Abstract
Purpose
The purpose was to investigate the paravascular abnormalities (PVA) around the retinal vascular arcades and their post-operative evolution in eyes with epiretinal membranes (ERM).
Methods
This is an observational case series. Fifty-seven eyes of 55 patients with concurrent PVA and ERM were studied (study group). Forty-one eyes in 41 patients with ERM but no PVA served as controls. Multiple optical coherence tomography (OCT) scans were made along the upper and lower arcades and across the fovea in each patient. Serial fundus photography and OCT scans were performed in eyes receiving an operation. All surgeries were performed by one surgeon. The incidence and location of paravascular retinal cysts, deep cystic spaces underneath the vessels, and paravascular retinal defects, as well as vitreoretinal interface changes, were determined and correlated with macular thickness.
Results
In the study group, paravascular retinal cysts were detected in 57 eyes (100 %), deep cystic spaces in nine eyes (15.8 %), and paravascular lamellar holes in 31 eyes (54.4 %). No case had a full-thickness hole. ERM adhesion to the PVA was noted in 16 eyes (28.1 %) and internal limiting membrane (ILM) changes over the PVA in 22 eyes (38.6 %). Compared with the control, the study group had significantly increased macular thickness. PVA, except lamellar holes, disappeared or decrease in severity after ERM and ILM removal surgery.
Conclusion
Different types of PVA are relatively common in eyes with ERM. Our findings suggest that PVA may develop secondary to ERM-induced macular thickening. Except for lamellar holes, most lesions decrease following an operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Paravascular abnormalities (PVA) in the posterior retina have been demonstrated in highly myopic eyes and idiopathic preretinal fibrosis. Shimada and associates [1] reported that paravascular retinal cysts, vascular microfolds, paravascular lamellar holes, and paravascular retinoschisis could be detected with optical coherence tomography (OCT) in highly myopic eyes, and vitreoretinal traction existed on the inner walls of retinal cysts in sections adjacent to the lamellar holes. They suggested the presence of paravascular lamellar holes might enhance the proliferative response of the internal limiting membrane (ILM), and might be an important causative factor for the development of a macular retinoschisis in highly myopic eyes. The same authors found that similar PVA were noted in cases of idiopathic preretinal fibrosis, and proposed that paravascular lamellar holes may also play a role in the idiopathic preretinal fibrosis development [2]. Muraoka and associates recently used the term paravascular inner retinal defect (PIRD) to describe the inner retinal defect along the major vessels in eyes with high myopia or epiretinal membranes (ERM) and discussed a possible correlation between the lesion and associated retinal dysfunctions [3].
Although PVA have been reported in eyes with ERM [3], the risk factors and clinical features have not been presented in detail. In addition, the evolution of these lesions after surgery remains unknown. In this study, we examine paravascular abnormalities in cases of ERM and analyze the prevalence, associated factors, and clinical features of these paravascular lesions. The lesions after ERM removal were compared with those before the surgery. Through this study, the interaction between PVA and ERM may be better understood.
Methods
For this study, we recruited consecutive patients with concurrent PVA and idiopathic ERM followed by the same surgeon (CM Yang) at National Taiwan University Hospital between January 2013 and December 2014 (the protocol approval number: 201508012RIND). ERM from non-idiopathic conditions such as high myopia, defined as a refractive error (spherical equivalent-SE) of more than −8 diopters (D) or an axial length > 26 mm, diabetic retinopathy, traumatic or post-surgical conditions were excluded. Forty-one eyes of 41 consecutive patients with ERM but no PVA during the same period were also recruited to serve as the control. All patients received complete ophthalmologic examinations including best-corrected visual acuity (BCVA), measurement of the axial length, anterior segment biomicroscopy, dilated fundus examination by indirect ophthalmoscopy, slit-lamp examination of the posterior fundus using a −78D lens, color fundus photography, and OCT examination. The study conformed to the tenets of the Declaration of Helsinki, and was approved by the National Taiwan University Hospital institutional review board.
OCT examinations were performed through a dilated pupil. Spectral-domain optical coherence tomography (SD-OCT) (CirrusTM HD-OCT, Carl Zeiss Meditec, Inc., Dublin, CA, USA; or RTVue® Model-RT100 version 3.5, Optovue, Inc., Fremont, CA, USA) on the macula was carried out in every case. Multiple linear OCT scans covering the upper and lower arcade and across the major retinal vessels were also performed. The OCT images were carefully examined for PVA, including the presence of paravascular retinal cysts, deep cystic space or schisis underneath the vessels, and paravascular lamellar retinal holes adjacent to the retinal vessel along the posterior vascular arcade. Vitreoretinal interface abnormalities including the presence of vitreoretinal adhesion/ traction on or adjacent to the retinal vessels, ERM adhesion to the PVA, and ILM changes at the PVA sites were also noted. Any ILM defect, ILM detachment, or ILM dehiscence was defined as an ILM change. Findings in the macular area, such as central macular thickness and the presence of a macular lamellar hole, were also analyzed.
Selected cases in the study group and the control group underwent vitrectomy with ERM and ILM peeling. The surgical indications were BCVA less than 16/20 or a significant symptomatic metamorphopsia severe enough to disturb daily activities. The surgical procedures consisted of 23-gauged pars plana vitrectomy with ERM removal and indocyanine green-assisted ILM peeling over the macular area. All cases that developed significant cataracts had phacoemulsification with posterior intraocular lens implantation during follow-up. Regular ophthalmic follow-up examinations were performed, including BCVA, fundus photography, and OCT on the macula and the arcade. All cases had a follow-up period of more than 1 year after surgery.
Statistical analysis
Student’s t-test was used to compare gender, laterality, number of eyes receiving operation, central macular thickness among eyes with different types of PVA, and various changes at the vitreoretinal junction. The Fisher exact probability test was used to compare the central macular thickness in the control and study groups, while the Mann–Whitney U test was used to compare the changes in logMAR after the operation and the central macular thickness between eyes with and without vitreoretinal change. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses. All data are shown in Mean ± S.D. P-values <0.05 are considered as statistically significant.
Results
The study group consisted of 57 eyes in 55 patients. There were 15 men and 40 women with a mean age of 62.7 ± 7.7 years (range: 47 to 81 years). The control group consisted of 41 eyes in 41 patients. There were 22 men and 19 women with a mean age of 62.5 ± 11.0 years (range: 41 to 87 years). Basic characteristics of the study and the control groups are summarized in Table 1. There were more female patients in the study group, and fewer cases in the control group had undergone surgery. Of note, the central macular thickness was significantly higher in the study group than in the control group (503.8 ± 102.1 vs. 384.1 ± 88.2; P < 0.001).
Locations and types of paravascular abnormalities
PVA were identified by fundus examination and color fundus photography in 40 of 57 eyes as spindle-shaped dark areas, multiple dark spots, or fissure-like dark lesions adjacent to retinal vessels. Paravascular retinal cysts were detected by OCT as intraretinal hyporeflective spaces lateral to the retinal vessels in all 57 eyes (Fig. 1a, b, c and d). In 35 of 57 (61.4 %) eyes with paravascular retinal cysts, the cysts existed along both the upper and lower temporal vessels of the retinal arcade. The cysts were distributed only along the upper retinal vessels in 17 eyes (29.8 %) and only along lower vessels in five eyes (8.8 %). The paravascular retinal cysts were detected along both retinal arteries and veins in 40 of 57 eyes (70.1 %), only along retinal arteries in four eyes (7 %), and only along retinal veins in 13 eyes (22.8 %).
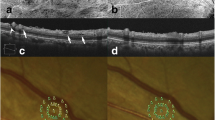
Paravascular abnormalities (PVA) in eyes with paravascular retinal cyst. a, b Right eye of a 62-year-old woman with epiretinal membrane (ERM) and PVA. Fundus photography showed grouped dark spots along the vessel while corresponding location on optical coherence tomography (OCT) demonstrated typical paravascular retinal cysts (circle). c, d Right eye of a 61-year-old woman with ERM and PVA. Fundus photography showed prominent ERM with PVA along arcades (dotted circles) and OCT demostrated elevated macula with paravascular cyst (asterisk). e Right eye of a 50-year-old woman with ERM and PVA. Horizontal OCT scan along the superior arcade revealed a paravascular lamellar hole (arrow); note that a cyst (asterisk) can be seen adjacent to the lamellar hole. f Left eye of a 63-year-old man with ERM and PVA. Vertical OCT scan demonstrated a paravascular deep cyst/schisis (open arrow)
Thirty-one of 57 eyes were found to have paravascular lamellar holes by OCT (Fig. 1e), while no eyes had full thickness paravascular retinal holes. The paravascular lamellar holes were always detected in close proximity to paravascular retinal cysts.
Paravascular deep cyst/schisis was located underneath the retinal vessels showing hyporeflective spaces containing vertical columns (Fig. 1f). This lesion was found in nine of 57 eyes and was associated with both paravascular retinal cysts and a lamellar hole.
Classification of paravascular abnormalities
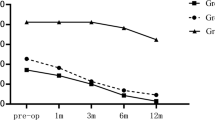
The patients with PVA were further classified into three groups based on the number of PVA types, including PVA 1 with paravascular cysts only; PVA 2 with paravascular cysts and lamellar holes and PVA 3 with paravascular cysts, lamellar holes, and deep cystic changes. Comparison of central macular thickness and age in patients with different types of PVA are summarized in Table 2. As shown in Fig. 2, results indicate that a more complicated PVA corresponds with a more significant increase in macular thickness.
Paravascular abnormalities (PVA) had positive correlation with central macular thickness (CMT). Central macular thickness in PVA 2 group (505.4 ± 95.6 μm, p < 0.001) and PVA 3 group (512.9 ± 139.9 μm, p = 0.004) are significantly increased in comparison to the non-PVA group (384.1 ± 88.2 μm), but that the in PVA 1 group (439.5 ± 131.2 μm) is not significantly increased. Despite this, there is a trend of increasing CMT through PVA1-3, the differences among these groups are insignificant. Non-PVA = eyes without any paravascular abnormalities; PVA 1 = paravascular cyst; PVA 2 = paravascular cyst and lamellar hole; PVA 3 = paravascular cyst, lamellar hole and deep cystic space. P value < 0.05 as significant by using Student’s t-test
Changes in the vitreoretinal junction refer to either the presence of vitreoretinal adhesion/traction on or adjacent to the retinal vessels, ERM adhesion to the PVA, or ILM changes at the PVA sites (Fig. 3). With OCT, vitreous adhesion/traction was found in five eyes (Fig. 3c and d), ERM adhesion in 16 eyes and ILM changes in 22 eyes in the PVA group. Both the ERM adhesion and ILM changes were observed more frequently in eyes with more complex PVA. The average central macular thickness was not significantly higher in eyes with changes in the vitreoretinal junction in the study group as demonstrated in Table 3.
Vitreoretinal interface abnormalities in eyes with paravascular abnormalities (PVA). Orientation of the optical coherence tomography (OCT) scan is shown in the right lower inlet. Vitreoretinal interface abnormalities refer to adhesions on or adjacent to the retinal vessels: a Left eye of a 53 year-old woman. Horizontal OCT scan along the superior arcade showed ERM adhesion at the paravascular abnormalities (PVA) sites (arrowhead); b Left eye of a 64 year-old woman. Vertical OCT scan of the superior arcade demonstrated internal limiting membrane (ILM) changes at the PVA sites (arrow); c, d Left eye of a 55 year-old woman. Vertical OCT scan showed vitreous adhesion to the PVA site (double-asterisk)
BCVA and PVA changes after operation
In the 31 eyes that underwent operation in the study group, there were eight eyes, 22 eyes, and one eye with PVA types 3, 2 and 1, respectively. Compared with the ten eyes receiving operations in the control group, the 31 eyes in the study group that had operations showed no significant differences in BCVA improvement as summarized in Table 1. Fundus examinations showed a disappearance or marked reduction of the dark streaks and macular thickness in all cases at 12-months post-operation (Fig. 4). OCT showed paravascular retinal cysts and deep cystic changes disappeared after operations (Figs. 5 and 6a, c), while lamellar holes remained, seemingly irreversible (Fig. 6b and d).
Evolution of paravascular abnormalities (PVA) and macular thickness in idiopathic epiretinal membrane (ERM) after surgery. Pre-operatively, a spindle-shaped dark areas or fissure-like lesions adjacent to retinal vessels (circle) and b, c thickened macula with cystic change (asterisk) in a case with idiopathic ERM on optical coherence tomography (OCT). d–f Post-operatively, the PVA lesions disappeared partially at 12-Month ophthalmoscopically and OCT demonstrates flat macula with no more cystic change
Evolution of paravascular abnormalities (PVA) in idiopathic epiretinal membrane (ERM) after surgery. a, b Pre-operatively, PVA as spindle-shaped dark areas along upper arcade with ERM shown on OCT. c En face OCT demonstrates areas of craters and pits adjacent to the vessels (dotted circle). d–f At 1-Month post-operatively, the partial resolution of PVA and ERM were observed ophthalmoscopically and on OCT
The resolution of paravascular cyst and irreversible change of lamellar hole after operation. a, b Left eye of a 53-year-old woman. Optical coherence tomography (OCT) showed disappearance of the paravascular cyst and deep cyst 1 month after operation. c Right eye fundus of a 70-year-old man. Pre-operatively, epiretinal membrane (ERM) adhesion (arrowhead) and lamellar hole (arrow) were seen. d Post-operatively, the ERM adhesion was released but the lamellar hole remained (arrow)
Discussion
In this study, we found various types of PVA may be present in cases of idiopathic ERM. With OCT, paravascular cysts were the most frequently seen abnormalities on either side of the vessels. In the most severe cases, evident schisis may develop under the vessels and cysts may extend between two parallel vessels, exhibiting a suspension bridge picture with two vessels acting as pylons. The cyst roofs on either side of the vessel may dehisce with stretching and result in a pit-like lamellar hole. Our study found only partial-thickness paravascular retinal holes but no full-thickness holes. This finding is shared by other previous reports [1, 3].
We found that the cysts and other types of PVA were most frequently associated with thickened macula induced by ERM and the greater thickness of the central macula, the more complex the PVA. Those cases without PVA showed significantly less central macular thickness. We postulated that an elevated macula may induce thinning of the adjacent retinal tissue in the arcade area; as the retinal vessels are more resistant to this stretching force, they stand out, forming microfolds and causing paravascular retinal tissue to split between the superficial and the deeper retinal layers, thus creating the paravascular cysts or schisis. Further tangential and oblique stretching forces from the ERM might eventually disrupt the inner cyst or schisis wall to form a lamellar hole. Shimada and associates found high prevalence of PVA in highly myopic eyes. It may be conceivable that an elongated eyeball in high myopia may induce retina thinning and through similar mechanisms as mentioned above contribute to the formation of PVA. In this study, ERM might or might not cover the area of PVA, indicating the force generated by the ERM and transferred to the paravascular area, but not necessarily the ERM itself, may be sufficient to induce PVA.
ILM abnormalities or ERM adhesion were sometimes found in PVA sites. As there was no significant difference in macular thickness among eyes without vitreoretinal changes and eyes with ERM adhesion and/or ILM abnormalities at PVA sites in our study as in Table 3, it suggests the ILM/ERM changes were more a direct consequence of ERM traction than a secondary change of central macular thickening. Less often, VR adhesions along the vessels may be found to be associated with PVA. In this study, five cases had OCT evidence of vitreoretinal adhesion along the arcade vessels in the study group. One of these five cases had a central macular thickness as low as 235 μm, suggesting that, although less common, this active traction alone may play a role in PVA formation in cases of idiopathic ERM. Previous studies showed cases with PVA in high myopia had VR adhesion [1, 4]. Their observations combined with our results suggest abnormal vitreoretinal traction alone or in association with a posterior retinal thinning from eyeball elongation may be the major reasons for PVA in high myopia, while paravascular retinal thinning secondary to the central macular thickening may be the major reason for the development of PVA in idiopathic ERM.
Whether PVA effects retinal function remains unclear [3, 5]. While the presence of PVA in ERM usually signified more prominent central elevation in our study, we did not, however, find the changes to be associated with worse visual prognosis. Recently, Muraoka and associates reported that 85 % of eyes with paravascular inner retinal defect of high myopia or ERM had visual field defects. However, no consistent visual field defect was detected corresponding to the PVA using different perimetry modalities. Whether this possible visual function change may be reversible by surgery or not requires further study.
In this study, we found that much of the PVA disappeared after ERM and ILM removal surgery. The only irreversible abnormality was paravascular lamellar macular holes. The reversibility of PVA suggests that they develop secondary to ERM traction, and once the causal force had been released, recovery from abnormal changes may be possible as long as there was no permanent damage. Because the presence of lamellar holes signifies tissue dehiscence or disruption, post-surgery recovery is more difficult in these cases. Shimada and associates suggested that paravascular lamellar holes may play a role in idiopathic preretinal fibrosis development. While our study could not entirely refute this proposal, the reversibility of many PV changes and the likely development of PV lamellar holes from PV cysts suggest the PVA were the results rather than the causes of ERM.
In our study, several patterns of change could be seen with fundoscopic examinations. The PVA most often occurred along the first or secondary branches of the retinal vessels. They may present as dark streaks adjacent to retinal vessels, dark shadows along the vessels, or nerve fiber layer defect-like changes between two vessels. These were in contrast with PVA in high myopic eyes, where in most cases only dark shadows along the vessels were found with fundus examinations. Similar morphological changes have been mentioned in the so-called retinal dimple or dissociated optic nerve fiber layer (DONFL) after ILM peeling [5–7], and the nerve fiber layer defect associated with glaucoma or other causes. DONFL only occurs in the previously ILM peeled area and presents as scattered dots or patches, forming a honeycomb pattern [8, 9]. DONFL has been recently suggested to represent Muller cell foot plate damage with resultant pitting on the retina surface instead of real nerve fiber splitting [10, 11]. The distribution and basic pattern of NFL differ considerably from PVA. A nerve fiber layer (NFL) defect may be similar to PVA; however, the dark streak in a NFL defect usually connects with the disc margin and the distribution is not related to vessels. The understanding of PVA in idiopathic ERM may be helpful in differentiating the lesions from other retinal abnormalities and avoid uncertainty during surgical manipulations.
This study is limited by its retrospective nature and the small case number. However, our results suggest that PVA were most likely seen in idiopathic ERM with significant central macular thickening. With the exception of lamellar holes, most of the PVA were reversible after surgical removal of ERM. Paravascular retinal thinning secondary to the central macular thickening may be the major mechanism for the development of PVA in idiopathic ERM. Additional cohort studies with longer follow-up periods are necessary to confirm the longitudinal changes in pathogenesis and associated retinal dysfunctions before and after surgery.
References
Shimada N, Ohno-Matsui K, Nishimuta A, Moriyama M, Yoshida T, Tokoro T, Mochizuki M (2008) Detection of paravascular lamellar holes and other paravascular abnormalities by optical coherence tomography in eyes with high myopia. Ophthalmology 115(4):708–717. doi:10.1016/j.ophtha.2007.04.060
Shimada N, Ohno-Matsui K, Yoshida T, Mochizuki M (2010) Presence of paravascular lamellar holes in patients with idiopathic premacular fibrosis. Br J Ophthalmol 94(2):263, 265–266. doi:10.1136/bjo.2008.156141
Muraoka Y, Tsujikawa A, Hata M, Yamashiro K, Ellabban AA, Takahashi A, Nakanishi H, Ooto S, Tanabe T, Yoshimura N (2015) Paravascular inner retinal defect associated with high myopia or epiretinal membrane. JAMA Ophthalmol 133(4):413–420. doi:10.1001/jamaophthalmol.2014.5632
Forte R, Cennamo G, Pascotto F, de Crecchio G (2008) En face optical coherence tomography of the posterior pole in high myopia. Am J Ophthalmol 145(2):281–288. doi:10.1016/j.ajo.2007.09.022
Ikuno Y, Gomi F, Tano Y (2005) Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. Am J Ophthalmol 139(3):462–467. doi:10.1016/j.ajo.2004.09.078
Ito Y, Terasaki H, Takahashi A, Yamakoshi T (2005) Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular holes. Opthalmology 112(8):1415–1420. doi:10.1016/j.ophtha.2005.02.023
Sakimoto S, Ikuno Y, Fujimoto S, Sakaguchi H, Nishida K (2014) Characteristics of the retinal surface after internal limiting membrane peeling in highly myopic eyes. Am J Ophthalmol 158(4):762–768. doi:10.1016/j.ajo.2014.06.024, e761
Pichi F, Lembo A, Morara M, Veronese C, Alkabes M, Nucci P, Ciardella AP (2014) Early and late inner retinal changes after inner limiting membrane peeling. Int Ophthalmol 34(2):437–446. doi:10.1007/s10792-013-9831-6
Steel DH, Dinah C, Habib M, White K (2015) ILM peeling technique influences the degree of a dissociated optic nerve fibre layer appearance after macular hole surgery. Graefes Arch Clin Exp Ophthalmol 253(5):691–698. doi:10.1007/s00417-014-2734-z
La Heij EC, Dieudonne SC, Mooy CM, Diederen RM, Liem AT, van Suylen RJ, Hendrikse F (2005) Immunohistochemical analysis of the internal limiting membrane peeled with infracyanine green. Am J Ophthalmol 140(6):1123–1125. doi:10.1016/j.ajo.2005.05.054
Lim JW, Kim HK, Cho DY (2011) Macular function and ultrastructure of the internal limiting membrane removed during surgery for idiopathic epiretinal membrane. Clin Exp Ophthalmol 39(1):9–14. doi:10.1111/j.1442-9071.2010.02377.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Liu, HY., Hsieh, YT. & Yang, CM. Paravascular abnormalities in eyes with idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol 254, 1723–1729 (2016). https://doi.org/10.1007/s00417-016-3276-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3276-3