Abstract
Purpose
To describe the spectral-domain optical coherence tomographic (SD-OCT) features of inflammatory choroidal neovascular membranes (iCNV) in multifocal choroiditis and punctate inner choroidopathy, and to compare them to those of the acute inflammatory lesions in the same underlying diseases. This is a retrospective, consecutive, observational case series.
Methods
Each patient underwent a comprehensive eye examination, fundus photography, and fluorescein angiography (FA) on the initial visit. SD-OCT features of iCNV were reviewed at presentation and 4 weeks later, and were compared to SD-OCT features of the inflammatory lesions. There were ten eyes with iCNV and eight eyes with the acute lesions of chorioretinitis.
Results
All iCNV had a sub-retinal pigment epithelium (sub-RPE) component and a subretinal or retinal component that infiltrated the outer retinal layers to different extents. All iCNV had associated fluid exudation, and all showed RPE and inner segment/outer segment junction layer (IS/OS) disruption. On the other hand, approximately half of the inflammatory lesions were confined between Bruch’s membrane and RPE; the rest showed infiltration into the outer retinal layers in a pattern similar to iCNV, with no fluid exudation but with associated choroidal hyperreflectivity. In most of them, disruption of RPE and IS/OS was also noted.
Conclusions
The acute lesions of chorioretinitis can be difficult to distinguish from iCNV based on clinical examination and FA. However, iCNV demonstrate characteristic SD-OCT features not seen with the inflammatory lesions. These findings may help to differentiate these two entities that typically require different treatments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammatory choroidal neovascular membrane (iCNV) is a sight-threatening complication of retinochoroidal inflammatory and infectious conditions. It is a fairly rare condition. Perentes et al. described its occurrence in 1.9 % of patients with uveitis and in 4.8 % of those with posterior uveitis [1].
Fluorescein angiography (FA) remains the gold standard test for the initial diagnosis of CNV. However, the advent of optical coherence tomography (OCT) has revolutionized the diagnosis and treatment of retinal diseases especially those involving the macular region. Spectral-domain OCT (SD-OCT) with its high acquisition speed allows an axial resolution of 3 to 7 μm in the eye. With its enhanced sensitivity and specificity, it detects fine in-vivo morphological changes in retinal microstructure, thus improving the diagnostic and decision-making abilities of ophthalmologists [2]. Hassentein and Meyer have recently reported a high correlation between histological structure of the retina and the image acquired by SD-OCT [3].
Inflammatory CNV develops in several conditions of posterior uveitis such as punctate inner choroidopathy (PIC) and multifocal choroiditis (MFC), which are both included in the white dot syndrome spectrum. A cross-sectional study of 66 patients with MFC and 13 patients with PIC compared the clinical characteristics at presentation between these two diseases. A greater frequency of CNV at presentation was found among patients with PIC than those with MFC (76.9 vs 27.7 %, p = 0.002). The presence of CNV was the most common reason for poor visual acuity (VA) at presentation in both MFC and PIC [4]. When CNV develops sequential to posterior uveitis, prompt therapy is essential.
The common practice nowadays for the management of iCNV relies on the use of intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents. Anti-VEGF therapy was reported to provide long-term vision maintenance in more than 90 % of patients with neovascular age-related macular degeneration and substantial visual improvement in 25 to 40 % of patients [5, 6]. Inflammatory chorioretinal lesions may also have the potential for adverse visual consequences. Treatment of inflammatory lesions relies on using immunosuppressant therapy.
Making the distinction between inflammatory lesions and iCNV is mandatory for the therapeutic approach. Both FA and OCT serve as diagnostic adjuncts. In this study, we investigated a consecutive series of patients with iCNV and inflammatory lesions secondary to MFC and PIC, and we sought to characterize the SD-OCT features of iCNV and to compare them to those of the inflammatory lesions occurring in the same disease entities.
Patients and methods
This observational case series included ten eyes of ten patients (seven females, three men; median age 30 years, range 6–37 years) with newly diagnosed iCNV who presented at the uveitis clinics of two tertiary referral centers between 2008 and 2012. The second group included eight eyes of six patients who presented in the same time period with acute inflammatory chorioretinal lesions (four females, two men; median age 30 years, range 21–36 years). All these six patients harbored also iCNV, and were part of the iCNV group studied (Tables 1, 2, and 3). All patients underwent comprehensive work-up ruling out any underlying systemic inflammatory or infectious etiology, full clinical examination, and imaging using FA and OCT. The OCT scans from our study patients were captured using Spectralis HRA + OCT™ (Heidelberg Engineering Inc., Vista, CA, USA), which provides an axial resolution of 3.9 μm and a transverse resolution of 14 μmin tissue [7].
Inflammatory CNV was defined by its clinical signs (e.g., grayish subretinal lesion with subretinal, intraretinal hemorrhage plus/or exudation with serosanguinous fluid accumulation) and angiographic features (i.e., leakage). Inflammatory lesions were defined by the appearance of chorioretinal lesions in the absence of clinical signs of iCNV and with mild leakage on FA. Multiple yellow-white idiopathic inflammatory lesions in the fundus were classified as either MFC or PIC using diagnostic criteria based on the original descriptions of these two conditions [8, 9]. Fluorescein angiograms were read separately by two of the authors (RA, MK), and the ones that were agreed on were included in the study as iCNV or inflammatory lesions.
For patients presenting with iCNV, treatment consisted of intravitreal bevacizumab (Avastin-1.25 mg/0.05 ml) combined with systemic anti-inflammatory therapy, whereas systemic anti-inflammatory therapy was instituted for patients with acute sight-threatening chorioretinitis.
The data were retrieved from the patients’ medical records and included: age at presentation, gender, best-corrected visual acuity (VA) on Snellen chart at presentation and 4 weeks later, etiology, and treatment. SD-OCT features of iCNV that were analyzed at presentation and 4 weeks later included lesion dimensions, associated fluid exudation, location, retinal pigment epithelium (RPE) and inner segment/outer segment junction layer (IS/OS) disruption, and choroidal hyperreflectivity. The same features were analyzed for the inflammatory lesions. The research adhered to the tenets of the Declaration of Helsinki, and was approved by the institutional review boards of both hospitals. Student’s t-test was used for visual acuity analysis and OCT measurement analysis before and after treatment.
Results
Patients’ characteristics and underlying etiology
Inflammatory CNV and the inflammatory lesions were more commonly encountered in females than males (Table 1). Median age was 30 years, and was similar in the two groups. The underlying disease was bilateral in around 2/3 of the cases in each group. The left eye was involved in 80 % of the cases with iCNV, while inflammatory lesions were equally distributed in the right and the left eyes. Inflammatory CNV was secondary to PIC in 60 % of the eyes and to MFC in 40 % of the eyes, whereas PIC and MFC were each the underlying etiology for the inflammatory lesions in 50 % of the eyes.
Treatment and visual outcome
All patients presenting with iCNV were treated with intravitreal bevacizumab injection (1.25 mg/0.05 ml). Nine of the 11 events were also treated with systemic immunosuppressants, as two patients declined systemic therapy. Five patients received prednisone, two received methotrexate orally in addition to prednisone, one received azathioprine and prednisone, and one received cyclosporine-A in addition to prednisone. Mean VA at presentation was 6/15 (range 1/36–6/6), improving to a mean of 6/7.5 after a 4-week period (range 6/20–6/6) (p = 0.011). Mean VA for the inflammatory lesions at presentation and 4 weeks later was 6/9 (range 6/45–6/6), and it was not found to be significantly different from that of the iCNV group (p = 0. 14).
With regard to patients presenting with the inflammatory lesions, four out of 11 events were observed with no therapeutic intervention, as these lesions were not felt to be presenting an immediate threat to vision. Three of the inflammatory lesions were treated with prednisone, two with methotrexate orally and prednisone, one with azathioprine and prednisone, and one with cyclosporine-A and prednisone.
Characteristics of lesions
Number of events studied
The total number of iCNV events studied was 11, as in one eye, two events that occurred 20 months apart were included. In eyes with the inflammatory lesions, 11 events as well were analyzed; in one of the eyes, two events that occurred at a 4-month-interval were included, and in the fellow eye of the same patient, three events that occurred simultaneously were analyzed (Tables 1, 2 and 3).
Lesion location in relation to foveal center by OCT
More than half of the iCNV (54.5 %, 6/11 of the events) were juxtafoveal in location, 27.3 % (3/11) were subfoveal and 18.2 % (2/11) were extrafoveal. The majority of the inflammatory lesions were extrafoveal in location (63.6 %, 7/11), 27.3 % (3/11) were subfoveal, and 9.1 % (1/11) were peripapillary in location.
Lesion structure and associated fluid exudation
All iCNV were heterogeneous in contour and content, whereas all inflammatory lesions were homogenous.
Intraretinal (IRF) and/or subretinal fluid (SRF) were observed in each of the iCNV, while none of the inflammatory lesions had any associated fluid exudation. In around half of the iCNV (54.5 %, 6/11 of the events) both IRF and SRF exudation occurred simultaneously, in 27.3 % (3/11) only IRF was noted, and in 18.2 % (2/11) only SRF was observed. At a 4-week interval, fluid exudation resolved in eight out of the 11 events (72.7 %); IRF with SRF remained in only one case (9.1 %), whereas IRF only with no SRF was detected in two events (18.2 %).
Lesion dimensions
Inflammatory CNVs were significantly bigger in dimensions in comparison to the inflammatory lesions; mean height of iCNV at presentation was 183.5 μm (median 155 μm, range 111–415 μm) whereas mean height of the inflammatory lesions was 101.5 μm (median 100 μm, range 42–205 μm) (p = 0.017). Furthermore, the base of the iCNV was significantly larger than that of the inflammatory lesions; its mean base was 1157.8 μm (median 892 μm, range 369–2,416 μm) and that of the inflammatory lesions was 433.8 μm (median 370 μm, range 92–1,210 μm) (p = 0.0047).
Four weeks later, iCNV regressed significantly; mean height was 113 μm (median 79 μm, range 65–233 μm) (p = 0.04) and mean base was 660.2 μm (median 654.5 μm, range 223–1,015 μm) (p = 0.047).
The mean height of the inflammatory lesions at 4 weeks was 89.4 μm (median 50 μm, range 0–283 μm) (p = 0.71), whereas mean base at 4 weeks was 317.1 μm (median 299 μm, range 0–832 μm) (p = 0.34).
Lesion location
All the iCNVs had a sub-RPE component [located between RPE layer and Bruch’s membrane (BM)] and a retinal or subretinal component. Approximately half of the iCNVs (54.5 %, 6/11) were composed of a sub-RPE component and a retinal component extending till the level of the OPL (Fig. 1a, see also Figs. 3 and 4). In 27.3 % (3/11) of iCNVs, there was a sub-RPE component and a subretinal component well-demarcated by the external limiting membrane (ELM) (Fig. 1b). In 18.2 % (2/11) of iCNV there was a sub-RPE component with a retinal component infiltrating into the ONL (Fig. 1c). In one eye of those in Fig. 1a and one eye of those in Fig. 1b, the retinal/subretinal component developed on top of an RPE scar (Fig. 1d). In these two eyes, the underlying etiology was MFC.
Location of inflammatory choroidal neovascular membrane (iCNV). a A dome-shaped heterogeneous iCNV (arrow) is seen with a sub-RPE component and a retinal component extending up to the level of the outer plexiform layer (asterisk). Intraretinal fluid is also noted with severe RPE and IS/OS disruption. b A dome-shaped sub-RPE component is seen (hollow arrow) with a subretinal component limited by the external limiting membrane (asterisk). Subretinal fluid (double arrow) is noted on either side of the lesion with increase in the thickness and the reflectivity of the outer nuclear layer and the outer plexiform layer (black arrow). c A sub-RPE component is seen underneath a disrupted RPE layer (hollow arrow) with a retinal component that has extended beyond the external limiting membrane into the outer nuclear layer (black arrow). Subretinal fluid is noted (double arrow) as well as IS/OS disruption. Increased thickness and reflectivity of the outer nuclear and outer plexiform layers (asterisk) is well demonstrated. d A heterogeneous lesion (arrow) is demonstrated with its retinal part extending to the level of the outer plexiform layer (pointed v), growing on a disrupted scarred RPE layer
With regard to the inflammatory lesions, the majority (45.5 %, 5/11) were well-demarcated lesions located between RPE and an intact BM (Fig. 2a). In 36.4 % (4/11) of the events, there was a sub-RPE component and a retinal component; in two of these cases, the retinal component infiltrated into the ONL, and in two of them it extended till the OPL (Fig. 2b and c respectively). In two eyes (18.1 %), a retinal component without sub-RPE component was demonstrated; in one eye the retinal lesion extended till the OPL, and in one eye it was subretinal, well-delineated by the ELM (Fig. 2d and e respectively).
Location of the inflammatory lesions. a A well-demarcated dome-shaped homogenous lesion (asterisk) in a patient suffering from punctate inner choroidopathy is shown located between RPE and intact Bruch’s membrane. Choroidal hyperreflectivity is well demonstrated here. Adjacent to it is an inactive lesion with atrophic changes in outer retinal layers. b A sub-RPE component is seen with a retinal component infiltrating into the outer nuclear layer. Choroidal hyperreflectivity is well shown here. c A sub-RPE component is seen with a retinal part extending till the level of the outer plexiform layer (asterisk). Disruption of the RPE and IS/OS is also noted with slight choroidal hyperreflectivity. d A dome-shaped retinal lesion is seen extending between the RPE complex and the outer plexiform layer. Choroidal hyperreflectivity is shown as well. Adjacent to it is an inactive RPE scar. e A placoid-shaped homogenous subretinal lesion is seen limited by the external limiting membrane. A disrupted RPE layer with choroidal hyperreflectivity is also demonstrated
Additional SD-OCT features
We have looked into additional SD-OCT features of the iCNV complex and the inflammatory lesions. RPE disruption and IS/OS disruption was noted in all of the iCNV and in most of the inflammatory lesions (82 and 91 % respectively). However, choroidal hyperreflectivity was noted in all of the inflammatory lesions, and in only a third of iCNV (Fig. 2).
Inflammatory CNV-associated RPE disruption and IS/OS disruption resolved at the 4-week interval in 20 and in 27.3 % of the lesions respectively. Choroidal hyperreflectivity was present in 36.4 % of the lesions at presentation, and appeared in 45.5 % of them 4 weeks later.
Clinical examples
Figure 3 illustrates an example of juxtafoveal CNV secondary to PIC in a 36-year-old female presenting with blurring of vision in the left eye. Upon presentation, VA was 6/10. SD-OCT demonstrated a heterogeneous lesion with sub-RPE and a retinal component extending till the OPL. There was associated IRF and disrupted IS/OS and RPE layer. The patient was treated with intravitreal bevacizumab injection in addition to prednisone. One month later, VA improved to 6/7.5. Total regression of the retinal component was noted, and the sub-RPE component was well-visualized, homogenous, with a restored IS/OS, RPE and complete fluid resorption.
The upper part displays a juxtafoveal heterogeneous CNV in a young female with PIC. A sub-RPE component is seen with a disrupted RPE layer and IS/OS layer. A retinal component is also seen extending till the level of OPL. The lower part shows the lesion 4 weeks post-treatment. A well-demarcated homogenous lesion is seen well-delineated between RPE and Bruch’s membrane. Intraretinal fluid was completely resorbed, and RPE and IS/OS were restored
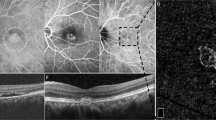
Figures 4 and 5 illustrate the color fundus characteristics, FA and SD-OCT features of iCNV in two patients secondary to PIC, while Fig. 6 shows the imaging characteristics of an inflammatory lesion secondary to PIC.
a Color fundus photograph showing a yellowish juxtafoveal CNV (hollow arrow) with small white juxtapapillary retinal inflammatory lesions (black arrow). b Fluorescein angiogram showing marked leakage from the CNV with fuzzy borders (hollow arrow) and mild leakage from the inflammatory juxtapapillary lesion (black arrow). c SD-OCT shows the CNV (hollow arrow) disrupting RPE and extending up to the level of the outer plexiform layer with adjacent intraretinal and subretinal fluid. The inflammatory lesion (black arrow) is seen adjacent to inactive RPE scar also extending till the level of the outer plexiform layer with no associated fluid exudation but with choroidal hyperreflectivity
a Color fundus photograph showing a gray-white deep retinal lesion (CNV) superonasal to fovea with two dot retinal hemorrhages on its lower edges. Two punctate atrophic retinal lesions are noted inferior and inferotemporal to fovea. b Late-phase fluorescein angiogram showing the leaking CNV superonasal to fovea (arrow) and staining of the atrophic lesions inferior and inferotemporal to fovea. c SD-OCT showing the heterogenous CNV (arrow) with the sub-RPE part and the retinal part extending into the outer nuclear layer, and in d the associated intraretinal fluid is illustrated
a Color fundus photograph showing an active retinal inflammatory lesion (hollow arrow) in a patient with punctate inner choroidopathy and one atrophic retinal lesion superior to fovea and two atrophic lesions inferior to optic disc. b Fluorescein angiogram demonstrating mild leakage from the active lesion that remains with distinct borders (hollow arrow) and staining of the other atrophic lesions. c SD-OCT showing the active inflammatory lesion (hollow arrow) with a sub-RPE component and a retinal component extending till the level of the outer plexiform layer. No intraretinal or subretinal fluid is observed
Discussion
In this study, we aimed to characterize SD-OCT features of iCNV at presentation and to track the changes observed 4 weeks later after the institution of medical therapy. Also, their features were compared to SD-OCT characteristics of inflammatory lesions occurring in the same underlying diseases, PIC and MFC, culminating thus in a pretty homogenous group of patients. Spaide et al. [10] in a recent paper discussed redefining MFC and PIC through multimodal imaging. The authors found that since both conditions target the same essential structures in the same phenotypic manner and, when active, are treated the same way, there seemed to be limited clinical utility in trying to differentiate them, and suggested that PIC was a subset of MFC. Kedhar et al. [4] reported that the presence of CNV was the most common reason for poor VA at presentation in both MFC and PIC.
This study demonstrates that all iCNV had a sub-RPE component and a subretinal or retinal component that infiltrated the outer retinal layers to different extents. All iCNV had associated fluid exudation, and all showed RPE and IS/OS disruption.
On the other hand, approximately half of the inflammatory lesions were confined between an intact BM and RPE; the rest showed infiltration into the outer retinal layers in a pattern similar to iCNV, but with no fluid exudation. In all of the inflammatory lesions, there was an associated choroidal hyperreflectivity. And in most of them, similar to what was described for iCNV, there was disruption of RPE and IS/OS. These findings comply with what was recently reported by Spaide et al. [10] on inflammatory lesions in MFC and PIC; as some of the solid RPE detachments appeared to rupture through RPE resulting in an outpouring of infiltrate into the outer retina. These findings were further confirmed by Zhang et al. [11] who noted that active lesions (stage 3) typically occur beneath the plane of OPL, and that they never destroy the layer structure of the inner retina.
Despite Gass’s definition [12] of distinct CNV growth patterns [type 1 (sub-RPE) in AMD vs type 2 (sub-retinal) in inflammation], this study clearly illustrates that the growth pattern of iCNV is no different from that occurring in AMD; from the subRPE space, through a breach in RPE, into either the subretinal space or into the outer retinal layers. Grossniklaus [13], who described the histopathologic findings of CNV secondary to different underlying diseases (AMD, POHS, myopia, idiopathic and pattern dystrophy), displayed that the cellular and the extracellular CNV components (RPE, vascular endothelium, fibrocytes, macrophages, photoreceptors, erythrocytes, lymphocytes, myofibroblasts, collagen, fibrin) were similar among the different etiologies, and concluded that CNVs represent a stereotypic, nonspecific, wound-repair response to a specific stimulus, similar to granulation tissue proliferation.
In exudative AMD, Park et al. [14] reported using Fourier-domain OCT, a combined subretinal and sub-RPE growth pattern of various degrees in 71 % of eyes. Likewise, our study, through the technique of SD-OCT which enables precise visualization of in-vivo retinal morphology, illustrates clearly CNV growth through a breach in RPE. SD-OCT demonstrated the growth of the neovascular complex into the subretinal space or into the outer retinal layers. A similar pattern was also displayed for the inflammatory lesions, as some of them did grow into the outer retinal layers. It is believed that in the early stages of inflammation, these lesions are composed of inflammatory infiltrate, and that the neovascular component develops sequential to the pro-angiogenic properties of some of the inflammatory mediators produced in this process, thus leaking fluid with associated retinal hemorrhages in some instances [15].
Spectral-domain OCT in this study enabled fine delineation of iCNV features not previously noted by other OCT machines. For example, Kotsolis [16] reported on FA and OCT features of iCNV in 20 eyes with MFC; juxtafoveal location was the most common in more than half of the cases, with a similar distribution to our group; however, IRF and/or SRF was detected in only 53.8 %, and this contradicts our results, as IRF/SRF could be detected in 100 % of eyes with iCNV in our study. This difference in results could be attributed to using different models of OCT machine. Kotsolis used both a Stratus OCT camera (Carl Zeiss Meditec, Dublin, CA, USA) and a Spectralis HRA + OCT, whereas our study relied solely on using Spectralis HRA + OCT™ (Heidelberg Engineering Inc., Vista, CA, USA). The high resolution obtained with modern OCT machines has allowed an almost “histopathologic” view of the retina, and this has changed our appreciation and understanding of the pathogenesis of different disease entities.
In addition, all inflammatory lesions showed a characteristic choroidal hyperreflectivity which was observed in only one third of iCNV. This feature had been previously reported by Vance et al. [17] in three acutely inflamed lesions of MFC, but was absent in myopic CNV. The authors hypothesized that this feature could be the result of increased light penetrance through disrupted photoreceptor and RPE layers. We believe that choroidal hyperreflectivity is a reflection of inflammatory infiltrate in the choroidal layer, as well as disruption in the outer retinal layers and RPE. A recent histopathological and electron microscopic analysis [18] of excised PIC-associated CNV demonstrated a focus of inner choroidal inflammatory cells which was composed of mature small lymphocytes. The same study displayed the occurrence of pigment granules extracellularly from focally damaged choroidal melanocytes. Spaide et al. [10] described slight thickening of the choroid underlying some acute lesions, but it did not appear to be consistently affected. Similarly, Zhang et al. [11] demonstrated increasing choroidal thickness in the active phase and thinning at the late regression stage.
Four weeks following the institution of medical therapy, restoration of IS/OS was noted in one patient, and RPE layer restoration occurred in two patients (as illustrated in Fig. 3). This has been previously noted in PIC [19] and in other inflammatory conditions such as multiple evanescent white dot syndrome (MEWDS). For example, one publication by Li and Kishi [20] which evaluated OCT findings in seven eyes with MEWDS, illustrated that all showed a disrupted or irregular IS/OS junction line of varied extent that was restored over a mean follow-up of 3.4 months. Spaide et al. [21] also evaluated 18 eyes with MEWDS, AZOOR, or MFC, and showed that there was loss of IS/OS layer that improved over time in some of the eyes correlating with functional outcome. In this study, disruption of IS/OS and RPE layers occurred in both iCNV and the inflammatory lesions; thus, this characteristic does not represent a differentiating feature between the two conditions. We speculate that with prompt treatment and before the ensue of atrophic outer retinal changes, most lesions would show restoration of IS/OS and RPE corresponding to the improvement in visual function.
In conclusion, advances in OCT technology have enhanced our understanding of the different retinal pathologies, and in this study SD-OCT delineated distinctive features for iCNV and inflammatory chorioretinal lesions, thus enabling precise approach for prompt diagnosis and treatment.
References
Perentes Y, van Tran T, Sickenberg M, Herbort CP (2005) Subretinal neovascular membranes complicating uveitis: frequency, treatments, and visual outcome. Ocul Immunol Inflamm 13:219–224
Regatieri CV, Branchini L, Duker JS (2011) The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 42(Suppl):S56–S66
Hassenstein A, Meyer CH (2009) Clinical use and research applications of Heidelberg retinal angiography and spectral domain optical coherence tomography—a review. Clin Exp Ophthalmol 37:130–143
Kedhar SR, Thorne JE, Wittenberg S, Dunn JP, Jabs DA (2007) Multifocal choroiditis with panuveitis and punctate inner choroidopathy: comparison of clinical characteristics at presentation. Retina 27:1174–1179
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355:1432–1444
Kiernan D, Mieler W, Hariprasad S (2010) Spectral-domain optical coherence tomography: a comparison of modern high resolution retinal imaging systems. Am J Ophthalmol 149:18–31
Watzke RC, Packer AJ, Folk JC, Benson WE, Burgess D, Ober RR (1984) Punctate inner choroidopathy. Am J Ophthalmol 98:572–584
Dreyer RF, Gass DJ (1984) Multifocal choroiditis and panuveitis. A syndrome that mimics ocular histoplasmosis. Arch Ophthalmol 102:1776–1784
Spaide RF, Goldberg N, Freund KB (2013) Redefining multifocal choroiditis and panuveitis and punctate inner choroidopathy through multimodal imaging. Retina 33:1315–1324
Zhang X, Zuo C, Li M, Chen H, Huang S, Wen F (2013) Spectral-domain optical coherence tomographic findings at each stage of punctate inner choroidopathy. Ophthalmology 120:2678–2683
Gass JD (1994) Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol 118:285–298
Grossniklaus HE, Hutchinson AK, Capone A Jr, Woolfson J, Lambert HM (1994) Clinicopathologic features of surgically excised choroidal neovascular membranes. Ophthalmol 101:1099–1111
Park SS, Truong SN, Zawadzki RJ, Alam S, Choi SS, Telander DG et al (2010) High-resolution Fourier-domain optical coherence tomography of choroidal neovascular membranes associated with age-related macular degeneration. Invest Ophthalmol Vis Sci 51:4200–4206
Dhingra N, Kelly S, Majid MA, Bailey CB, Dick AD (2010) Inflammatory choroidal neovascular membrane in posterior uveitis—pathogenesis and treatment. Indian J Ophthalmol 58:3–10
Kotsolis AI, Killian FA, Ladas ID, Yannuzzi LA (2010) Fluorescein angiography and optical coherence tomography concordance for choroidal neovascularisation in multifocal choroiditis. Br J Ophthalmol 94:1506–1508
Vance SK, Khan S, Klancnik JM, Freund KB (2011) Characteristic spectral-domain optical coherence tomography findings of multifocal choroiditis. Retina 31:717–723
Pachydaki SI, Jakobiec FA, Bhat P, Sobrin L, Michaud NA, Seshan SV et al (2012) Surgical management and ultrastructural study of choroidal neovascularization in punctate inner choroidopathy after bevacizumab. J Ophthalmic Inflamm Infect 2:29–37
Channa R, Ibrahim M, Sepah Y, Turkcuoglu P, Lee JH, Khwaja A et al (2012) Characterization of macular lesions in punctate inner choroidopathy with spectral domain optical coherence tomography. J Ophthalmic Inflamm Infect 2:113–120
Li D, Kishi S (2009) Restored photoreceptor outer segment damage in multiple evanescent white dot syndrome. Ophthalmology 116:762–770
Spaide RF, Koizumi H, Freund KB (2008) Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol 146:111–120
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amer, R., Priel, E. & Kramer, M. Spectral-domain optical coherence tomographic features of choroidal neovascular membranes in multifocal choroiditis and punctate inner choroidopathy. Graefes Arch Clin Exp Ophthalmol 253, 949–957 (2015). https://doi.org/10.1007/s00417-015-2930-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2930-5