Abstract
Background
In Amyotrophic Lateral Sclerosis (ALS) patients with SOD1 mutation the intrathecal administration of tofersen slowed down the progression of disease in a controlled clinical study, but results were not statistically significant.
Methods
In this multicentre, observational study, we evaluated a cohort of 27 ALS-SOD1 patients who were treated with tofersen, focussing on 17 patients who were followed for at least 48 weeks (median period of 84 weeks, range 48–108). We compared the clinical slopes, as measured by ALSFRS-R, MRC scale and Forced Vital Capacity, during tofersen treatment with retrospective data at 1 year prior to therapy. Cerebrospinal fluid (CSF) and serum neurofilament light chains (NFL) were measured in all patients.
Results
Cumulative evaluation of the ALSFRS-R and MRC progression rates showed a statistically significant change during treatment with respect to the period prior to therapy (p = 0.023 and p = 0.007, respectively). The analysis of individual patients showed that nine of the seventeen patients substantially stabilized or slightly improved. Four patients deteriorated during treatment, while in the remaining patients the very slow course did not allow to identify significant changes. CSF and serum NFL concentration markedly decreased in the near totality of patients.
Increased levels of white blood cells and proteins in the CSF were found in 60% of patients. Such alterations were clinically asymptomatic in all but two patients who showed an acute pure motor radiculitis, which responded to steroid therapy.
Conclusions
Clinical findings and NFL analysis strongly suggest that tofersen may have a disease-modifying effect in a subset of SOD1-ALS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 2–3% of amyotrophic lateral sclerosis (ALS) cases are caused by mutations in the gene encoding superoxide dismutase 1 (SOD1) in which a toxic gain of function of the mutant SOD1 protein is consistently considered the disease mechanism [1, 2]. Tofersen is an antisense oligonucleotide designed to induce RNase mediated degradation of SOD1 mRNA and consequently to reduce the synthesis of both wild type and mutated SOD1 protein [3]. In a phase 3 trial, testing the safety and effect of intrathecal administration of Tofersen, a reduction in the decline of the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) scores from baseline was observed in treated patients compared to placebo, but results were not statistically significant [4, 5]. The concentrations of SOD1 in cerebrospinal fluid (CSF) and of neurofilament light chains (NFL) in plasma were significantly reduced with respect to placebo [4, 5].
In the present study, we describe our experience of 27 SOD1 ALS patients treated with tofersen, focussing on 17 patients who were followed for at least 48 weeks (median 84 weeks, range 48–108).
Methods
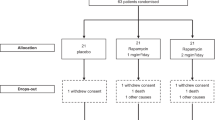
Since the Expanded Access Program became available in Italy, in November 2021, tofersen treatment opportunity was discussed with 42 SOD1 ALS patients followed at five NeMO (NeuroMuscular Omnicenter) centers, including Rome, Milan, Trento, Brescia and Ancona (Fig. 1). Twenty-seven patients accepted to start tofersen treatment. Fifteen patients were not treated for the following reasons: four because of the advanced stage of their disease (invasive pressure positive ventilation by tracheostomy), eleven refused because the course of their disease was very slow and supposedly the burden of the treatment overweighed the potential benefits.
Tofersen treatment was initiated with three loading 100 mg doses administered intrathecally at 2 week intervals followed by maintenance 100 mg doses injected every 4 weeks, according to the dosing regimen of the RCT VALOR trial [4]. A 25-gauge, 90 mm Whitacre spinal needle was used to access the subarachnoid space.
Of the 27 patients in which Tofersen was started, five patients were excluded due to incomplete data collection and two for patient’s decision of discontinuation after few weeks of treatment. Three patients died within 6 months since first injection, and they will be described separately. The remaining 17 patients were followed for a period ≥ 48 months and were included in our analysis.
All patients were evaluated every 12 weeks by ALS functional rating scale-revised (ALSFRS-R), forced vital capacity (FVC) examination, expressed as a percentage of the predicted value and medical research council (MRC) scale. An MRC megascore was calculated by summing the scores of 22 muscles listed in supplementary Table 1.
These patients were already followed at one of the NeMO sites where they were regularly evaluated every 1–3 months since the diagnosis. Thus, a pre-baseline clinical evaluation, at 52 weeks (± 4) before the start of therapy, was available for all cases but one (patient n. 15) for whom the evaluation was made 6 months before. A pre-treatment ALSFRS-R progression rate (ALSFRSr-PR) was calculated using the following formula: [ALSFRS-R at pre-baseline evaluation—ALSFRS-R at first administration] divided by the number of months between pre-baseline evaluation and first application. During the treatment period, the ALSFRSr-PR was calculated by dividing the difference of ALSFRS-R scores between the beginning and the last examination by the number of months. Pre-and post-treatment MRC-progression rate was calculated in the same manner.
The Wilcoxon signed-rank test was used to compare the disease progression rate before treatment with the disease progression rate after treatment (in terms of both ALSFRS-r and MRC points lost per month).
The concentrations of neurofilament light chains in the CSF (CSF-NFL) and serum (sNFL) were investigated in all patients at baseline and every 12 weeks. NFL analysis was performed with Simple Plex cartridges using the Ella apparatus (ProteinSimple, San Jose) according to the manufacturer’s instructions. The local ethical committee approved this study and all patients signed an informed consent.
Results
Clinical course
Demographic, clinical and genetic aspects are described in Table 1. Twelve patients were males (70.5%), five (29.5%) were females. Ten patients had a family history of ALS and seven were sporadic cases. The mean age of disease onset was 51.5 years (range 34–72); the mean age at tofersen start was 58.6 years (range 47–74). All patients had spinal onset, except one with bulbar signs as first symptoms. The mean interval of time between symptoms onset and starting therapy was 87.2 months (range 12–219). At baseline, eleven patient used non-invasive ventilation (NIV), no patient had tracheostomy and two patients were fed by gastric feeding tube. The median duration of follow-up during treatment was 84 weeks (IQR 66–96) with a range of 48–108 weeks. Cumulative evaluation of the progression rate, as measured by ALSFRS-R and MRC scale, showed a statistically significant change during treatment with respect to the period prior to therapy (p = 0.023 and p = 0.007, respectively) (Table 2). The analysis of individual patients showed that nine of seventeen patients (53%) substantially stabilized or slightly improved during tofersen treatment compared to the clinical worsening observed in the pre-treatment period (patients 1, 2, 3, 4, 9, 12, 13, 14, and17) (Fig. 2 and Table 3). Respiratory function remained stable in all patients during the therapy (Fig. 2 and Table 3). The period of stabilization in these nine patients lasted a mean of 88 weeks (range 60–108).
Clinical course in individual patients, as measured by ALSFRS-R, MRC and FVC at pre-baseline, at baseline and during treatment (left). Graphs also show NFL levels (pg/ml) in CSF and serum at baseline and during treatment (right). These nine patients showed significant stabilization or small improvement. NFL concentration were markedly reduced in six patients while increased in patient 4; patient 2 and 3 showed a substantial increase after initial decline
Of the remaining patients, two (patients 7 and 15) continued to worsen during the first weeks of treatment, but after the 36–48th week, both ALSFRS and MRC remained stable until last examination (Fig. 3, Table 3). Respiratory function declined in both patients. In patient 15 no further respiratory tests were available from the 36th week on, but the number of hours in NIV remained constant during treatment. Clinical outcome of patient 8 will be described in the side effect session.
Clinical slopes in patients with deterioration during treatment. One patient (patient 16) showed a dramatic worsening associated with marked decrease of serum and CSF NFL concentration. In the remaining patients an apparent clinical stabilization was observed after the 48th week. NFL concentration was reduced in these patients; patient 8 showed an increase in correspondence of radiculitis episode
One patient (patient 16) deteriorated rapidly with a progression rate apparently greater than prior to therapy (Fig. 3). One month after the first injection, he was admitted to the emergency department due to COVID-19 pneumonia and was tracheotomised for respiratory failure. The relentless and remarkable progression resulted in the decision to suspend treatment 16 months after the initial injection. He was still alive 1 year after drug interruption. In the remaining four patients (patient 5, 6, 10 and 11) the very slow course did not allow to identify significant changes between the pre- and post-treatment periods in both functional and respiratory assessments (Fig. 4, Table 3).
Three patients (patients 18–20, not included in the analysis) died shortly after the initiation of treatment (Table 4). At baseline, two of them had very severe impairment of both respiratory and limbs muscles while the third patient had a moderate weakness of upper limbs with normal respiratory function. Patients 19 died suddenly after 15 weeks since the beginning of therapy.
Side effects
Four patients reported post-dural puncture headache. In all of them, headache occurred as a single episode over the several injections that were carried out and only in one case treatment was needed. Seven patients reported pain in their limbs with an apparent radicular distribution.
Analysis of CSF white-cell count and of total protein content was performed in 15 patients (supplementary Table 2). At baseline, median protein concentration was 53 mg/dl (IQR 33–68, range 28–80) with 10 patients (66.7%) showing mildly elevated (> 40 mg/dl) values while white-cell count was < 3 mm3 in all cases. After tofersen therapy 9 patients showed an increase of both proteins and white-cell counts, consistent with aseptic meningoradiculitis. Notably, in five patients (patient 1, 2, 8, 10, 17) the increase of white cells was marked (> 30) and persistent over time. These alterations remained clinical asymptomatic in all patients but two. Patient 8 and 10 showed a rapid deterioration of muscular strength in their quadriceps and tibio-peroneal muscles a few days after the 9th and 13th injection, respectively, without any sensory symptoms. CSF examination showed marked increase of proteins (432 mg /dl in patient 8 and 238 mg /dl in patient 10) and of cells (160 cells/mm3 in patient 8 and 55 cells/mm3 in patient 10). At baseline, CSF protein concentrations were 54 mg/dl and 68 mg/dl, respectively, with no cells. Magnetic resonance imaging (MRI) of the spinal cord, performed in both patients, excluded myelitis while showed contrast enhancement in the lumbar roots in patient 8. A lumbar radiculitis was suspected and a course of steroid treatment was undertaken with clinical improvement in both patients. Patient 10, who had a very slow disease course, decided to discontinue treatment at the 48th week. He showed a complete remission of the acute worsening and his condition remained stable over the following 12 months (Fig. 4).In patient 8 (Fig. 3), tofersen injections were continued in association to 50 mg/day of prednisone with marked but incomplete improvement, with a concomitant reduction of CSF proteins (112 mg/dl) and cells (18 cells/mm3). After four additional injections, an attempt to reduce the dosage of steroids was followed by slight worsening of the quadriceps strength with an elevation of protein concentration and of cells count (supplementary Table 2). The increase of steroid dosage again determined an improvement. At the 15th and 16th infusions, we delayed the time interval between injections to 8 weeks, but a coincidental, abrupt reduction of the FVC was observed and NIV was started. No substantial change in limb strength was detected. Thus, the 4 week interval was reintroduced and since then, a 25 mg/day dosage of steroids was maintained with additional 50 mg/day dose 1 day before and 3 days after the injection. Over the following months, his clinical condition remained stable but CSF alterations persisted.
Neurofilaments
After tofersen initiation, CSF–NFL concentration markedly declined in 14/17 patients with a mean 61% reduction (range 49–79%) of the baseline value (Figs. 2–4). The nadir was reached around the 12–36th week of treatment and concentrations remained stable over the follow-up in eleven patients while in three patients (patients 2, 3 and 8) a consistent increase was observed.
In one patient (patient 6) CFS–NFL concentration remained unchanged during treatment while in two patients (patients 4 and 10) NFL concentration increased.
Changes of sNFL concentration roughly mirrored those observed in the CSF.
Discussion
In this work, we report on a multi-center study of 17 ALS SOD1 patients who were treated with tofersen over a mean time of 84 weeks (range 48–108 weeks). The long-term period of observation and the availability of clinical data at 1 year before baseline provided the opportunity to compare ALSFRS-R, MRC scale and FVC slopes between pre- and on-treatment periods.
We found that tofersen therapy was associated with a significant change of the clinical course with respect to the pre-treatment period. This result was driven by nine patients (53%) in which the ALSFRS-R or the MRC scores remained stable or slightly improved during treatment over a mean of 88 weeks (range 60–108). Notably, these patients had clearly declined over 1 year period before therapy (Fig. 2).
Four patients continued to worsen during treatment (Fig. 3). Whether in the course of the degenerative process initiated by mutant SOD1 there may be a point of no return, where therapeutics targeting disease-causing mechanisms are not beneficial remains to be elucidated. Patient 16, with fast progression over the year preceding therapy, declined dramatically during treatment, resulting in the decision to suspend treatment after 16 months. The possibility that in this patient COVID-19 contributed to motor impairment with an unknown mechanism may not be excluded [6]. In three of these patients, clinical slopes declined over the first 36–48 weeks but an apparent stabilization was observed over the following months. Notably, patients participating to the open label extension of the VALOR study, showed a tendency to stabilize after the 40th week [5]. Further follow-up is needed to establish whether a delayed effect of tofersen may be identified.
In our study, we utilized a combination of ALSFRS-R, MRC scale and FVC to evaluate disease course. ALSFRS-R and MRC scale are widely used in the clinical practice and clinical trials, but they are unlikely to represent accurate measurement of ALS progression. However, their combined use may reduce the risk of errors, most likely [7]. MRC scale may be useful in patients with slow course, as small even though significant losses of strength might lead to no noticeable change in ALSFRS-R, as they do not cause noteworthy effects on daily life.
Overall, tofersen was well tolerated and most side effects observed in our patients were previously reported [5, 8]. Post-injection headache was very rare and lower limb pain, with radicular distribution, occurred in 40% of patients but they never limited the continuation of therapy. We found increased levels of white blood cells and proteins in the CSF in 60% of our patients, consistent with a drug-related aseptic meningitis. Such alterations, which have been previously reported in 58–73% of patients, are generally asymptomatic but rare patients are described manifesting as reversible myeloradiculitis [5, 8]. In our series, only two patients showed clinical signs, consistent with a pure motor focal radiculitis responsive to steroid treatment. As far as we know, this side effect has never been reported previously.
The evidence of a marked reduction of CSF and serum NFL concentrations in 82% of our patients (Figs. 2, 3, 4), confirms previous results [5, 8], indicating that tofersen interferes with mechanisms of axonal degeneration, most likely. Notably, both in our series and in the literature, an increase of CSF NFL was observed in some patients after the initial decline or, occasionally, soon after tofersen initiation. A possible explanation for such findings is that drug-related meningoradiculitis may lead to an alteration of the blood–brain barrier permeability, allowing more NFL to enter the CSF. Unexpectedly, patients who continued to deteriorate during therapy showed a reduction of NFL concentrations. On the other hand, patient 4, who harbored an intronic mutation, showed a clinical response to Tofersen, but NFL concentration increased consistently. The reasons for the discrepancy between biological effect and clinical benefit, remain to be clarified.
This work was an observational cohort study, inherently limited by the absence of a control group. However, the availability of retrospective data obtained in highly specialized ALS clinics allowed to identify disease trajectories in the pre-baseline period, thus assuming each patient as his/her own control. In addition, limitations in evaluating our results include the interpretation of stable disease as evidence of treatment response, because plateaus or small reversals may be observed in the natural history of ALS [7, 9]. Reportedly, the duration of these phases is short while very rare patients show sustained stabilization, as observed in our series.
Conclusions
Our long-term follow-up of a cohort of SOD1 ALS patients treated with tofersen, showed a stabilization or mild improvement of clinical course in a significant proportion of patients. This results together with the evidence of a reduction of CSF and serum NFL concentrations, strongly suggest that tofersen had a disease modifying effect, at least in a subgroup of patients.
Further studies are needed to confirm our results and hopefully to understand if factors predicting drug responsiveness may be identified.
Data availability
Data of this study are available from the corresponding author upon reasonable request by a qualifed researcher.
References
Müller K, Brenner D, Weydt P et al (2018) Comprehensive analysis of the mutation spectrum in 301 German ALS families. J Neurol Neurosurg Psychiatry 89:817–827. https://doi.org/10.1136/jnnp-2017-317611
Saccon RA, Bunton-Stasyshyn RK, Fisher EM, Fratta P (2013) Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain 136:2342–2358. https://doi.org/10.1093/brain/awt097
McCampbell A, Cole T, Wegener AJ et al (2018) Antisense oligonucleotides extend survival and reverse decrement in muscle response in ALS models. J Clin Invest 128:3558–3567. https://doi.org/10.1172/JCI99081
Miller T, Cudkowicz M, Shaw PJ et al (2020) Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med 383:109–119. https://doi.org/10.1056/NEJMoa2003715
Miller TM, Cudkowicz ME, Genge A et al (2022) Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med 387:1099–1110. https://doi.org/10.1056/NEJMoa2204705
Li X, Bedlack R (2021) COVID-19-accelerated disease progression in two patients with amyotrophic lateral sclerosis. Muscle Nerve 64:E13–E15. https://doi.org/10.1056/NEJMoa220470510.1002/mus.27351
Hu N, Shen D, Yang X, Cui L, Liu M (2022) Plateaus and reversals evaluated by different methods in patients with limb-onset amyotrophic lateral sclerosis. J Clin Neurosci 97:93–98. https://doi.org/10.1056/NEJMoa220470510.1016/j.jocn.2022.01.016
Wiesenfarth M, Dorst J, Brenner D et al (2024) Effects of tofersen treatment in patients with SOD1-ALS in a “real-world” setting–a 12-month multicenter cohort study from the German early access program. EClin Med. https://doi.org/10.1016/j.eclinm.2024.102495
Bedlack RS, Vaughan T, Wicks P, Heywood J, Sinani E, Selsov R, Macklin EA, Schoenfeld D, Cudkowicz M, Sherman A (2016) How common are ALS plateaus and reversals? Neurology 86:808–812. https://doi.org/10.1056/NEJMoa220470510.1212/WNL.0000000000002251
Funding
This work was funded by ICoMM ONLUS, Association for ALS research and by Catholic University (Linea D1 to MS and SL).
Author information
Authors and Affiliations
Contributions
Conceptualization and design of the study: MS, FC, VS, RZ, MF, MC and AC. Acquisition and analysis of data: all authors. Statistical analysis: AL. Drafting of the manuscript: all authors.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest in relationship to this work. MS received advisory board and speaker honoraria from Biogen.
Ethics approval
This study involves human participants and was approved by local ethical committee (approval Prot. ID 5059). Participants gave informed consent to participate in the study before taking part.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sabatelli, M., Cerri, F., Zuccarino, R. et al. Long-term treatment of SOD1 ALS with tofersen: a multicentre experience in 17 patients. J Neurol 271, 5177–5186 (2024). https://doi.org/10.1007/s00415-024-12437-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12437-7