertheless, it might strengthen the role of colour pupillography in glaucoma diagnostics. Due to pupillary escape, the AUC for the PIPR with longer stimulus durations can only be calculated for the time starting a few seconds after stimulus offset.
The significantly reduced maximal relative amplitudes during light exposure, especially for the blue 4 s condition, but also for the red stimulus, further underline the evidence for a disturbed function and interaction of ipRGCs (intrinsic and synaptic), RGCs and rods and cones. Similar considerations of a loss of both synaptic and melanopsin driven responses were recently proposed for unilateral glaucoma [18]. Moreover, to our knowledge, this study is the first to address the specific group of subjects with ocular hypertension - in an exploratory intent. Although there seemed to be slight differences between OH and normals (especially concerning the pupillary escape behavior described above), we could not confirm statistically significant differences between the two groups. Taking into consideration that the only general conspicuousness of OH patients is the statistically elevated IOP > 21 mmHg compared to normals, it is not surprising that colour pupillography tests failed to distinguish these two groups per se – as all current structural and functional ophthalmological tests do as well. Nevertheless, as OH patients have a cumulative risk of 9.5 % for the development of glaucoma within 60 months [2], there is clinical interest to identify this minor subgroup to appropriately treat those patients. Pitsas et al. just recently showed that around 20 % of their OH cohort could be classified as abnormal in the global Moorfields regression analysis of HRT measurements and similarly around 20 % had defects in the short-wavelength automatic perimetry (SWAP), while all showed normal Humphrey white on white visual fields. Although no statistical correlation between HRT and SWAP was detected and the overlap of abnormal HRT parameters and SWAP defects was only 4.1 %, those OH patients might belong to the eyes with an increased risk for glaucoma conversion [19].
In our OH sample, two single subjects are outliers with elevated slope values of a pupillary escape up to a maximum of 5.21 (maximum normals 1.82). This may indicate a risk factor for conversion into manifest glaucoma- a speculation that cannot be answered by the present study and definitely needs to be confirmed in a larger sample and longitudinal investigations. Yet, functional abnormalities in pupillary light reflexes as the pupillary escape behavior might be a hint for somehow altered interactions between different photoreceptors and the neuroretinal network that also might occur even without any detectable structural damage.
The present study revealed statistically significant correlations between pupillary parameters and visual field defects. Larger visual field defects were correlated with an elevated SORRS, reduced MRAs, and a reduced PIPR. Within the glaucoma group, these results were less pronounced and statistically not significant. One reason could be the degree of overlap (see Fig. 6) due to the interindividual variability of the pupil light reflex – a factor that we cannot get completely rid of, but we tried to optimally consider by calculating and comparing the relative values. Additionally, a variable amount of damaged optic nerve fibers is suggested before the detection of visual field defects by standard perimetry [20], thus making a correlation between colour pupillography and standard perimetry not optimal.
Our measurements were mainly performed in the morning, yet it was not feasible to measure every subject exactly at the same time during the day. Therefore, a known circadian rhythm of the PIPR [21] may have confounded our results, but we regard this limitation to have minor relevance.
One limitation of the present study that we want to discuss is the selection of our glaucoma group. Our cohort was supposed to comprise glaucoma patients with manifest glaucomatous visual field defects of different severity. Those patients - fortunately- are usually under IOP-lowering treatment to avoid further irreversible damage. We excluded all patients with a history of previous glaucoma surgery on the measured eye to avoid any possible influence on the iris sphincter. Thus, all glaucoma patients were under therapy with IOP-lowering eyedrops that might also have had a disturbing influence on the pupil reaction. On the other hand, considering that we still revealed statistically significant differences between the glaucoma group and the normal group in several pupillary parameters, this indicates even more robust values.
Likewise, our glaucoma sample represented a relatively natural cohort of advanced glaucoma patients strengthening the potential application value of colour pupillography in clinical routine. However, we are totally aware that these pupillographic tests are not (yet) suitable for clinical routine decisions in identifying or treating individual patients – mainly due to the above-mentioned interindividual variability of the human pupil light reflex making it currently impossible to distinguish between “glaucoma”, “OH”, and “normal” with sufficient sensitivity and specificity.
There has been an ongoing debate about which stimulus parameters best assess ipRGC activity. Recently, it has been shown that full-field chromatic stimulation is better than central field stimulation in assessing ipRGC function [22]. In that experiment no further increase in PIPR was observed when the stimulus duration increased from 400 to 1000 ms. However, according to our results above, a 4 s stimulus provides additional information other than the PIPR. So in addition to showing a remarkable PIPR to blue stimulation, a 4 s stimulus can also reveal different pupillary dynamics (especially a pupillary escape behavior for red stimulation) between the normal, OH and glaucoma groups that would consequently indicate that it is a viable stimulus condition for glaucoma diagnostics.
In conclusion, our data showing a significantly reduced PIPR suggest a specific impairment of the melanopsin-driven pathway of ipRGCs in advanced glaucoma. Furthermore we observed reduced maximal relative amplitudes and a remarkable pupillary escape behavior using the 4 s red stimulus in glaucoma indicating a disturbed synaptic function and interaction between the outer photoreceptors, RGCs, and ipRGCs. We introduced a promising new parameter, the SORRS, characterizing this pupillary escape phenomenon in manifest glaucoma.
References
Friedman DS, Wilson MR, Liebmann JM, Fechtner RD, Weinreb RN (2004) An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol 138(3 Suppl):19–31
Kass MA, Heuer DK, Higginbotham EJ et al (2002) The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 120:701–713
Skorkovská K, Kelbsch C, Blumenstock G, Wilhelm H, Wilhelm B (2012) Glaucoma screening by means of pupil campimetry. Klin Monbl Augenheilkd 229(11):1097–1102
Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollaq MD (2000) A novel human opsin in the inner retina. J Neurosci 20(2):600–605
Dacey DM, Liao HW, Peterson BB et al (2005) Melanopsin expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433:749–754
Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295(5557):1070–1073
Hattar S, Liao HW, Takao M, Berson DM, Yau KW (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295:1065–1070
Hattar S, Lucas RJ, Mrosovsky N et al (2003) Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424:76–81
Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM (2007) Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis Res 47:946–954
Schmidt TM, Do MTH, Dacey D, Lucas R, Hattar S, Matynia A (2011) Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci 31(45):16094–16101
Qiu X, Kumbalasiri T, Carlson SM et al (2005) Induction of photosensitivity by heterologous expression of melanopsin. Nature 433:745–749
Lucas RJ, Douglas RH, Foster RG (2001) Characterization of a novel ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci 4:621–626
Brown TM, Gias C, Hatori M et al (2010) Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol 8, e1000558
Li RS, Chen B, Tay DK, Chan HHL, Pu M, So K (2006) Melanopsin-expressing retinal ganglion cells are more injury-resistant in a chronic ocular hypertension model. Invest Ophthalmol Vis Sci 47:2951–2958
Kankipati L, Girkin CA, Gamlin PD (2011) The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci 52:2287–2292
Feigl B, Mattes D, Thomas R, Zele AJ (2011) Intrinsically photosensitive (Melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci 52:4362–4367
Esterman B (1967) Grid for scoring visual fields. Arch Ophthalmol 77:780–786
Nissen C, Sander B, Milea D et al (2014) Monochromatic pupillometry in unilateral glaucoma discloses no adaptive changes subserved by the ipRGCs. Front Neurol 5:15
Pitsas C, Papaconstantinou D, Georgalas I, Halkiadakis I (2015) Relationship between short-wavelength automatic perimetry and Heidelberg retina tomograph parameters in eyes with ocular hypertension. Int J Ophthalmol 8(5):1013–1017
Gordon MO, Beiser JA, Brandt JD et al (2002) The ocular hypertension treatment study: baseline factors that predict the onset of open-angle glaucoma. Arch Ophthalmol 120(6):714–720, discussion 829–830
Zele AJ, Feigl B, Smith SS, Markwell EL (2011) The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One 6(3), e17860. doi:10.1371/journal.pone.0017860
Lei S, Goltz HC, Chandrakumar M, Wong AMF (2014) Full-field chromatic pupillometry for the assessment of the postillumination pupil response driven by Melanopsin-containing retinal ganglion cells. Invest Ophthalmol Vis Sci 55:4496–4503
Acknowledgments
We thank Dr. Anne Kurtenbach for her help with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Egon Schumacher-Stiftung, Germany, a private foundation without commercial interest, provided financial support in the form of labour and material costs funding. The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the local institutional ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kelbsch, C., Maeda, F., Strasser, T. et al. Pupillary responses driven by ipRGCs and classical photoreceptors are impaired in glaucoma. Graefes Arch Clin Exp Ophthalmol 254, 1361–1370 (2016). https://doi.org/10.1007/s00417-016-3351-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3351-9