Abstract
Background
To report functional and high-resolution retinal imaging abnormalities, including adaptive optics (AO) throughout the course of acute macular neuroretinopathy (AMNR).
Methods
Two female patients (four eyes) with a diagnosis of AMNR were observed at the Clinical Investigation Center, CHNO des Quinze-Vingts, Paris, France. The patients underwent detailed ophthalmic examination including best-corrected visual acuity, slit-lamp examination, kinetic and static perimetry, full-field and multifocal electroretinogram, infrared reflectance, autofluorescence imaging and spectral-domain optical coherence tomography (SD-OCT) and AO fundus imaging at presentation and during follow-up.
Results
Both cases showed concomitant loss of integrity of the outer retinal structures on SD-OCT, and marked abnormalities on AO imaging with disruption of the visibility of the cone mosaic. In the first case, photoreceptor damage was seen to progress during several weeks before healing. In both cases, there were persistent morphological abnormalities of photoreceptors 1 year after onset.
Conclusion
This study further highlights the value of AO fundus imaging to facilitate detection, mapping, and monitoring of damage to the cone outer segments during AMNR. In particular, residual damage to the cone mosaic can be precisely documented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute macular neuroretinopathy (AMNR) is a rare maculopathy that was first reported by Bos and Deutman in 1975 [1]. It occurs predominantly in young women and is typically bilateral [2]. It is characterized by a sudden onset of visual disturbances that may follow a flu-like illness. Main symptoms consist of central or paracentral scotomata. Funduscopic examination shows barely visible dark red-brownish wedge-shaped lesions [1]. These subtle fundoscopic changes are better seen with infrared reflectance (IR) using confocal systems showing lobular hypo-reflective lesion, a major help for the diagnosis [3–9]. Recent reports using high-resolution spectral-domain optical coherence tomography (SD-OCT) have shown abnormalities at the level of the outer retina [5, 7, 10–16] suggested by functional tests [17]. The term of AMOR for acute macular outer retinopathy has been proposed to more closely relate to the site of injury [5, 14]. Central static perimetry as well as multifocal electroretinogram are useful adjuncts to document macular dysfunction correlated with structural abnormalities [10, 12, 14, 18–20]. The etiology of AMNR is unclear, and common reports of flu-like illness preceding symptoms would suggest a viral cause [2, 4, 5, 7, 10, 19]. A case of AMNR following infusion of anti-thymocyte globulin has also been reported, raising the issue of cytokine release as a potential pathogenic mechanism [21]. Other triggering factors have been associated with AMNR, including oral contraceptives [22], vasoconstrictive drugs [19, 23, 24], hypotensive episodes [25, 26], or contrast dye intravenous injections [27] suggesting a possible vascular etiology. Supporting this latter hypothesis, a recent study from Sarraf et al., applying SD-OCT, outlined two distinct phenotypes with hyper-reflective lesions located either above (type 1, also known as paracentral acute middle maculopathy) or below (type 2) the plexiform layer, the authors proposing the occlusion of the superficial or deep retinal capillary plexus, respectively, as a putative pathogenic mechanism [28]. On the other hand, Hirooka et al., showed evidence of impaired circulation in the choroid that may be indicative of inflammation present at the early stages of AMNR [29]. Therefore, the exact underlying pathogenic mechanisms that may involve vascular and/or inflammatory process remain unclear, and a precise characterization of retinal abnormalities may help better understand this rare disorder. Adaptive optics (AO), by correcting optical aberrations using a wavefront sensor, allows the direct visualization of the cone photoreceptor mosaic in the macular area [30–33]. Here, we report functional assessment and multimodal imaging including adaptive optics findings to follow retinal changes in two patients with AMNR type 2 [28].
Materials and methods
The study adhered to the tenets of the Declaration of Helsinki and was approved by the local ethics committee. Patients were included after written informed consent was obtained. Each patient underwent full ophthalmic examination. Best-corrected visual acuity was measured using the EDTRS (Early Treatment Diabetic Retinopathy Study) chart. Kinetic and static perimetry within the central 10° were performed. Multifocal electroretinography (mfERG) was performed using a 61-hexagon stimulation pattern and ERG-jet lens recording electrodes, and incorporated the ISCEV recommendations (Veris II for Multifocal ERG) [34]. Imaging comprised infrared reflectance (IR), fundus autofluorescence (FA) imaging at 488 nm, and spectral-domain optical coherence tomography (SD-OCT) (HRAII® and Spectralis® OCT, Heidelberg Engineering, Dossenheim, Germany). The AO camera was based on a 52-actuator electromagnetic deformable mirror and a 1024-lenslet Shack–Hartmann sensor (rtx camera, Imagine Eyes, Orsay, France). A superluminescent diode operating at 750 nm was focused at the retina to probe optical aberrations. A 4x4° retinal area was flood-illuminated by a pulsed infrared LED, and imaged by a lownoise CCD camera. AO images were taken at the eccentricities from 0° to 6° temporal and nasal from fovea. Cone counting was performed on a 500 x 500μm retinal surface in the diseased area using custom-designed software [35].
Results
Patient 1
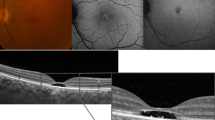
A 17-year-old female presented with a sudden onset and bilateral central scotomata and decreased vision in the left eye of 1 week duration, which was preceded by a flu-like illness. Past medical history was unremarkable, and the patient was not on any medication or oral contraceptive. Visual acuity at presentation was 20/20 in the right eye and 20/63 in the left eye after correction of a mild hyperopia. Anterior segments were unremarkable. Fundus examination revealed parafoveal ill-defined dark-reddish round lesions in both eyes (Fig. 1a — a). Perimetry showed bilateral paracentral decrease in retinal sensitivity (Fig. 2c). Multifocal ERG responses showed decreased amplitudes in response to the central and paracentral hexagons more pronounced for the left eye (Fig. 2a). IR showed bilateral well-defined juxtafoveal petal-shaped hypo-reflective areas (Fig. 1a — b) whereas FAF was unremarkable (Fig. 1a — c). SD-OCT revealed parafoveal areas of irregular reflectivity within the outer retina (Fig. 1a — d and e). These focal changes were localized in the parafoveal region in the right eye, which might explain VA conservation for this eye, whereas changes were mainly in the foveal region on the left. Focal reflective abnormalities of the outer retinal structures were associated to hyper-reflective dots within the outer nuclear layer (ONL). Fluorescein and indocyanine green angiography did not show any abnormalities (not shown)..
Case 1 fundus abnormalities at presentation (A) and 8-month follow-up (B). (a) Color fundus photographs show subtle changes with parafoveal ill-defined dark-reddish round lesions of both maculae. (b) Infrared reflectance images show bilateral well-defined juxtafoveal petal-shaped hypo reflective areas, with a decrease in size and sharpness at 8 months. (c) Fundus autofluoresence images are normal both at presentation and follow-up. Optical coherence tomography: horizontal scan of the right (d) and left (e) eye show parafoveal focal areas of irregular reflectivity, from the outer plexiform layer (dashed arrow) to the interdigitation zone, and a relatively preserved retinal pigment epithelium/Bruch’s complex (filled arrow). Furthermore, parafoveal hyper-reflective dots were present within the outer retina at presentation, better seen on the horizontal scan of the right eye (d). Residual hypo-reflectivity of the ellipsoid and interdigitation zones (filled arrow) and moderate focal thinning of the outer nuclear layer (dashed arrow) are visible at 8 months (e)
Follow-up of macular function disturbances in case 1: Multifocal electroretinogram (a) at presentation, (b) at 10-month follow-up shows decreased amplitudes in responses to central hexagons. Automated static perimetry (10–2 Humphrey visual field) (c) at presentation reveals paracentral decrease in retinal sensitivity, with relative improvement at 10-month follow-up (d)
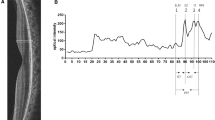
AO imaging at presentation (Fig. 3) revealed sharply demarcated areas of cone mosaic disturbances, with patchy hypo- and hyper-reflectivity corresponding with changes in reflectivity both on IR and SD-OCT. During follow-up examinations, visual acuity improved to 20/15 in the right eye and 20/20 in the left eye at 4 months, remaining stable thereafter. The patient, however, continued to perceive scotomatas on both eyes which were confirmed by static perimetry and multifocal ERG (Fig. 2b, d). Follow-up images showed focal areas of hypo-reflectivity of the ellipsoid and interdigitation zones, with a corresponding discrete thinning of the ONL (Fig. 1b — d and e, supplementary figure). AO imaging (Fig. 4) showed an initial extension of the area of cone mosaic disorganization at 1 month which became blurred, before progressive healing. At 12 months, persistent alteration of the cone mosaic and the discrete thinning of the ONL on SD-OCT document the permanent loss of cone photoreceptor. Cone counting in the diseased retinal area (Fig. 4d, Table 1) showed an initial decrease in cone density at 1 month, with a progressive increase over time.
Follow-up images of the right eye for case 1. (a) Infrared reflectance shows progressive attenuation of the hypo-reflective macular lesion. (b) Optical coherence tomography reveals hypo-reflectivity of the ellipsoid and interdigitation zones, with focal thinning of the outer nuclear layer. (c, d) Adaptive optics images taken from the 2° nasal 2° superior from the foveal center show a sharply demarcated at presentation, and then ill-defined region of hypo-reflectivity that could correspond to the loss or disorganization of inner/outer segment. The lesion size increased at month 1, then slowly decreased over time. The normal photoreceptor mosaic progressively reappears in the border of the lesion. The patches of hyper- and hypo-reflectivity within the parafoveal lesion are noteworthy. All scale bars show 100 μm
Patient 2
A 22-year-old female presented with sudden bilateral central scotomata following an episode of flu-like symptoms. Past medical history was unremarkable, and the patient was not on any medication or oral contraceptive. She was referred to our center 4 weeks after onset of symptoms.
Visual acuity was 20/16 in both eyes without correction. Slit-lamp examination was unremarkable, and fundus examination showed a subtle darkening of both central maculae. Multifocal ERG showed mildly decreased amplitudes in response to peri-central hexagons (Fig. 5a). These changes were well correlated with decreased retinal sensitivity. IR revealed wedge-shaped parafoveal hyporeflectivity and only subtle changes in FAF (Fig. 6). SD-OCT showed abnormal reflexivity restricted to the outermost retinal structures (Fig. 6). AO imaging was not available at presentation.
Follow-up of macular function disturbances in case 2. Multifocal electroretinogram (a) at presentation, (b) at 9-month follow-up shows decreased amplitudes in responses to central hexagons. Automated static perimetry (10° central Octopus 101) (c) at 9 months shows paracentral decrease in retinal sensitivity, with relative improvement at 18-month follow-up (d)
Case 2 fundus abnormalities at presentation and 9 months follow-up. Infrared reflectance images (IR) show bilateral well-defined juxta foveal petal-shaped hypo reflective areas, with a decrease in size and sharpness at 9 months. Fundus autofluoresence images (AF) show very subtle changes within the macular pigment area on both eyes which decrease in follow-up. Optical coherence tomography (OCT): horizontal scans show parafoveal loss of reflectivity of the external limiting membrane, the ellipsoid and interdigitation zones at presentation, better seen on the horizontal scan of the left eye (arrows). Very subtle hyporeflectivity of the ellipsoid zone with moderate focal thinning of the outer nuclear layer is visible at 9 months
At 9 months, symptoms and visual acuity were stable with persisting central scotomata and focal decreased in amplitude at the multifocal ERG (Fig. 5b). Static perimetry showed peri-central areas of decreased retinal sensitivity in both eyes (Fig. 5c), which partially improved after 18 months (Fig. 5d). IR and SD-OCT showed subtle areas of decreased reflectivity. AO imaging revealed an ill-defined cone mosaic in the area corresponding with the hyporeflective zone on IR (Fig. 6). In addition, sparse hyper-reflective dots were also present within the area of cone mosaic structural loss (Fig. 7).
Discussion
The two cases presented here are the third report of AO imaging during AMNR [36, 37], and the first applying a flood-illuminated technique with long-term follow-up. AO imaging demonstrated disturbances of the cone mosaic, which initially increased in size then progressively resolved, yet with persisting damage at 12 months. Taken together, our findings are consistent with photoreceptors being primarily affected in AMNR, resulting in permanent loss of cones [5, 7, 10–14, 37]. These permanent changes should be taken into account when counseling patients with this disorder.
The cause of photoreceptor lesions in AMNR is not known. Our first case reveals hyper-reflective dots within the ill-defined outer retinal structures on SD-OCT at the acute phase, which disappeared at 1 month, an early sign previously reported [10, 12, 16, 38, 39] with an intact retinal pigment epithelium/choriocapillaris complex. This hyper-reflectivity may represent localized edema or fluid collection [10]. Alternatively, the hyper-reflective dots documented here could correspond to inflammatory infiltrates, this reinforcing the viral/infectious hypothesis of AMNR. They could also represent outer segment structures that were shed from the injured photoreceptors. The parafoveal location and peculiar lobular pattern of AMNR lesion is also intriguing, and distinct from other presumed inflammatory disorders that also affect the outer retina, such as multiple evanescent white dot syndrome (MEWDS) or acute zonular occult outer retinopathy (AZOOR), with which AMNR has previously been associated under the entity of AZOOR-complex diseases [40]. The relative sparing of the very central avascular foveal zone, its lobular pattern and the reports of AMNR triggered by hemodynamic changes [19, 23–26] could suggest a vascular component to AMNR. Normal fluorescein angiography, however, would indicate that retinal vessels are not the major site of injury in AMNR pathogenesis, with a recent report implicating choroidal vasculature [29].
Furthermore, since most cases of AMNR occur in young women, with some reports of association with oral contraceptives [22], an hormonal component to the pathogenic mechanism could be suggested. These potential contributing factors are shared with some disorders of the AZOOR complex. The precise interplay between the possible viral/inflammatory, hemodynamic, and hormonal components, and their role in photoreceptor injury in AMNR, remain to be discovered.
AMNR also displays intriguing characteristics with the enhancement of the macular lesions on infrared reflectance. This would support the idea of systematically performing this non-invasive imaging technique in cases of acute, bilateral scotomata and normal fundus, especially if occurring in a young woman. For Mirshahi et al. [9], AMNR would be associated with reduced reflectance of the inner retina, alterations in the outer layers leading to subtle secondary changes in the optical properties of the retinal surface such as in retinal thickness. The preservation of inner retinal structures reported by us and others [5, 7, 10–16] does not support this hypothesis. More recently, Fawzi et al. suggested the origin of IR abnormalities within retinal pigment epithelium/outer segment interdigitation abnormalities [38]. Using AO, we were able to demonstrate that IR changes in AMNR were, at least in part, related to direct photoreceptor disturbances, hyporeflective lobular macular lesions on IR being correlated with areas of disorganized cone photoreceptor mosaic in our two cases.
Conclusion
AO confirms that cone cells are one of the primary sites of injury in AMNR, and that photoreceptor damage can be observed over more than a year after onset, explaining the persistence of visual symptoms. Although a new semiological description is needed to better interpret abnormalities detected with this new technique, AO will take an increasing place in retinal disease assessment, and more specifically in cases of photoreceptor diseases.
References
Bos PJ, Deutman AF (1975) Acute macular neuroretinopathy. Am J Ophthalmol 80(4):573–584
Turbeville SD, Cowan LD, Gass JD (2003) Acute macular neuroretinopathy: a review of the literature. Surv Ophthalmol 48(1):1–11
Amin P, Cox TA (1998) Acute macular neuroretinopathy. Arch Ophthalmol 116(1):112–113
Gandorfer A, Ulbig MW (2002) Scanning laser ophthalmoscope findings in acute macular neuroretinopathy. Am J Ophthalmol 133(3):413–415
Hughes EH, Siow YC, Hunyor AP (2009) Acute macular neuroretinopathy: anatomic localisation of the lesion with high-resolution OCT. Eye 23(11):2132–2134
Gomez-Torreiro M, Gomez-Ulla F, Bolivar Montesa P, Rodriguez-Cid MJ (2002) Scanning laser opthalmoscope findings in acute macular neuroretinopathy. Retina 22(1):108–109
Neuhann IM, Inhoffen W, Koerner S, Bartz-Schmidt KU, Gelisken F (2010) Visualization and follow-up of acute macular neuroretinopathy with the spectralis HRA+OCT device. Graefes Arch Clin Exp Ophthalmol 248(7):1041–1044
Kuznik-Borkowska A, Cohen SY, Broido-Hooreman O, Gaudric A (2006) Acute macular neuroretinopathy. J Fr Ophtalmol 29(3):319–322
Mirshahi A, Scharioth GB, Klais CM, Baatz H (2006) Enhanced visualization of acute macular neuroretinopathy by Heidelberg retina tomography. Clin Exp Ophthalmol 34(6):596–599
Chan WM, Liu DT, Tong JP, Law RW, Lam DS (2005) Longitudinal findings of acute macular neuroretinopathy with multifocal electroretinogram and optical coherence tomography. Clin Exp Ophthalmol 33(4):439–442
Monson BK, Greenberg PB, Greenberg E, Fujimoto JG, Srinivasan VJ, Duker JS (2007) High-speed, ultra-high-resolution optical coherence tomography of acute macular neuroretinopathy. Br J Ophthalmol 91(1):119–120
Maschi C, Schneider-Lise B, Paoli V, Gastaud P (2011) Acute macular neuroretinopathy: contribution of spectral-domain optical coherence tomography and multifocal ERG. Graefes Arch Clin Exp Ophthalmol 249(6):827–831
Vance SK, Spaide RF, Freund KB, Wiznia R, Cooney MJ (2011) Outer retinal abnormalities in acute macular neuroretinopathy. Retina 31(3):441–445
Yeh S, Hwang TS, Weleber RG, Watzke RC, Francis PJ (2011) Acute macular outer retinopathy (AMOR): a reappraisal of acute macular neuroretinopathy using multimodality diagnostic testing. Arch Ophthalmol 129(3):365–368
Baumuller S, Holz FG (2012) Early spectral-domain optical coherence tomography findings in acute macular neuroretinopathy. Retina 32(2):409–410
Azar G, Wolff B, Cornut PL, Sahel JA, Mauget-Faysse M (2012) Spectral domain optical coherence tomography evolutive features in acute macular neuroretinopathy. Eur J Ophthalmol 22(5):850–852
Sieving PA, Fishman GA, Salzano T, Rabb MF (1984) Acute macular neuroretinopathy: early receptor potential change suggests photoreceptor pathology. Br J Ophthalmol 68(4):229–234
Browning AC, Gupta R, Barber C, Lim CS, Amoaku WM (2003) The multifocal electroretinogram in acute macular neuroretinopathy. Arch Ophthalmol 121(10):1506–1507
Corver HD, Ruys J, Kestelyn-Stevens AM, De Laey JJ, Leroy BP (2007) Two cases of acute macular neuroretinopathy. Eye 21(9):1226–1229
Maturi RK, Yu M, Sprunger DT (2003) Multifocal electroretinographic evaluation of acute macular neuroretinopathy. Arch Ophthalmol 121(7):1068–1069
Munch IC, Traustason S, Olgaard K, Larsen M (2012) Acute macular neuroretinopathy in relation to anti-thymocyte globulin infusion. Acta Ophthalmol 90(4):e321–e322
Miller MH, Spalton DJ, Fitzke FW, Bird AC (1989) Acute macular neuroretinopathy. Ophthalmology 96(2):265–269
O'Brien DM, Farmer SG, Kalina RE, Leon JA (1989) Acute macular neuroretinopathy following intravenous sympathomimetics. Retina 9(4):281–286
Desai UR, Sudhamathi K, Natarajan S (1993) Intravenous epinephrine and acute macular neuroretinopathy. Arch Ophthalmol 111(8):1026–1027
Leys M, Van Slycken S, Koller J, Van de Sompel W (1991) Acute macular neuroretinopathy after shock. Bull Soc Belge Ophtalmol 241:95–104
Stilma JS, de Lange JJ, Crezee FC (1987) Bilateral central scotoma with preservation of central vision in 2 patients following caesarean section under spinal anesthesia. Doc Ophthalmol Adv Ophthalmol 67(1–2):59–68
Guzak SV, Kalina RE, Chenoweth RG (1983) Acute macular neuroretinopathy following adverse reaction to intravenous contrast media. Retina 3(4):312–317
Sarraf D, Rahimy E, Fawzi AA, Sohn E, Barbazetto I, Zacks DN, Mittra RA, Klancnik JM Jr, Mrejen S, Goldberg NR, Beardsley R, Sorenson JA, Freund KB (2013) Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol 131(10):1275–1287
Hirooka K, Saito W, Noda K, Ishida S (2014) Enhanced-depth imaging optical coherence tomography and laser speckle flowgraphy in a patient with acute macular neuroretinopathy. Ocul Immunol Inflamm 22(6):485–489
Liang J, Williams DR, Miller DT (1997) Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis 14(11):2884–2892
Pallikaris A, Williams DR, Hofer H (2003) The reflectance of single cones in the living human eye. Invest Ophthalmol Vis Sci 44(10):4580–4592
Roorda A (2000) Adaptive optics ophthalmoscopy. J Refract Surg 16(5):S602–S607
Chui TY, Song H, Burns SA (2008) Adaptive-optics imaging of human cone photoreceptor distribution. J Opt Soc Am A Opt Image Sci Vis 25(12):3021–3029
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Palmowski-Wolfe AM (2008) ISCEV guidelines for clinical multifocal electroretinography (2007 edition). Doc Ophthalmol Adv Ophthalmol 116(1):1–11
Loquin KBI, Nakashima K, Rossant F, Boelle P-Y, Paques M (2011) Photoreceptor detection in in-vivo adaptive optics images of the retina: towards a simple interactive tool for the physicians. IEEE International Symposium on Biomedical Imaging (ISBI), Chicago, pp 191–194
Hansen SO, Cooper RF, Dubra A, Carroll J, Weinberg DV (2013) Selective cone photoreceptor injury in acute macular neuroretinopathy. Retina 33(8):1650–1658
Mrejen S, Pang CE, Sarraf D, Goldberg NR, Gallego-Pinazo R, Klancnik JM, Sorenson JA, Yannuzzi LA, Freund KB (2014) Adaptive optics imaging of cone mosaic abnormalities in acute macular neuroretinopathy. Ophthalmic Surg Lasers Imaging Retina 45(6):562–569
Fawzi AA, Pappuru RR, Sarraf D, Le PP, McCannel CA, Sobrin L, Goldstein DA, Honowitz S, Walsh AC, Sadda SR, Jampol LM, Eliott D (2012) Acute macular neuroretinopathy: long-term insights revealed by multimodal imaging. Retina 32(8):1500–1513
Feigl B, Haas A (2000) Optical coherence tomography (OCT) in acute macular neuroretinopathy. Acta Ophthalmol Scand 78(6):714–716
Gass JD, Hamed LM (1989) Acute macular neuroretinopathy and multiple evanescent white dot syndrome occurring in the same patients. Arch Ophthalmol 107(2):189–193
Acknowledgments
The authors are grateful to patients reported in this study, to the staff from Imagine Eyes for their technical support, and to the orthoptists, nurses, and study coordinators from the CIC503, as well as to the librarian of Quinze-Vingts hospital for her assistance with reference articles.
None of the co-authors have a commercial relationship in relation associated with the data presented in this study.
The project was financially supported by the ANR TecSan 09–09 iPhot and Foundation Fighting Blindness (FFB FFB center grant C-GE-0912-0601-INSERM02), and by IA FFB grant N°: CD-CL-0808-0466-CHNO).
Conflict of interest statement
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Isabelle Audo and Kiyoko Gocho contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary figure
Outer nuclear layer mapping for case 1 at presentation and 9 months and case 2 at 9 and 18 months after onset revealed ONL thinning. (PDF 1592 kb)
Supplementary material
video showing close-up follow-up adaptive optics images of Fig. 4 from the left eye for Case 1, taken from the 2° nasal 2° superior from the foveal center, shows similar pattern of abnormalities as for the right eye, i.e., sharp hyporeflective lesion at presentation, increased in lesion size then decrease with persistent abnormalities at M12 and patchy hyper reflective dots. Scale bar shows 100 μm. (GIF 2924 kb)
Rights and permissions
About this article
Cite this article
Audo, I., Gocho, K., Rossant, F. et al. Functional and high-resolution retinal imaging monitoring photoreceptor damage in acute macular neuroretinopathy. Graefes Arch Clin Exp Ophthalmol 254, 855–864 (2016). https://doi.org/10.1007/s00417-015-3136-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3136-6