Abstract
Purpose
To investigate changes in choroidal thickness following vitrectomy in eyes with epiretinal membrane (ERM) and macular hole (MH)
Methods
Choroidal thicknesses at the fovea and at 1 and 3 mm superior, inferior, temporal, and nasal to the fovea were measured using enhanced-depth imaging optical coherence tomography (EDI-OCT) before and 1 week, 1, 3, 6, and 12 months after vitrectomy. In 95 eyes with ERM and 56 with MH, the thicknesses were compared between baseline and each postoperative visit. The postoperative thickness changes from baseline were compared between the ERM and MH groups. Clinical factors associated with choroidal thickness change were also evaluated.
Results
All choroidal thicknesses were significantly increased 1 week after vitrectomy compared to baseline in the ERM group (i.e. mean subfoveal choroidal thickness increased from 218 to 233 μm, P < 0.001). In the MH group, the increase was statistically significant only for inferior choroidal thicknesses. Such significant increase in choroidal thicknesses was not observed from 1 month postoperative in both groups. Postoperative SFCT change was significantly associated with combined cataract extraction (P = 0.026) and surgical indication (P = 0.010) at 1 week postoperative and with baseline SFCT at all postoperative visits (all P < 0.05).
Conclusions
Choroidal thickness may temporarily increase following vitrectomy at the early postoperative period and subsequently decrease to the baseline value. Compared to the ERM group, the MH group showed location-dependent choroidal thickness change at early postoperative period, which might be explained by position-dependent tamponading effect of gas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The choroid, which constitutes the middle vascular ocular layer between the outermost sclera and the innermost retina, plays an important role in the pathogenesis of many diseases of the posterior segment of the eye. Traditional imaging modalities such as indocyanine green angiography and B-scan ultrasonography have limited image resolution, but optical coherence tomography (OCT) enhanced visualization of the choroid. Choroidal thickness has been measured noninvasively in diverse retinal and choroidal diseases using enhanced-depth imaging OCT (EDI-OCT) or swept-source OCT (SS-OCT), which enables a more accurate measurement of the thickness by providing much clearer choroidal images than conventional time or spectral-domain OCT [1–5].
Changes in choroidal thickness have been observed in several retinal and choroidal diseases, such as high myopia, central serous chorioretinopathy, polypoidal choroidal vasculopathy, age-related maculopathy, and Vogt-Koyanagi-Harada disease by using EDI-OCT [1, 3, 4, 6]. From these findings, several authors have suggested that choroidal thickness changes are associated with changes in choroidal circulation or inflammation [2, 4, 5, 7]. The changes of choroidal thickness following retinal surgery was recently reported using EDI-OCT by Kimura and associates [8]. They showed that subfoveal choroidal thickness (SFCT) temporarily increases after segmental scleral buckling surgery. Michalewska et al. recently reported choroidal thickness change following vitrectomy, no significant difference in early postoperative periods, but significant decrease in the thickness at 3 months after vitrectomy in eyes with epiretinal membrane (ERM) [9]. However, little is known about the long-term choroidal thickness changes following vitrectomy, in which various surgical procedures are performed depending on surgical indications, and thus diverse factors may affect postoperative choroidal thickness change.
In the present study, we investigated the postoperative changes of choroidal thickness up to 12 months following vitrectomy for ERM and macular hole (MH), in which surgical procedures and operating times are similar among cases, compared to other surgical indications for vitrectomy. By comparing choroidal thickness change between the ERM and MH groups, we aimed to find a difference in the thickness change between the two indications and also discuss the origin of the difference.
Methods
Subjects
The medical records of 148 eyes of 144 consecutive patients with ERM and 81 eyes of 80 consecutive patients with MH who were (1) treated with pars plana vitrectomy (PPV) from January 2012 to October 2013 and followed up for ≥6 months were retrospectively reviewed. Patients were excluded if they had a history of ocular trauma or intraocular surgery other than cataract extraction (n = 12 ERM and 7 MH), other coexistent macular or retinal diseases such as retinal vein occlusion, diabetic retinopathy, and myopic foveoschisis (n = 19 ERM and 7 MH), high myopia defined as refractive errors > −6 diopters (D) and axial length >26.0 mm (n = 16 ERM and 8 MH), or poor-quality OCT images (n = 6 ERM and 3 MH, respectively). In total, 95 eyes of 93 patients with idiopathic ERM (ERM group) and 56 eyes of 55 patients with idiopathic MH (MH group) were included in this study (Fig. 1). Forty-four eyes with ERM and 25 with MH were followed up for ≥12 months; the data on choroidal thickness at 12 months postoperative were obtained from the patients. This study was approved by the Institutional review board (IRB) of the Seoul National University Bundang Hospital, and the study was conducted according to the tenets of the Declaration of Helsinki.
Surgical procedures
Vitrectomy for MH and ERM was performed by two experienced retinal surgeons (S.J.W and K.H.P.) using identical procedures. A 23-gauge transconjunctival sutureless vitrectomy (TSV) was performed using the Accurus 800CS surgical system (Alcon Surgical, Fort Worth, TX, USA) with a contact lens system (Hoya Corp, Tokyo, Japan). In patients with a visually significant cataract, phacoemulsification with implantation of an intraocular lens was performed before TSV. Peeling of the ERM or internal limiting membrane (ILM) was carried out by using end-gripping forceps (Alcon, Fort Worth, TX, USA). ILM peeling was performed for all eyes with MH and 26 eyes with ERM after staining the ILM with 0.05 % indocyanine green (ICG, Dong In Dang Pharmaceutical, Shiheung-Si, Kyunggi-Do, Korea). For gas tamponade, 18 % sulfahexafluoride (SF6) was used in eyes with MH. After the MH surgery, the patients were instructed to maintain a facedown position for 1 week. Antibiotics and steroid, 1 % prednisolone acetate (Pred Forte®; Allergan Inc., Irvine, CA, USA) and 0.5 % levofloxacin (Cravit; Santen, Osaka, Japan), were topically instilled four times a day postoperatively.
Examinations
Patients underwent comprehensive ophthalmologic examinations, including best-corrected visual acuity measurements using Snellen charts, slit-lamp examination, intraocular pressure (IOP) measurement by noncontact tonometer (KT-500 automated tonometer, Kowa Co. Ltd., Tokyo, Japan), and indirect ophthalmoscopy, at the preoperative and postoperative visits. The refractive errors were measured with the Topcon KR8800 autorefractometer (Topcon Inc., Tokyo, Japan) and axial length was measured in all patients with the IOL Master 500 (Carl Zeiss Meditec Inc., Jena, Germany) before vitrectomy. The IOP at the preoperative and 1-week, 1-, 3-, 6-, and 12-month postoperative visits were reviewed and used for our analyses.
OCT images obtained before the operation and at 1 week, 1, 3, 6, and 12 months after the surgery were reviewed for our analyses. Full-thickness choroidal images were obtained with the patient in an upright position by using EDI-OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) with eye-tracking and image-averaging systems, as described by Spaide et al. [10] OCT scans were performed by a single trained examiner; the OCT camera was positioned close enough to the eye to obtain an inverted image. Two high-quality 10.5-mm horizontal and vertical line scans were obtained through the fovea. The eye tracking system and automatic software were used to detect and maintain the same scanning position through the fovea and, in turn, to place follow-up scans precisely on the same location. We confirmed the location and position of the scans with simultaneous real-time scanning laser ophthalmoscope (SLO) infrared reflectance images of the retina during the measurement of choroidal thickness.
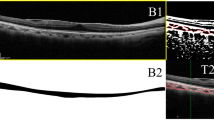
The choroid was defined as the layer between the outer border of the retinal pigment epithelium and the inner border of the sclera (Fig. 2), as described in previous studies [11, 12]. The thickness was measured by using the manual caliper provided by the software in the device. This was carried out at the fovea (subfoveal choroid) and at points 1 mm and 3 mm superior, inferior, nasal, and temporal to the fovea.
Enhanced-depth imaging optical coherence tomography images showing the choroidal thickness at the subfovea, inferior, and superior location at baseline and at 1 week and 1, 3, and 6 months after vitrectomy in eyes treated with gas tamponade for macular hole (a) and without gas tamponade for epiretinal membrane (b). The two eyes are matched samples with respect to age (61 and 64 years old), sex (male), preoperative subfoveal choroidal thickness (both 205 μm), and surgical procedures except for gas tamponade (vitrectomy with internal limiting membrane peeling)
As our cases with MH were treated with short-acting gas tamponade using SF6, choroidal thickness in eyes of the MH group from 1 postoperative month, when intravitreal gas was completely absorbed, could be measured. At 1 postoperative week, however, the superior choroidal thickness 3 mm from the fovea could not be measured because of remaining intravitreal gas in eight of 56 (14 %) patients with MH. Subfoveal and 1-mm temporal and nasal choroidal thicknesses could not be measured for the same reason in three (5 %) patients at the postoperative period and 1-mm superior thickness was not measurable in four of 56 (7 %) patients.
The OCT measurements of thicknesses were independently performed twice in a masked fashion and the average values of the two measurements were used for analysis. The intraclass correlation coefficient (ICC) was used to examine reproducibility, i.e., agreement between the two measurements, in choroidal thickness measurements. Data regarding the time of day at which SD-OCT was performed were also collected.
Statistical analyses
Descriptive statistics were performed for patient demographics, underlying disease, refractive error, and axial lengths. The Student’s t test was used for comparing the baseline characteristics and choroidal thicknesses between the ERM and MH groups. The thicknesses at the subfoveal, superior, inferior, temporal, and nasal choroid were compared between the baseline values and those at subsequent postoperative visits by using the paired t test. The thickness changes from baseline (thickness at follow-up minus that at baseline) were compared between the MH and ERM groups by using the Student’s t test. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Continuous values are presented as mean ± standard deviation.
Results
Demographic and clinical characteristics
The clinical characteristics of the patients included in our study are presented in Table 1. The mean ages were 66.2 ± 8.7 years (range, 35–84) and 63.6 ± 8.1 years (range, 44–83) in the ERM and MH groups, respectively. Combined cataract extraction and TSV was performed in 48 (51 %) and 34 (61 %) eyes with ERM and MH, respectively. Mean axial length was 23.6 ± 1.0 mm (range, 21.3-26.0) in the ERM group and 23.6 ± 1.2 mm (range, 21.8-26.0) in the MH group, respectively. Myopia was present in 32 (34 %) and 22 (39 %) eyes with ERM and MH, respectively. Mean time of day of OCT image acquisition at baseline and postoperative visits ranged from 11:38 AM to 12:52 PM in the ERM group and from 11:26 to 12:40 PM in the MH group. There was no significant difference in demographic and baseline clinical characteristics between the two groups (Table 1). There were no serious postoperative complications such as retinal detachment or endophthalmitis following the macular surgery with or without combined cataract extraction. Postoperative hypotony (IOP < 6 mmHg) was observed in one patient at week 1 and in none at subsequent postoperative periods.
Choroidal thicknesses following vitrectomy for ERM and MH
The choroidal thickness measurements showed good reproducibility, with the intraclass correlation coefficient (ICC) ranging between 0.952 and 0.988. For example, the ICC of SFCT was 0.988 [95 % confidence interval (CI), 0.983–0.992] at baseline and 0.987 (95 % CI, 0.979–0.991) at 1 postoperative week.
Photographic examples showing choroidal thickness changes following vitrectomy for ERM and MH in matched patients are presented in Fig. 2. At 1 postoperative week, the subfoveal and 1- and 3-mm superior choroidal thicknesses in the MH group decreased (Fig. 2a); in contrast, those in the ERM group all increased (Fig. 2b). In the photographic examples, the choroidal thickness at all locations decreased to values that were similar to those of the baseline in both ERM and MH groups from the 1-month visit and gradually decreased up to the 6-month visit.
The temporal changes of the mean choroidal thicknesses at multiple locations (subfoveal, temporal, nasal, superior, and inferior choroid) are shown in Fig. 3 (subfoveal choroidal thickness) and Fig. 4 (perifoveal choroidal thickness) and Table 2. The thickness at baseline showed no significant difference at all measured locations between the ERM and MH groups (all P > 0.05). In both groups, similar temporal patterns of choroidal thickness were noted in most choroidal locations (Figs. 3 and 4); the thickness increased at 1 week and decreased subsequently. However, the superior 3-mm choroidal thickness in the MH group showed a decreased choroidal thickness at 1 postoperative week compared to baseline, although statistically insignificant (Fig. 4 and Table 2). Significant differences in choroidal thickness were observed between baseline and 1 week postoperative visits for all choroidal locations in the ERM group (all P < 0.05) and only for the inferior choroid in the MH group (P = 0.040 and 0.023 for 1-mm and 3-mm inferior choroid, respectively). Between baseline and 6 months, there were significant differences in the subfoveal (P = 0.007) and 1-mm temporal (P = 0.010) choroidal thickness in the ERM group and in the 1-mm temporal (P = 0.024) and nasal (P = 0.001) and 3-mm superior (P = 0.005) thickness in the MH group.
Baseline and postoperative subfoveal choroidal thickness over 12 months following vitrectomy for epiretinal membrane (ERM group) and macular hole (MH group). The ERM group shows a remarkable increase in choroidal thickness at 1 postoperative week compared to the MH group. The error bars in the left column denote the upper or lower bound of the 95 % confidence interval. Data at 12 months were available in 44 and 25 eyes for the ERM and MH groups, respectively
Baseline and postoperative subfoveal choroidal thickness over 12 months following vitrectomy for epiretinal membrane (ERM group) and macular hole (MH group) at multiple locations, including 1 mm (left) and 3 mm from the fovea (right). The two groups show a remarkable difference in superior choroidal thickness, especially 3 mm from the fovea, at 1 postoperative week. Data at 12 months were available in 44 and 25 eyes for the ERM and MH groups, respectively
The temporal changes of the mean choroidal thicknesses in the eyes treated with and without combined cataract extraction are shown in Supplementary Fig. 1. In the ERM group, both the eyes with and without combined cataract surgery showed temporary choroidal thickness increase at 1 week, which was more remarkable in the eyes treated with combined cataract surgery. The difference between baseline and 1-week thickness was statistically significant in the subfoveal (P = 0.048) and 1-mm superior (P = 0.013) and inferior choroid (P = 0.014) among the ERM eyes treated with vitrectomy only. In the MH eyes treated with vitrectomy only, there was no significant difference between baseline and 1-week thickness in any choroidal location.
The mean choroidal thickness changes at early (1 week) and late postoperative periods (6 months) are compared between the ERM and MH groups (Table 3). At the early postoperative period, the changes were comparable between the ERM and MH groups at the temporal, nasal, and inferior locations (all P > 0.05). However, the subfoveal (13.7 ± 26.5 μm and 0.9 ± 16.8 μm in the ERM and MH group, respectively) and superior choroidal thickness change at 1 mm (17.9 ± 30.7 μm and 3.2 ± 30.6 μm) and 3 mm (11.8 ± 30.8 μm and 0.2 ± 28.4 μm) were significantly different between the two groups (P = 0.001, 0.010, and 0.044 for the subfoveal, 1-mm superior, and 3-mm superior thickness, respectively) at the early postoperative period. At the late postoperative period, however, the changes were comparable between the ERM and MH groups at all choroidal locations.
Factors influencing subfoveal choroidal thickness change following vitrectomy
The effect of clinical factors and surgical parameters, such as surgical indication (ERM or MH), combined cataract extraction, age, sex, axial length, baseline SFCT, and IOP change (follow-up minus baseline IOP), in postoperative SFCT change was evaluated. SFCT change at 1 week was significantly associated with surgical indication (regression coefficient B = −14.3, P = 0.010), combined cataract extraction (B = 13.0, P = 0.026), and baseline SFCT (B = −0.063, P = 0.047). The SFCT change was significantly associated with baseline SFCT at 1 (B = −0.110, P = 0.043), 3 (B = −0.111, P = 0.019), and 6 months (B = −0.129, P = 0.006).
Discussion
This study showed choroidal thickness changes following vitrectomy in two common indications of macular surgery, ERM and MH. Overall, the two groups showed similar temporal change over a 12-month postoperative period. The thickness increased at 1 postoperative week and subsequently decreased to the baseline values. Interestingly, there were some discrepancies in the changes at early postoperative period between the two indications; the MH group showed no significant difference in choroidal thickness except the inferior choroid between baseline and 1 week after surgery, whereas the ERM group showed significantly increased choroidal thickness at all the choroidal locations 1 week after vitrectomy.
Several factors may affect postoperative choroidal thickness following vitrectomy, although little is known about what clinical/surgical factors may affect the thickness. To control the effect of various surgical procedures on choroidal thickness, ERM and MH were selected for the investigation of choroidal thickness change following vitrectomy because (1) the surgery for ERM and MH does not require the complicated procedures of vitrectomy, and (2) in each indication, the procedures do not vary widely among the cases. It may minimize the inter-individual variability in choroidal thickness changes attributable to different surgical procedures among cases. Furthermore, the baseline and demographic characteristics were comparable between the two groups, which enabled the comparison of postoperative choroidal thickness change between two different surgical indications.
Remarkably, choroidal thickness at specific locations showed significantly different postoperative change between the ERM and MH groups at early postoperative period. For example, postoperative change in choroidal thicknesses at subfoveal and superior location showed significant difference between the ERM and MH groups. We believe that the difference in choroidal thickness changes at week 1 between the two groups may derive from the different surgical procedure between ERM and MH surgery, gas tamponade, as (1) most of the eyes with MH had intravitreal gas, mostly less than 50 % volume of the vitreous cavity, only at the 1-week postoperative visit in our study and (2) only at the early postoperative period, there is significant difference in choroidal thickness change between the two surgical indications. Further, difference in 1-week choroidal thickness was also observed between 41 patients with proliferative diabetic retinopathy treated by vitrectomy without gas tamponade and 28 with rhegmatogenous retinal detachment treated by vitrectomy with gas tamponade, who had surgery at our institution during the same time period (Supplementary Fig. 3).
The gas in the vitreous cavity tamponades the retina and may also compress the underlying choroid. As the choroid has elastic properties [13], the intravitreal gas is expected to decrease postoperative choroidal thickness by exerting compressive force on the choroid. Its effect on postoperative choroidal thickness may depend on the choroidal location, with the subfoveal and superior choroid showing greater effects than other locations due to patients’ prone (as recommended for early postoperative period) and upright (during OCT examination) positions, respectively. Thus, significant difference in postoperative subfoveal and superior choroidal thickness change between the ERM and MH groups may be partly explained by the effect of gas. However, the inferior choroid in the MH group may be affected least by the tamponading gas for the patient’s positions, which is compatible with our result that the inferior choroid showed similar postoperative change in both the ERM and MH groups. Thus, location-dependent difference in choroidal thickness change between the ERM and MH groups suggest that the discrepancy may be associated with the effect of gas, tamponading the retina and also the underlying choroid, which requires further studies for confirmation. From the suggested relationship between the tamponading effect of gas and the location of the choroid, the choroidal thickness change, calculated by using EDI-OCT measurements, might indicate the tamponading effect mediated by intravitreal gas in patients who underwent vitrectomy with intravitreal gas injection, which also requires further investigation.
A few recent studies showed no significant difference in choroidal thickness at early (<10 days) postoperative periods in eyes with idiopathic ERM or MH [9, 14]. However, due to small sample size and short follow-up period in the studies, the findings from these studies should be confirmed in a study with a longer follow-up on a larger number of patients. Indeed, the temporal pattern of choroidal thickness in the studies was generally similar to our findings on choroidal thickness at the subfovea and 1 mm from the fovea; however, statistical analyses showed different results on the significance of short-term choroidal thickness change at several locations between the previous studies and ours. The difference in measured time (7 vs. 10 days), sample size, or proportion of the eyes treated with combined cataract surgery between previous studies and ours might result in the difference.
Our study has several limitations which require careful interpretation on our results. Because of its retrospective design, it has the intrinsic drawback of possible selection bias. Additionally, it is well known that choroidal thickness shows diurnal variation [15–17], but in this retrospective study, it was impossible to control diurnal variation in every patient. Tan et al. showed that the highest mean choroidal thickness was obtained at 9:00 AM and the mean choroidal thickness then decreased progressively over the subsequent time points to 5:00 PM. The thickness change among the eyes with a mean baseline SFCT of 372.2 μm was 10.55 μm between 11:00 AM and 1:00 PM in the study. Because Tan et al. showed that subjects with a thin choroid (≤300 μm), which includes most of our cases, had a smaller diurnal variation in subfoveal thickness, the thickness changes due to diurnal variation between the earliest (11:26 AM, 1 week) and latest mean times of OCT acquisition (12:40 PM, 6 months) in the MH group and between 11:38 AM (1 week) and 12:52 PM (1 month) in the ERM group is expected to be less than 10.55 μm. The differences in mean OCT acquisition time between the baseline and 1 week postoperative in our study were only 30 and 22 min (examined later at baseline in both groups) for the ERM and MH groups, respectively. The choroidal thickness increase of 13.7 μm following vitrectomy in the ERM group would not be explained by diurnal variation because (1) the time difference, 30 min, is not sufficient to produce such a degree of choroidal thickness change, and (2) the choroidal thickness at 1 week is expected to be less than that at baseline due to diurnal variation as the mean time of OCT acquisition at 1 week is later than that at baseline. This indicates that the choroidal thickness increased at 1 week after vitrectomy as a postoperative change rather than because of diurnal variation.
Furthermore, the effect of combined cataract extraction should be carefully considered because over than half of the patients with ERM and MH had undergone combined cataract extraction and cataract surgery can cause significant increase in postoperative choroidal thickness [18, 19]. However, in the subgroup analyses presented in Supplementary Fig. 1, vitrectomy without cataract surgery in the ERM group also resulted in significant thickness increase in subfoveal and 1-mm superior and inferior choroid in the ERM group at 1 week postoperative visit; therefore, vitrectomy itself may increase choroidal thickness unless its effect was negated by gas, as shown in the MH group. Additionally, the difference in the proportion of ILM-peeled eyes may be a potential source of bias in comparison of choroidal thickness changes between the ERM and MH groups. However, we believe that the difference in mechanical trauma during surgery between ILM peeling in the MH or ERM group and ERM peeling only in the ERM group would be insignificant, thus it is unlikely that the difference significantly affected our results on choroidal thickness. Indeed, there were no significant differences in choroidal thickness at any location at any postoperative time between the eyes with and without ILM peeling. (Supplementary Fig. 2) Although there has been no evidence that the dye used for ILM peeling affects choroidal thickness change following macular surgery, the use of other intraocular dyes might result in different results.
In conclusion, our study showed temporary choroidal thickness increase at all locations in the ERM group and a location-dependent choroidal thickness change at the early postoperative period in the MH group. This difference between the two indications may result from the effect of gas tamponade to decrease postoperative choroidal thickness. Further prospective studies with a larger sample size are required to confirm the effect of intravitreal gas in choroidal thickness.
References
Chung SE, Kang SW, Lee JH, Kim YT (2011) Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 118:840–845. doi:10.1016/j.ophtha.2010.09.012
Imamura Y, Fujiwara T, Margolis R, Spaide RF (2009) Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 29:1469–1473. doi:10.1097/IAE.0b013e3181be0a83
Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S (2011) Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 249:1123–1128. doi:10.1007/s00417-011-1620-1
Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF (2010) Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 117:1792–1799. doi:10.1016/j.ophtha.2010.01.023
Maruko I, Iida T, Sugano Y, Oyamada H, Sekiryu T, Fujiwara T, Spaide RF (2011) Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina 31:510–517. doi:10.1097/IAE.0b013e3181eef053
Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T (2011) Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 151(594–603), e591. doi:10.1016/j.ajo.2010.10.030
Vance SK, Imamura Y, Freund KB (2011) The effects of sildenafil citrate on choroidal thickness as determined by enhanced depth imaging optical coherence tomography. Retina 31:332–335. doi:10.1097/IAE.0b013e3181eef0ae
Kimura M, Nishimura A, Yokogawa H, Okuda T, Higashide T, Saito Y, Sugiyama K (2012) Subfoveal choroidal thickness change following segmental scleral buckling for rhegmatogenous retinal detachment. Am J Ophthalmol. doi:10.1016/j.ajo.2012.05.010
Michalewska Z, Michalewski J, Adelman RA, ZawiSlak E, Nawrocki J (2015) Choroidal thickness measured with swept source optical coherence tomography before and after vitrectomy with internal limiting membrane peeling for idiopathic epiretinal membranes. Retina 35:487–491. doi:10.1097/IAE.0000000000000350
Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146:496–500. doi:10.1016/j.ajo.2008.05.032
Sogawa K, Nagaoka T, Takahashi A, Tanano I, Tani T, Ishibazawa A, Yoshida A (2012) Relationship between choroidal thickness and choroidal circulation in healthy young subjects. Am J Ophthalmol 153(1129–1132), e1121. doi:10.1016/j.ajo.2011.11.005
Kimura M, Nishimura A, Yokogawa H, Okuda T, Higashide T, Saito Y, Sugiyama K (2012) Subfoveal choroidal thickness change following segmental scleral buckling for rhegmatogenous retinal detachment. Am J Ophthalmol 154:893–900. doi:10.1016/j.ajo.2012.05.010
Friberg TR, Lace JW (1988) A comparison of the elastic properties of human choroid and sclera. Exp Eye Res 47:429–436
Fujiwara A, Shiragami C, Fukuda K, Nomoto H, Shirakata Y, Shiraga F (2012) Changes in subfoveal choroidal thickness of epiretinal membrane and macular hole before and after microincision vitrectomy surgery. Nihon Ganka Gakkai Zasshi 116:1080–1085
Brown JS, Flitcroft DI, Ying GS, Francis EL, Schmid GF, Quinn GE, Stone RA (2009) In vivo human choroidal thickness measurements: evidence for diurnal fluctuations. Invest Ophthalmol Vis Sci 50:5–12. doi:10.1167/iovs.08-1779
Tan CS, Ouyang Y, Ruiz H, Sadda SR (2012) Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 53:261–266. doi:10.1167/iovs.11-8782
Chakraborty R, Read SA, Collins MJ (2011) Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci 52:5121–5129. doi:10.1167/iovs.11-7364
Ohsugi H, Ikuno Y, Ohara Z, Imamura H, Nakakura S, Matsuba S, Kato Y, Tabuchi H (2014) Changes in choroidal thickness after cataract surgery. J Cataract Refract Surg 40:184–191. doi:10.1016/j.jcrs.2013.07.036
Noda Y, Ogawa A, Toyama T, Ueta T (2014) Long-term increase in subfoveal choroidal thickness after surgery for senile cataracts. Am J Ophthalmol. doi:10.1016/j.ajo.2014.05.016
Financial support
None
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter, or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Choroidal thickness before and after vitrectomy for epiretinal membrane (ERM group) and macular hole (MH group) in the subgroup operated (A) with and (B) without combined cataract extraction. (A) The ERM and MH groups show a temporary increase in choroid thickness at 1 week postoperative visit. Data at 12 months were available in 21 and seven eyes for the ERM and MH groups, respectively. (B) Compared to the eyes treated with vitrectomy combined with cataract extraction, those treated with vitrectomy only show less remarkable choroidal thickening at 1 week postoperative visit in the ERM group. Such a temporary increase in choroidal thickness is not observed in the MH group. Data at 12 months were available in 23 and 18 eyes for the ERM and MH groups, respectively. The error bars in the left column denote the upper or lower bound of the 95 % confidence interval. (JPEG 87 kb)
Supplementary Figure 2
Subfoveal choroidal thickness following vitrectomy in eyes with epiretinal membrane (ERM) treated with and without internal limiting membrane (ILM) peeling. There are no significant differences at any postoperative time between the two groups. (all P > 0.05) The error bars in the left column denote the upper or lower bound of the 95 % confidence interval. (JPEG 43 kb)
Supplementary Figure 3
Choroidal thickness before and after vitrectomy for proliferative diabetic retinopathy (PDR group) treated by vitrectomy without gas tamponade and rhegmatogenous retinal detachment (RRD group) treated by vitrectomy with gas tamponade. The PDR group shows a temporary increase in choroidal thickness at 1 week postoperative visit, whereas such an increase is rarely observed in the RRD group. The error bars in the left column denote the upper or lower bound of the 95 % confidence interval. (JPEG 57 kb)
Rights and permissions
About this article
Cite this article
Ahn, S.J., Woo, S.J. & Park, K.H. Choroidal thickness change following vitrectomy in idiopathic epiretinal membrane and macular hole. Graefes Arch Clin Exp Ophthalmol 254, 1059–1067 (2016). https://doi.org/10.1007/s00417-015-3154-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3154-4