Abstract
Purpose
To evaluate the effects of pharmacologically induced mydriasis and miosis on kinetic perimetry findings in normal participants.
Methods
Thirty-eight eyes of 38 healthy young participants underwent kinetic perimetry (Octopus 900 perimeter) with III4e, I4e, I3e, I2e, and I1e stimuli. For each participant, 24 predetermined meridians with 15° intervals were automatically tested with a velocity of 3°/s under normal, mydriatic, and miotic conditions. Mydriasis and miosis were induced by one drop of 0.4 % tropicamide and 2 % pilocarpine hydrochloride, respectively. The isopter area and kinetic sensitivity were compared between the three pupil conditions.
Results
The average pupil size in the normal condition was 5.6 ± 0.9 mm, and it significantly increased to 8.5 ± 0.7 mm after mydriasis (p < 0.01) and decreased to 3.4 ± 0.8 mm after miosis (p < 0.01). Compared to the normal pupil, the isopter area of the dilated pupil was not significantly different under the III4e stimulus; however, it significantly decreased under the I4e, I3e, I2e, and I1e stimuli (p < 0.01). Compared to the normal pupil, the isopter area of the constricted pupil significantly decreased (p < 0.01) with the III4e stimulus and significantly increased with the I3e and I2e stimuli (p < 0.05).
Conclusions
For both pupil conditions, kinetic sensitivity at each meridian showed a similar trend to the isopter area under each stimulus. The isopter area of the dilated pupil generally decreased, whereas the isopter area of the constricted pupil showed various findings. Therefore, careful attention should be paid to changes in the isopter area associated with changes in the pupil size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kinetic perimetry is generally performed using the Goldmann perimeter [1], which requires the examiner to manually control the moving stimulus. Therefore, perimetric results are dependent on examiner skill because it is difficult to precisely match stimulus velocity or measurement meridians among examiners [2]. Thus, inherent examiner bias hinders the accuracy of manual kinetic perimetry. To remove this bias, a few automated kinetic perimeters have been developed [3, 4], which can be used to conduct an unbiased examination.
Many studies have reported a decrease in the sensitivity of static perimetry within 30° of the visual field of pharmacologically dilated [5–10] and constricted [5, 11, 12] pupils. Although a previous study reported that kinetic perimetry induced constriction of isopters in constricted pupils [13, 14], to our knowledge, no such investigation has been carried out in dilated pupils. In addition, in these studies [13, 14], the relationship between pupil size and kinetic perimetry was investigated with the manual Goldmann perimeter. Therefore, an extensive study using automated kinetic perimetry must be performed to clarify the relationship between pupil size and kinetic perimetry findings.
To this end, we conducted the present study to evaluate the effects of pharmacologically induced dilated and constricted pupil on kinetic perimetry findings in healthy participants by using automatic kinetic perimetry.
Materials and methods
Thirty-eight healthy young participants, eight men and 30 women with a mean age of 24.2 ± 4.4 years, who had undergone automated kinetic perimetry, were included in this study. This study followed the tenets of the Declaration of Helsinki. Each participant provided written informed consent after the ethics committee of the Kitasato University School of Allied Health Science approved the study (No. 2013-25).
All participants underwent comprehensive ophthalmic examinations, including non-cycloplegic refraction testing, visual acuity testing at 5 m using a Landolt ring chart, intraocular pressure measurement, ocular axial length measurement, and slit-lamp and fundus examination by a glaucoma specialist. The participants, who had a corrected visual acuity of 20/20 or better, intraocular pressures of 21 mmHg or less, normal optic disc appearance, open angle, and no ophthalmic disease that could affect the visual field test results, were included in this prospective study.
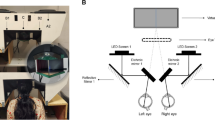
Automated kinetic perimetry was performed with the Octopus 900 perimeter (Haag-Streit, Koeniz, Switzerland). The measurement conditions for automated kinetic perimetry were calibrated automatically to the same measurements as those for the Goldmann perimeter with a background luminance of 10 cd/m2 (31.4 asb). The Goldmann stimulus sizes and intensities of III4e, I4e, I3e, I2e, and I1e were used. The stimulus velocity was 3°/s according to a previous investigation [15], and the stimuli were presented in the following order: III4e, I4e, I3e, I2e, and I1e. Figure 1 shows the measurable area of the perimeter and the starting locations of a moving stimulus. The stimulus test locations were 24 predetermined meridians with a 15° interval. The stimuli were presented randomly from the extreme periphery of the normal age-corrected kinetic sensitivity to the center. The fixation of each participant in this study was monitored with a display, according to previous reports [16–18]. Although the Octopus 900 perimeter adjusts for reaction time by adjusting the isopter area according to the response time to the stimulus, in this study, we did not adjust the reaction time because intra-participant, and not inter-participant, comparisons of kinetic sensitivity were performed among the three pupil conditions.
Area measured by kinetic perimetry and the starting locations of a moving stimulus at each meridian. The area outlined by the dashed line was measured by the Octopus 900 perimeter. The III4e stimulus has been used as an example to show the starting locations with a moving stimulus at each meridian. The stimulus is presented randomly on each meridian from the extreme periphery of normal age-corrected kinetic sensitivity to the center. If the normal age-corrected kinetic sensitivity is outside the measurable area (dashed line), the starting location is set to the extreme end of the measurable area on the same meridian. The I4e, I3e, I2e, and I1e stimuli were also measured using the same method
For each participant, the eye with the lower amount of astigmatism was selected as the study eye. If the amount of astigmatism was the same in both eyes, the eye with the lower myopia was chosen as the study eye. The selected eye of each participant was assessed by expert examiners (KH, CK, and AY). All participants underwent automated kinetic perimetry under the following conditions: normal, dilated, and constricted pupil. Automated kinetic perimetry was performed with the pupil in the normal condition at the beginning, yielding baseline measurements, and with dilated and constricted pupils in a random order over 2 days. Mydriasis and miosis were achieved by 0.4 % tropicamide and 2 % pilocarpine hydrochloride, respectively. These eye drops were instilled, one drop at a time, half an hour before the examination. To focus accurately on the dome-shaped radius of 30 cm, refraction and visual acuity were determined after pupillary constriction or dilation, and the refraction was corrected for near vision (30 cm) by using disposable soft contact lenses. In addition, the size of the pupil was measured by capturing a screenshot on the display screen of the perimeter, 1 min after each measurement. Left-eye meridians were evaluated as mirror images for concurrent analysis of data from the left and right eye.
Next, the isopter area and kinetic sensitivity at each meridian of the dilated and constricted pupils were compared with those of the normal pupil. Kinetic sensitivity, expressed in degrees, indicated the point at which the participant responded to the kinetic stimulus, relative to the fixation point.
The exclusion criteria were as follows: obvious fixation loss during the examination, poor fit of the corrective contact lenses, or occurrence of side effects due to mydriatic and miotic eye drops.
Statistical analysis
All data were compiled in Microsoft Excel and analyzed using the statistical software packages SPSS version 21.0 (IBM Japan, Ltd., Tokyo, Japan) and G*Power3 version 3.1.7 (Franz Faul, Universität Kiel, Germany). The current Octopus 900 perimeter does not display the coordinate axes for expressing the kinetic sensitivity, and it does not measure the pupil size. Therefore, kinetic sensitivity was calculated in degrees from the fixation point, and pupil size was measured with the free ImageJ software version 1.47v (Wayne Rasband, National Institutes of Health, Bethesda, MD).
The Bonferroni test was used to compare the isopter area and kinetic sensitivity between the dilated and constricted conditions and the normal condition. A sample size calculation revealed that 28 participants were required for the three repeated measurements within a single subject. Power calculation was performed, assuming an effect size of 0.25, α error = 0.05, power (1-β error) = 0.80, and non-sphericity correction (1 / [the number of repeated measurement-1]) of 0.5.
Results
No participants were excluded by the exclusion criteria. Thus, 17 right eyes and 21 left eyes with a mean axial length of 25.0 ± 1.4 mm were analyzed. Table 1 shows the changes in spherical equivalent, visual acuity, intraocular pressure, and pupil size under each pupil condition. Compared with the pupil size of 5.6 ± 0.9 mm in the normal condition, the size of the dilated and constricted pupils significantly increased by 2.9 mm and decreased by 2.2 mm, respectively (p < 0.01). Moreover, compared with the refraction of −4.19 ± 3.46 D in the normal condition, the refraction after mydriasis and miosis significantly changed toward hyperopia of 0.23 D (p = 0.01) and myopia of 0.94 D (p < 0.01), respectively. Compared with the visual acuity of −0.24 ± 0.06 in the normal condition, the visual acuity of under-dilated pupils slightly decreased to 0.02 of LogMAR (p = 0.04). No significant difference in intraocular pressure was observed between the three conditions.
Table 2 and Fig. 2 show the changes in the isopter area and kinetic sensitivity at each meridian under each pupil condition. Compared with the isopter area under normal pupil conditions, the isopter area of dilated pupils was not significantly different with the III4e stimulus, whereas the isopter areas with the I4e, I3e, I2e, and I1e stimuli significantly decreased by 805.4, 1106.7, 874.4, and 274.3 degree2, respectively (all p < 0.01). Furthermore, compared to the normal pupil, the isopter area with the III4e stimulus in the constricted pupil significantly decreased by 508.2 degree2 (p < 0.01), whereas the isopter areas with the I3e and I2e stimuli significantly increased by 445.9 and 319.1 degree2, respectively (both p < 0.05). For the dilated pupil, although the kinetic sensitivity at two meridians under the III4e stimulus slightly increased and decreased, respectively, compared to the normal pupil, the kinetic sensitivity at each meridian under each stimulus showed a similar trend to isopter area under each stimulus (all p < 0.05). The percent change in the kinetic sensitivity at each meridian for each stimulus under dilated and constricted pupil conditions compared with the normal pupil condition is shown in Fig. 3.
Kinetic sensitivity at each meridian of each stimulus under normal, dilated, and constricted conditions. Data are shown as the mean and standard deviation for all participants. The mean kinetic sensitivity measured with each stimulus is shown in the lower right part of each panel. The symbols △ and ▼ were used to indicate that the kinetic sensitivity significantly increased and decreased compared to the normal pupil, respectively
Discussion
For the dilated pupil, both isopter area and kinetic sensitivity at each meridian decreased under I4e, I3e, I2e, and I1e stimuli; the decrease in isopter area was especially marked under low-intensity stimuli such as I3e, I2e, and I1e. A previous study involving static perimetry within 30° showed that the mean deviation or mean defect significantly decreases by approximately −1 to −3 dB for the dilated pupil when compared to the normal pupil [6–8, 19]. Although the results of this study cannot be directly compared to those of previous studies because of differences in the measurement method and areas, the tendency for decreasing sensitivity was similar to that reported in the previous study using static perimetry. This similarity is probably due to the influence of the increase in spherical aberration [20], a phenomenon in which the parallel rays of incident light do not converge at the same point after passing through the lens. In fact, it is believed that decreasing sensitivities in the central to middle area could be attributed to the increase in spherical aberration. When visual field testing of the dilated pupil is performed, the kinetic perimetry findings should be carefully interpreted, especially in the case of glaucoma patients receiving pilocarpine therapy, which can affect the findings.
Compared with the normal pupil, both isopter area and kinetic sensitivity at each meridian of the constricted pupil slightly increased under the I3e and I2e stimuli. A previous study using kinetic perimetry [13] reported that compared with the normal pupil, isopter area of the constricted pupil under the I4e, I3e, and I2e stimuli decreased by −24, −33, and −65 %, respectively. However, the current study showed contrasting results: in this study, the isopter area under the I3e and I2e stimuli increased by +6.9 and +11.6 %, respectively. These contrasting results are considered to be associated with differences in pupil size between the previous [13] and current studies. Spherical aberration decreases with decreasing pupil size, and conversely, diffraction increases with decreasing pupil size [20]. In previous studies, the pupil area and diameter after a 30-min instillation of the miotic agent were 2.74 mm2 and 1.4 mm, respectively [13, 14], and it is thought that the decrease in isopter area is influenced by the increase in diffraction. However, it is also known that visual performance is the best when the pupil diameter is approximately 3.5 mm because aberration and diffraction are markedly decreased at this pupil size [20]. In the current study, the isopter area of the miotic pupil increased under I3e and I2e stimuli, indicating that the average pupil size of 3.4 mm, at which aberration and diffraction were the smallest, was most suited to optimal visual performance. On the other hand, the isopter area of the constricted pupil under the III4e stimulus was smaller than that of the normal pupil. This finding can be attributed to a decrease in the amount of incident light from the extreme periphery of the visual field associated with pupil constriction, rather than diffraction. Although previous reports did not evaluate the extreme periphery of the visual field using a stimulus size of III4e [13, 14], the isopter area of II4e and I4e decreased under miosis compared with that under a normal pupil condition. Although this may be attributed to diffraction, considering that the pupil size was less than 2 mm, the current study showed a finding similar to that of previous studies [13, 14]. Our study suggests that kinetic sensitivity at the middle to central area increases, but the overall extent of the visual field is slightly narrow under moderate miosis.
Previously, several ophthalmic drops that affect pupil size were used in glaucoma patients, such as pilocarpine, epinephrine, and dipivefrine, and these eyedrops were generally instilled to decrease the intraocular pressure. Therefore, visual field findings were carefully assessed in patients using these ophthalmic drops. Currently, however, these ophthalmic drops are not prescribed as often, and prostaglandin or β blocker agents are generally prescribed for the treatment of glaucoma. Therefore, less attention is now paid to pupil diameter. Interestingly, among the different glaucoma therapeutics, it has been reported that 0.15 % [21, 22] and 0.2 % [23, 24] brimonidine has a miotic effect in addition to an intraocular pressure-lowering effect, constricting the pupil from 5–6 to 3–4 mm. It is known that a pupil size of around 3.5 mm is associated with the best visual performance because the effect of aberration and diffraction are the lowest. Therefore, visual field findings must be carefully evaluated before and after instillation of brimonidine.
This study has the following limitations. First, only young participants were included in this study. The baseline pupil size varies according to age. It is generally recognized that the pupil size decreases with increasing age. Therefore, variations in kinetic sensitivity measured with mydriatic and miotic agents might be different in elderly participants. Second, only participants with normal vision, and no regions of abnormal vision, were evaluated in this study. Although a previous study has reported that the scotoma area does not change before and after instillation of miotic agents in patients with glaucoma [14], further investigation in this context is warranted because manual kinetic perimetry was used in that study [14]. Third, the bias in the order of stimulus presentation and test conditions may have affected the results. In the current study, the largest and most intense stimulus (III4e) was presented first, and all participants underwent the testing under the normal pupil condition first. However, these biases are expected to have a small impact, if any, because participants who had previously undergone automated kinetic perimetry were included in the current study to exclude the learning effect.
In conclusion, under dilated pupil, the isopter area generally decreases, and under constricted pupil, the peripheral isopter area decreases. However, the isopter area of the constricted pupil increases in the middle to central region. These results suggest the possibility that moderate miosis would be conducive to increased isopter area in the middle under low-intensity stimuli. Thus, during the evaluation of kinetic perimetry findings, the changes in isopter area due to alterations in the pupil size must be carefully considered.
References
Goldmann H (1945) Grundlagen exakter perimetrie. Ophthalmologica 109:57–70. doi:10.1159/000300224
Trobe JD, Acosta PC, Shuster JJ, Krischer JP (1980) An evaluation of the accuracy of community-based perimetry. Am J Ophthalmol 90:654–660
Weinreb RN, Perlman JP (1986) The effect of refractive correction on automated perimetric thresholds. Am J Ophthalmol 101:706–709
Johnson CA, Keltner JL (1987) Optimal rates of movement for kinetic perimetry. Arch Ophthalmol 105:73–75
Lindenmuth KA, Skuta GL, Rabbani R, Musch DC (1989) Effects of pupillary constriction on automated perimetry in normal eyes. Ophthalmology 96:1298–1301
Kudrna GR, Stanley MA, Remington LA (1995) Pupillary dilation and its effects on automated perimetry results. J Am Optom Assoc 66:675–680
Park HJ, Youn DH (1994) Quantitative analysis of changes of automated perimetric thresholds after pupillary dilation and induced myopia in normal subjects. Korean J Ophthalmol 8:53–60
Mendivil A (1997) Influence of a dilated pupil on the visual field in glaucoma. J Glaucoma 6:217–220
Wood JM, Wild JM, Bullimore MA, Gilmartin B (1988) Factors affecting the normal perimetric profile derived by automated static threshold LED perimetry. I. Pupil size. Ophthalmic Physiol Opt 8:26–31
Herse PR (1992) Factors influencing normal perimetric thresholds obtained using the Humphrey Field Analyzer. Invest Ophthalmol Vis Sci 33:611–617
Edgar DF, Crabb DP, Rudnicka AR, Lawrenson JG, Guttridge NM, O’Brien CJ (1999) Effects of dipivefrin and pilocarpine on pupil diameter, automated perimetry and LogMAR acuity. Graefes Arch Clin Exp Ophthalmol 237:117–124
Webster AR, Luff AJ, Canning CR, Elkington AR (1993) The effect of pilocarpine on the glaucomatous visual field. Br J Ophthalmol 77:721–725
McCluskey DJ, Douglas JP, O’Connor PS, Story K, Ivy LM, Harvey JS (1986) The effect of pilocarpine on the visual field in normals. Ophthalmology 93:843–846
Kee CW, Youn DH (1987) The influence of miotics on the visual field. Korean J Ophthalmol 1:52–58
Hirasawa K, Shoji N, Okada A, Takano K, Tomioka S (2014) Evaluation of stimulus velocity in automated kinetic perimetry in young healthy participants. Vis Res 98:83–88. doi:10.1016/j.visres.2014.03.010
Nowomiejska K, Vonthein R, Paetzold J, Zagorski Z, Kardon R, Schiefer U (2010) Reaction time during semi-automated kinetic perimetry (SKP) in patients with advanced visual field loss. Acta Ophthalmol 88:65–69. doi:10.1111/j.1755-3768.2008.01407.x
Schiefer U, Strasburger H, Becker ST, Vonthein R, Schiller J, Dietrich TJ, Hart W (2001) Reaction time in automated kinetic perimetry: effects of stimulus luminance, eccentricity, and movement direction. Vis Res 41:2157–2164
Wakayama A, Matsumoto C, Ohmure K, Inase M, Shimomura Y (2011) Influence of target size and eccentricity on binocular summation of reaction time in kinetic perimetry. Vis Res 51:174–178. doi:10.1016/j.visres.2010.11.002
Lindenmuth KA, Skuta GL, Rabbani R, Musch DC, Bergstrom TJ (1990) Effects of pupillary dilation on automated perimetry in normal patients. Ophthalmology 97:367–370
Freeman MH, Hull CC, Charman WN (2003) Chapter 15: the eye as an optical instrument. Butterworth Heinemann, Edinburgh
Gerente VM, Biondi AC, Barbosa CP, Lottenberg CL, Paranhos A Jr (2007) Effect of brimonidine tartrate 0.15 % on scotopic pupil: controlled trial. J Ocul Pharmacol Ther 23:476–480. doi:10.1089/jop.2007.0017.R1
Thordsen JE, Bower KS, Warren BB, Stutzman R (2004) Miotic effect of brimonidine tartrate 0.15 % ophthalmic solution in normal eyes. J Cataract Refract Surg 30:1702–1706. doi:10.1016/j.jcrs.2003.12.037
Besada E, Reed K, Najman P, Shechtman D, Hardigan P (2011) Pupillometry study of brimonidine tartrate 0.2 % and apraclonidine 0.5 %. J Clin Pharmacol 51:1690–1695. doi:10.1177/0091270010385932
Kim JM, Park KH, Kim CY, Kim HK, Kim TW, Kim MS (2011) Effects of brimonidine timolol fixed combination therapy on anterior ocular segment configuration. Jpn J Ophthalmol 55:356–361. doi:10.1007/s10384-011-0046-y
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Clinical trial registration
We registered this study with UMIN. (http://www.umin.ac.jp/). ID: UMIN000013248. Date of registration: 2014/02/24.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hirasawa, K., Shoji, N., Kobashi, C. et al. Effects of mydriasis and miosis on kinetic perimetry findings in normal participants. Graefes Arch Clin Exp Ophthalmol 253, 1341–1346 (2015). https://doi.org/10.1007/s00417-015-3048-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3048-5