Abstract
Video head impulse tests (video-HITs) are commonly used for vestibular evaluation; however, the results can be contaminated by various artifacts, including technical errors, recording problems, and participant factors. Although video-HITs can be used in patients with Parkinson’s disease (PD), the effect of neck rigidity has not been systematically investigated. This study aimed to investigate the effect of neck rigidity on video-HIT results in patients with PD. We prospectively recruited 140 consecutive patients with PD (mean age ± standard deviation = 68 ± 10 years, 69 men) between September 2021 and April 2024 at Korea University Medical Center. The video-HIT results were compared with those of 19 age- and sex-matched healthy participants. Neck rigidity was stratified as a subdomain of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale motor part (MDS-UPDRS-III). In 59 patients, the vestibulo-ocular reflex (VOR) gain was overestimated in at least one canal plane (58/140, 41%), mostly in the anterior canal (AC, n = 44), followed by the horizontal (HC, n = 15) and posterior canals (PC, n = 7). VOR gain overestimation was also observed in patients with no (18/58, 35%), subtle (20/58, 34%), or mild (17/58, 29%) neck rigidity. Multivariable logistic regression analysis showed that VOR overestimation was positively associated with neck rigidity (odds ratio [OR] [95% confidence interval] = 1.51 [1.01–2.25], p = 0.043). The head velocities of patients decreased during head impulses for the AC (p = 0.033 for the right AC; p = 0.014 for the left AC), whereas eye velocities were similar to those of healthy participants. Our findings suggest that neck rigidity may be a confounder that can contaminate video-HIT results. Thus, the results of video-HITs, especially for the AC, should be interpreted with the context of head velocity during head impulses in patients with neck rigidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Video head-impulse tests (video-HITs) allow for the quantitative assessment of semicircular canal function [1,2,3]. Since their introduction [4], video-HITs have been at the forefront of vestibular evaluations [1, 5]. However, they are vulnerable to potential sources of artifacts, which occur in approximately 30–55% of tests [6, 7]. These artifacts may occur due to technical errors (e.g., head bouncing and goggle slippage), recording problems (trace oscillation, trace loss, miscalibration), or participant factors (rigid neck, blinking, inattention) [6, 8,9,10]. Further, these artifacts can be indistinguishable from covert saccades unless the raw data are thoroughly examined, thereby confounding the results [1, 8]. Additionally, nystagmus or saccadic oscillation/intrusion—which is commonly accompanied by central vestibulopathy—can interfere with the quantitative recording of the video-HITs [11].
Rigidity is a cardinal symptom of Parkinson’s disease (PD) [12]. It is clinically assessed by passively flexing and extending a patient’s appendage [12, 13]. Numerous studies have revealed that video-HITs aid in the diagnosis of PD and other Parkinson-plus syndromes. However, excessive neck rigidity can make it difficult for testers to perform video-HITs in patients with PD. Surprisingly, the effect of neck rigidity on video-HITs has not been delineated. Instead, data have been simply sorted out by testers [14], or video-HITs have been performed during the ON-state in patients with PD [15]. In this context, the influence of neck rigidity remains unknown, which awaits clarification for future studies among patients with PD.
Methods
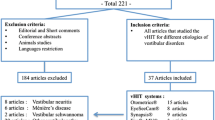
We prospectively recruited 169 consecutive patients with PD at the Korea University Medical Center between September 2021 and April 2024. Each patient underwent video-HITs at the initial assessment before PD-specific medications were administered [14]. PD was diagnosed according to the Movement Disorder Society (MDS) criteria [16]. We excluded patients with a history of central or vestibular disorders, including vestibular neuritis (n = 7), posterior circulation stroke (n = 5), Meniere’s disease (n = 2), and vestibular migraine (n = 2), which could affect the video-HIT results. Those who failed to achieve optimal peak head acceleration due to excessive neck rigidity (n = 8; six had grade 4 neck rigidity and two had grade 3) were excluded. We also excluded five patients whose video-HIT data were not available because of other artifacts during impulses (large head bounce, exaggerated blinking, and lid and eye position artifacts). Finally, 140 patients (mean age ± standard deviation [SD] = 68 ± 10 years; 69 men) were included in the analysis.
Video-HITs
Head and eye movements were recorded using video-HITs (SLVNG, SLMED, Seoul, South Korea) as described previously [17]. All patients discontinued any medications that may have affected the results at least 48 h prior to the test.
For the test, the patient was seated 1.2 m in front of the target. The eye position was calibrated using a red dot sequentially presented from the center at 10° vertically and horizontally. Passive unpredictable, high-acceleration, and small-amplitude head rotations were then delivered in the plane of the horizontal canals (HCs). After evaluating the HCs, head impulses for the vertical canals were delivered with the patient’s head turned 30–40° to the left or right of the fixation point eccentrically, while being aligned to the plane of the right anterior-left posterior and left anterior-right posterior canals. Eye and head movement data were synchronously sampled at a rate of 120 Hz. Eye movements were included in analyses only when the peak head acceleration during the HIT was >2500°/s2 for the HCs and 1500°/s2 for the vertical canals. At least 10 valid head impulses were recorded in each direction after excluding trials with blinks and outliers [17].
To measure the vestibulo-ocular reflex (VOR) gain, the ratio of the area under the eye velocity curve was compared to the area under the head velocity curve. The VOR gains for each canal were measured for individual impulse trials as the ratio of the mean velocity of the eye divided by that of the head during the 40-ms time window centered at peak head acceleration. For statistical analyses, we calculated the mean gain for the HC, anterior canal (AC), and posterior canal (PC) on both sides. We also measured the peak velocities of the head and eyes during impulses in each canal plane.
We initially recruited 20 age- and sex-matched healthy participants and excluded one patient with a mild degree of neck rigidity. Except for one patient, none of the healthy participants had neck rigidity. The results of video-HITs were considered positive (abnormal) when they fell outside the normal range of VOR gain determined by assessing 19 healthy participants (mean age ± SD = 62 ± 6 years, 8 men) [14]. The peak head acceleration during HIT was >3000°/s2 for the HCs and 2000°/s2 for the vertical canals. The reference range was defined as mean ± 2 SD. The normal gain values were as follows: HC = 0.86–1.20; AC = 0.75–1.23; PC = 0.73–1.32 [14]. The eyelids were taped and lifted during the vertical impulses to prevent eyelid flicks as required [1, 8, 18].
In addition to the VOR gain measurements, the presence of reversed catch-up saccades was determined as previously described [19]. The reversed catch-up saccades were analyzed based on the velocity–time graphs of the impulses in all canal planes. They were determined to be present when (1) they were directed toward the direction of head rotation and (2) the peak eye velocity of the corrective saccades exceeded 60°/s with a cutoff value according to the main sequence of the saccades [20].
Assessment of neck rigidity and Parkinsonism
Neck rigidity was stratified as the subdomain of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale motor part (MDS-UPDRS-III) [13]. The MDS-UPDRS-III was evaluated at the initial visit, prior to the video-HITs or administration of PD-related medication.
As a subdomain of the MDS-UPDRS-III, neck rigidity was assessed during slow passive movement of the neck in a relaxed position and categorized into five subgroups: 0, no rigidity; 1, slight (rigidity only detected with the activation maneuver); 2, mild (rigidity detected without the activation maneuver, but full range of motion is easily achieved); 3, moderate (rigidity detected without the activation maneuver; full range of motion is achieved with effort); and 4, severe (rigidity detected without the activation maneuver and full range of motion not achieved). Additionally, the severity of motor disability in patients with PD was assessed using the MDS-UPDRS-III and Hoehn and Yahr (H&Y) scale [13].
Statistical analysis
Nominal independent variables were compared using the χ2 or Fisher’s exact test. Continuous independent variables were compared using the Mann–Whitney U test, Student’s t test, and analysis of variance (ANOVA) tests. For logistic regression analysis, significant variables were selected using the backward variable selection method. A p value of <0.05 was considered significant in the multivariable logistic regression analysis.
Statistical analyses were performed using R (version 3.4.0; The R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org), and the significance level was set at p < 0.05 (two-tailed).
Results
Clinical characteristics
The detailed clinical characteristics of the 140 patients are presented in Table 1. The disease duration ranged from 1 month to 12 years (median, interquartile range: 12 months, 6–18 months). The patients had comorbidities, including dyslipidemia (n = 63), hypertension (n = 59), diabetes mellitus (n = 35), depressive disorder (n = 33), anxiety disorder (n = 18), history of coronary artery occlusive disease (n = 13), and transient ischemic attack or cerebrovascular disease (n = 6). The neurologic sequelae of prior stroke were mostly minimal, with a modified Rankin scale score of 0 in 3 patients and 1 in 3 patients with dysarthria (n = 2) and hemiparesis (n = 2). None of the patients developed any red flag signs suggesting atypical Parkinsonian syndromes at the initial evaluation or during follow-up. The findings of 116 patients have been previously reported in part [14, 21].
Video-HITs
The VOR gain ranged from 0.66 to 1.43 in the right HC (RHC; mean ± SD = 1.06 ± 0.13), 0.54 to 1.51 in the left HC (LHC; 1.02 ± 0.14), 0.56 to 1.61 in the right AC (RAC; 1.09 ± 0.19), 0.62 to 1.73 in the left AC (LAC; 1.10 ± 0.19), 0.36 to 1.40 in the right PC (RPC; 1.01 ± 0.17), and 0.46 to 1.49 in the left PC (LPC; 1.01 ± 0.19).
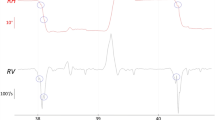
The VOR gain was normal in 43% of patients with PD (60/140, 43%). However, there was at least one decrease in the VOR gain in 28 patients as follows: any HC in 15 (right only, n = 5; left only, n = 6; both left and right, n = 4), any AC in 6 (right only, n = 4; both right and left, n = 2), or any PC in 15 (right only, n = 3; left only, n = 7; both right and left, n = 5). In contrast, 58 patients showed overestimation of VOR gain in at least one canal plane (58/140, 41%; six patients showed VOR overestimation in certain canal planes and decreased gain for other canals): any HC in 15 (right only, n = 8; left only, n = 2; both left and right, n = 5), any AC in 44 (right only, n = 18; left only, n = 10; both right and left, n = 16), or any PC in 7 (left only, n = 4; both right and left, n = 3) (Fig. 1). None of the patients exhibited reversed catch-up saccades during the impulses.
Results of video head-impulse tests in the patients. The VOR gain was normal in 60 patients (60/140, 43%); however, there was at least one VOR gain decrease in 28 patients: any HC in 15, any AC in six, any PC in 15. Fifty-eight patients showed an overestimation of VOR gain at least in one canal plane (58/140, 41%): any HC in 15, any AC in 44, or any PC in 7. LAC left anterior canal, LHC left horizontal canal, LPC left posterior canal, RAC right AC, RHC right HC, RPC right PC, VOR vestibulo-ocular reflex. * Six patients showed VOR overestimation in certain canal planes and also decreased gain for other canals
Overestimation of VOR gain and other clinical parameters
Patients with VOR gain overestimation had more severe neck rigidity than those without it (mean [95% confidence interval] = 2.1 [1.9–2.4] vs. 1.8 [1.6–2.0], p = 0.046; Table 2; Fig. 2). Otherwise, the clinical characteristics did not differ between patients with and without overestimation (Table 2).
Association of neck rigidity with the VOR gain of each canal
AC gains were positively correlated with neck rigidity (r = 0.200, p = 0.019), but not with other canals (r = −0.041, p = 0.630 for HC gains; r = −0.030, p = 0.722 for PC gains).
Multivariable logistic regression analyses showed that VOR overestimation was positively associated with neck rigidity (odds ratio [OR] [95% confidence interval] = 1.51 [1.01–2.25], p = 0.043; Table 3).
Eye and head velocities during impulses
The patients’ head and eye velocities are summarized in Table 4. The head velocities of patients were lower than those of healthy participants for the ACs (131 ± 33 vs. 157 ± 47 ms, p = 0.033 for the RAC; 131 ± 35 vs. 153 ± 39 ms, p = 0.014 for the LAC). The head velocity for the RAC differed with respect to neck rigidity (Fig. 3; F = 2.563, p = 0.031; a post hoc analysis to determine which group difference contributed to the overall difference was not available because only one patient had severe neck rigidity). The head velocity did not differ with respect to neck rigidity for the other canals (F = 0.206, p = 0.959 for the RHC; F = 0.125, p = 0.986 for the LHC; F = 2.060, p = 0.076 for the LAC; F = 0.427, p = 0.829 for the RPC; F = 0.644, p = 0.667 for the LPC). The eye velocities of the patients did not differ from those of the healthy participants (Table 4). Eye velocity did not differ with respect to neck rigidity (F = 0.705, p = 0.621 for the RHC; F = 0.114, p = 0.989 for the LHC; F = 0.713, p = 0.615 for the RAC; F = 0.382, p = 0.860 for the LAC; F = 0.586, p = 0.710 for the RPC; F = 0.644, p = 0.667 for the LPC; Fig. 3).
Head velocity for the right AC differed with respect to neck rigidity (F = 2.563, p = 0.031), yet not for the other canals (F = 0.206, p = 0.959 for the right HC; F = 0.125, p = 0.986 for the left HC; F = 2.060, p = 0.076 for the left AC; F = 0.427, p = 0.829 for the right PC; F = 0.644, p = 0.667 for the left PC). Meanwhile, eye velocity did not differ with respect to neck rigidity F = 0.705, p = 0.621 for the right HC: F = 0.114, p = 0.989 for the left HC: F = 0.713, p = 0.615 for the right AC: F = 0.382, p = 0.860 for the left AC; F = 0.586, p = 0.710 for the right PC: F = 0.644, p = 0.667 for the left PC). AC = anterior canal, HC = horizontal canal, PC = posterior canal
Discussion
The findings of this study can be summarized as follows: (1) The VOR was overestimated in 41% of patients with PD in at least one canal; (2) VOR gain overestimation was commonly observed, even in patients with slight or mild neck rigidity; (3) VOR overestimation was frequently found during impulses for the ACs compared to those for other canal planes; (4) VOR gain overestimation was associated with neck rigidity, irrespective of age, sex, body weight, disease duration, or H&Y scale score; and (5) patients with PD showed decreased head velocities during impulses for the AC compared to healthy participants.
Prior studies on video-HITs in PD
There have been contradictory results regarding video-HIT for PD. Some researchers reported bilaterally deficient responses in patients with advanced stages of PD compared to those with mild-to-moderate stages of PD [15, 22,23,24]. Similarly, other researchers have reported mild impairment of the canal function confined to the low-frequency detected only on caloric and rotatory chair tests [25,26,27,28]. However, recent studies pointed out that the VOR gain did not differ from those measured in age- and sex-matched controls [15, 29]. Moreover, when covariates related to PD were statistically controlled, VOR gain showed no association with the clinical severity or disease duration of PD [14]. This discrepancy may be due in part to the heterogeneity of the patients and different methods applied for video-HITs or gain measurements across studies. While not as robust as that found in other neural structures, alpha-synuclein can be found in the vestibular nucleus complex, nucleus prepositus hypoglossi, and deep cerebellar nuclei that participate in the central VOR pathway [30, 31]. This suggests that the VOR pathway may be involved as the disease progresses, and a deficient VOR may be observed at advanced stages of PD.
VOR gain overestimation in patients with PD
Although VOR gain increments were observed in 41% of our patients, they may not be actual increments given the absence of subsequent backup saccades in any of the patients (i.e., overestimation). Additionally, the diagnosis of PD already excludes the possibility of hyperactive HITs, which are a feature observed in PD-mimickers preferentially involving the vestibulocerebellum, such as multiple system atrophy (MSA) [19] or spinocerebellar ataxia [32]. Rather, relatively low head velocity during impulses would have been associated with VOR gain overestimation, primarily for the ACs.
Previously, Lv and colleagues found that VOR gain is increased in patients with PD compared to that in healthy participants, and this increment was associated with a higher UPDRS [28]. They reported that gain was measured up to 1.6–1.8 [28]. However, robust reversed catch-up saccades were not found in those patients either, implying that it may be an overestimation, likely due to neck rigidity, as observed in our patients. Our observations further indicate that VOR gain overestimation or its reciprocal as a derivative may be a potential indicator of neck rigidity in patients with PD.
Notably, the VOR gain overestimation was more prominent during head impulses for ACs than for PCs. This observation seems plausible given that the antigravity muscles (i.e. neck extensors) are hypertonic in patients with PD. Indeed, rigidity is explained by the abnormal activation of the postural control mechanism, which should be normally utilized during standing when equilibrium is threatened [33]. The Ib inhibitory interneurons are less active, whereas the Ia inhibitory interneurons are more active in PD, explaining the rigid extensor in the axial muscles [34]. The nucleus gigantocellularis, which receives projections from the substantia nigra, is disinhibited during the disease process to exert this phenomenon [35, 36]. Therefore, rigidity may be prominent during AC stimulation that necessitates flexion of the neck.
Neck rigidity during video-HITs: an obstacle for assessment of VOR but valuable for the quantification of neck rigidity
Our findings suggest that video-HIT results can be easily misleading in patients with PD, especially in the ACs. Notably, overestimation was observed even in patients without robust neck rigidity on extrapyramidal examination. Video-HITs require a high-acceleration head impulse to silence the inhibitory vestibular input from the opposite side [1]. By convention, the increased muscle tone may remain constant throughout the range of motion, regardless of speed. However, even subclinical and marginal rigidity can contaminate the results of video-HITs. Additionally, clinical assessment can be inherently subjective and open to bias. Contrary to conventional wisdom, detection of rigidity can be influenced by the range of motion or speeds during the examination. Notably, a low speed below 70°/s cannot be used to practically differentiate between normal tone and rigidity; an optimal speed and range of motion differ for each joint for assessment of rigidity [37]. This may explain the somewhat contradictory result where the greatest proportion of VOR gain overestimation was seen in the group with no neck rigidity in our study. This may not be an issue among those with severe neck rigidity that does not allow for high-acceleration head impulses; most of these cases were excluded from our study. However, clinicians should be wary of performing and interpreting video-HITs in patients with PD, even among those without significant axial rigidity. To avoid this, the presence of reversed catch-up saccades should be sought when encountering increased VOR gain [38,39,40]. Otherwise, a simple interpretation of the gain can be misleading to indicate central vestibulopathy in patients with Parkinsonism.
Recent studies have found that the gain difference between ACs and PCs can be utilized as a diagnostic marker in patients with hereditary and acquired cerebellar ataxia [19, 41]. Our observations suggest that the AC gain can easily be overestimated when Parkinsonism or neck rigidity is present, increasing the gap between the vertical canals and thereby masquerading as central vestibulopathy. Thus, the presence of perverted catch-up saccades (i.e., vertical bias during horizontal impulses) should be considered when differentiating neurodegenerative disorders that primarily involve the vestibulocerebellum [19].
Furthermore, our findings imply that the actual decrement can be masked in patients with Parkinsonism. Several studies report that the results of video-HITs may vary with respect to the canal plane, showing abnormal HITs for vertical canals in the presence of normal HCs [42], or vice versa [43]. This could hamper the analysis and correlation of clinical parameters with other studies on VOR gain for each canal in patients with PD or other Parkinsonian syndromes.
We speculate that VOR gain overestimation may not be confined to those with Parkinsonism but also to those who have problems relaxing the neck due to dystonia or neck pain [44]. Given that ptosis and pupil blocking lid can result in a VOR decrease for PCs in the elderly [8], the AC gain overestimation can be an obstacle for the interpretation of the results of video-HITs in older patients with neck problems.
Our study has some limitations. First, the effect of neck rigidity on video-HITs could have been underestimated owing to selection bias. Patients with severe neck rigidity were excluded from the initial enrollment because only one patient had severe (grade 4) neck rigidity. For this reason, the largest proportion of patients showing VOR gain overestimation was found among those with no neck rigidity or mild neck rigidity. Head impulses with a low peak head velocity (causing low acceleration) are not adequate for evaluating VOR function [45], which was excluded initially. This selection bias may have led to underestimation of the association between VOR gain overestimation and neck rigidity, which may explain the marginal statistical significance between the two. However, we speculate that they could hold significance for the evaluation of neck rigidity. Second, measuring neck rigidity using a clinical scale is inherently unreliable and open to bias. Instead, electromyography, accelerometers, gyroscopes, and myotonometry allow for non-invasive, objective measurement of rigidity [46,47,48]. Comparing the results of video-HITs with objective quantification of neck rigidity would be desirable. Third, although we found an association between neck rigidity and video-HIT gain, a causal relationship remains to be elucidated. To clarify this, it is desirable to compare the results of each patient during one’s on- and off-states.
In conclusion, neck rigidity is a potential confounder that can contaminate the results of video-HIT in patients with PD. VOR gain overestimation may be attributed to neck rigidity, especially when it affects the neck extensors, thereby affecting the results of video-HITs for ACs. Thus, clinicians should be wary of interpreting the results of video-HITs, especially for ACs, even in those with marginal neck rigidity on clinical examination.
Data availability
Anonymized data will be shared upon request from any qualified investigator.
References
Halmagyi G, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS (2017) The video head impulse test. Front Neurol 8:258
Weber K, Aw S, Todd M, McGarvie L, Curthoys I, Halmagyi G (2008) Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology 70:454–463
Curthoys IS, McGarvie LA, MacDougall HG, Burgess AM, Halmagyi GM, Rey-Martinez J, Dlugaiczyk J (2023) A review of the geometrical basis and the principles underlying the use and interpretation of the video head impulse test (vHIT) in clinical vestibular testing. Front Neurol 14:1147253
MacDougall H, Weber K, McGarvie L, Halmagyi G, Curthoys I (2009) The video head impulse test diagnostic accuracy in peripheral vestibulopathy. Neurology 73:1134–1141
MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP (2013) Application of the video head impulse test to detect vertical semicircular canal dysfunction. Otol Neurotol 34:974–979
Mantokoudis G, Tehrani ASS, Kattah JC, Eibenberger K, Guede CI, Zee DS, Newman-Toker DE (2015) Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurotol 20:39–50
Trinidad-Ruiz G, Rey-Martinez J, Matiño-Soler E, Batuecas-Caletrio A, Martin-Sanz E, Perez-Fernandez N (2020) Relevance of artifact removal and number of stimuli for video head impulse test examination. Ear Hear 41:1397–1406
Yoon HJ, Lee JH, Lee JH, Park E, Lee SU, Kim BJ, Kim JS (2024) Effects of pupil size in video head-impulse tests. J Neurol 271:819–825
McGarvie LA, Martinez-Lopez M, Burgess AM, MacDougall HG, Curthoys IS (2015) Horizontal eye position affects measured vertical VOR gain on the video head impulse test. Front Neurol 6:58
Castillo-Bustamante M, Pauna HF, da Costa MR, Gutierrez VA, Madrigal J (2024) Insights into vestibulo-ocular reflex artifacts: a narrative review of the video head impulse test (vHIT). Cureus 16:e55982
Koohi N, Mendis S, Lennox A, Whelan D, Kaski D (2022) Video head impulse testing: pitfalls in neurological patients. J Neurol Sci 442:120417
Berardelli A, Sabra A, Hallett M (1983) Physiological mechanisms of rigidity in Parkinson’s disease. J Neurol Neurosurg Psychiatry 46:45–53
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R (2008) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov disord 23:2129–2170
Hong JP, Kwon H, Park E, Lee SU, Lee CN, Kim BJ, Kim JS, Park KW (2024) The semicircular canal function is preserved with little impact on falls in patients with mild Parkinson’s disease. Parkinsonism Relat Disord 118:105933
Hawkins KE, Chiarovano E, Paul SS, Burgess AM, MacDougall HG, Curthoys IS (2022) Vestibular semicircular canal function as detected by video head impulse test (vHIT) is essentially unchanged in people with Parkinson’s disease compared to healthy controls. J Vestrib Res 32:261–269
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601
Kim SH, Lee SU, Cho BH, Cho KH, Yu S, Kim BJ, Kim JS (2023) Analyses of head-impulse tests in patients with posterior circulation stroke and vestibular neuritis. Neurology 100:e2374–e2385
Loeffler JD, Slatt B, Hoyt WF (1966) Motor abnormalities of the eyelids in Parkinson’s disease: electromyographic observations. Arch Ophthalmol 76:178–185
Kim JG, Kim SH, Lee SU, Lee CN, Kim BJ, Kim JS, Park KW (2022) Head-impulse tests aid in differentiation of multiple system atrophy from Parkinson’s disease. J Neurol 269:2972–2979
Leigh RJ, Zee DS (2015) The neurology of eye movements. Oxford University Press, New York
Hong JP, Baik K, Park E, Lee SU, Lee CN, Kim BJ, Kim JS, Park KW (2024) The vestibulospinal dysfunction has little impact on falls in patients with mild Parkinson’s disease. Parkinsonism Relat Disord 122:106081
Smith PF (2018) Vestibular functions and Parkinson’s disease. Front Neurol 9:1085
Lithgow BJ, Shoushtarian M (2015) Parkinson’s disease: disturbed vestibular function and levodopa. J Neurol Sci 353:49–58
Bohnen NI, Roytman S, Griggs A, David SM, Beaulieu ML, Müller ML (2022) Decreased vestibular efficacy contributes to abnormal balance in Parkinson’s disease. J Neurol Sci 440:120357
Reichert WH, Doolittle J, McDowell FH (1982) Vestibular dysfunction in Parkinson disease. Neurology 32:1133–1133
White OB, Saint-Cyr JA, Sharpe JA (1983) Ocular motor deficits in Parkinson’s disease: I. The horizontal vestibulo-ocular reflex and its regulation. Brain 106:555–570
Vitale C, Marcelli V, Furia T, Santangelo G, Cozzolino A, Longo K, Allocca R, Amboni M, Marciano E, Barone P (2011) Vestibular impairment and adaptive postural imbalance in parkinsonian patients with lateral trunk flexion. Mov Disord 26:1458–1463
Lv W, Guan Q, Hu X, Chen J, Jiang H, Zhang L, Fan W (2017) Vestibulo-ocular reflex abnormality in Parkinson’s disease detected by video head impulse test. Neurosci Lett 657:211–214
Hawkins KE, Rey-Martinez J, Chiarovano E, Paul SS, Valldeperes A, MacDougall HG, Curthoys IS (2021) Suppression head impulse test paradigm (SHIMP) characteristics in people with Parkinson’s disease compared to healthy controls. Exp Brain Res 239:1853–1862
Seidel K, Mahlke J, Siswanto S, Krüger R, Heinsen H, Auburger G, Bouzrou M, Grinberg LT, Wicht H, Korf HW (2015) The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol 25:121–135
Gibb W (1992) Neuropathology of Parkinson’s disease and related syndromes. Neurol Clin 10:361–376
Lee SU, Kim JS, Yoo D, Kim A, Kim HJ, Choi JY, Park JY, Jeong SH, Kim JM, Park KW (2022) Ocular motor findings aid in differentiation of spinocerebellar ataxia type 17 from Huntington’s disease. Cerebellum 22:1–13
Broussolle E, Krack P, Thobois S, Xie-Brustolin J, Pollak P, Goetz CG (2007) Contribution of Jules Froment to the study of parkinsonian rigidity. Mov Disord 22:909–914
Delwaide P, Pepin J-L, De Noordhout AM (1991) Short-latency autogenic inhibition in patients with parkinsonian rigidity. Ann Neurol 30:83–89
Kitai S (2011) Electrophysiology of the corpus striatum and brain stem integrating systems. In: Terjung R (ed) Comprehensive physiology. Wiley, New York, pp 997–1015
Chronister R, Walding J, Aldes L, Marco L (1988) Interconnections between substantia nigra reticulata and medullary reticular formation. Brain Res Bull 21:313–317
Teräväinen H, Tsui JK, Mak E, Calne DB (1989) Optimal indices for testing parkinsonian rigidity. Can J Neurol Sci 16:180–183
Choi JY, Kim JS, Jung JM, Kwon DY, Park MH, Kim C, Choi J (2014) Reversed corrective saccades during head impulse test in acute cerebellar dysfunction. Cerebellum 13:243–247
Lee S, Koo YJ, Kim HJ, Kim JS (2022) Pseudo-reversed catch-up saccades during head impulses: a new cerebellar sign. J Neurol 269:5651–5654
Kim K, Kim HJ, Choi JY, Kim JS (2022) Hyperactive and cross-coupled head impulse signs in recurrent strokes: clinical signs of global cerebellar dysfunction. J Neurol 269:1698–1700
Lee SU, Kim JS, Kim HJ, Choi JY, Park JY, Kim JM, Yang X (2020) Evolution of the vestibular function during head impulses in spinocerebellar ataxia type 6. J Neurol 267:1672–1678
Berkiten G, Tutar B, Atar S, Kumral TL, Saltürk Z, Akan O, Sari H, Onaran Ö, Biltekin Tuna Ö, Uyar Y (2023) Assessment of the clinical use of vestibular evoked myogenic potentials and the video head impulse test in the diagnosis of early-stage Parkinson’s disease. Ann Otol Rhinol Laryngol 132:41–49
Karababa E, Sonkaya AR, Satar B, Korkmaz H (2023) Role of the functional head impulse test in evaluating vestibulo-ocular reflex abnormalities in individuals with Parkinson’s disease. Clin Otolaryngol 48:881–887
Chen L, Halmagyi GM (2020) Video head impulse testing: from bench to bedside. Semin Neurol 40:5–17
McGarvie LA, MacDougall HG, Halmagyi GM, Burgess AM, Weber KP, Curthoys IS (2015) The video head impulse test (vHIT) of semicircular canal function—age-dependent normative values of VOR gain in healthy subjects. Front Neurol 6:154
Di Biase L, Summa S, Tosi J, Taffoni F, Marano M, Cascio Rizzo A, Vecchio F, Formica D, Di Lazzaro V, Di Pino G (2018) Quantitative analysis of bradykinesia and rigidity in Parkinson’s disease. Front Neurol 9:279364
Levin J, Krafczyk S, Valkovič P, Eggert T, Claassen J, Bötzel K (2009) Objective measurement of muscle rigidity in Parkinsonian patients treated with subthalamic stimulation. Mov Disord 24:57–63
Marusiak J, Jarocka E, Jaskolska A, Jaskolski A (2018) Influence of number of records on reliability of myotonometric measurements of muscle stiffness at rest and contraction. Acta Bioeng Biomech 20:123–131
Acknowledgements
This study was supported by the Basic Research Program through the National Research Foundation of Korea (NRF) funded by the MSIT (2022R1A4A1018869).
Author information
Authors and Affiliations
Contributions
Dr. Woo analyzed and interpreted the data and wrote the manuscript. Drs. Y. Kim, K. Baik, E. Park, C.N. Lee, S. Kwak, H. Park, J.S. Kim, and K.W. Park analyzed and interpreted the data, and revised the manuscript. Dr. S.U. Lee designed and conceptualized the study, interpreted the data, and revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Drs. Woo, Y. Kim, K Baik, S.U. Lee, E. Park, C.N. Lee, S. Kwak, H. Park, K.W. Park report no disclosures. JS Kim serves as an Associate Editor of Frontiers in Neuro-otology and on the editorial boards of Frontiers in Neuro-ophthalmology, Journal of Neuro-ophthalmology, Journal of Vestibular Research, and Clinical and Translational Neuroscience.
Ethical standard
This study followed the tenets of the Declaration of Helsinki and was performed according to the guidelines of the Institutional Review Board of Korea University Anam Hospital (2023AN0442).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woo, D., Kim, Y., Baik, K. et al. Neck rigidity: a pitfall for video head-impulse tests in Parkinson’s disease. J Neurol 271, 5223–5232 (2024). https://doi.org/10.1007/s00415-024-12488-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12488-w