Abstract
Objective
The aim of our study was to analyze the characteristics of patients with autoimmune encephalitis (AE) to identify prognostic factors associated with the development of drug-resistant epilepsy (DRE).
Methods
In this retrospective observational cohort study, we enrolled adult patients with AE between January 2016 and December 2022. The patients were categorized into two groups based on the presence or absence of DRE at the last follow-up. The predictors of the development of DRE were investigated using logistic regression analysis.
Results
Among 121 AE patients, 75.2% (n = 91) experienced acute symptomatic seizures, and 29.8% (n = 36) developed DRE at the last follow-up. On multivariate regression analysis, the factors associated with DRE were antibody negativity (OR 3.628, 95% CI 1.092–12.050, p = 0.035), focal seizure (OR 6.431, 95% CI 1.838–22.508, p = 0.004), refractory status epilepticus (OR 8.802, 95% CI 2.445–31.689, p = 0.001), interictal epileptiform discharges on EEG (OR 6.773, 95% CI 2.206–20.790, p = 0.001), and T2/FLAIR hyperintensity in the limbic system (OR 3.286, 95% CI 1.060–10.183, p = 0.039).
Conclusions
In this study, the risk of developing DRE was mainly observed among AE patients who were negative for antibodies or had focal seizures, refractory status epilepticus, interictal epileptiform discharges on EEG, and T2/FLAIR hyperintensity in the limbic system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune encephalitis (AE) is being increasingly recognized as one of the major causes of acute symptomatic seizures [1]. According to previous studies, approximately 70% of patients with AE have seizures, and most of them respond well to immunotherapy [2]. Patients with AE usually achieve seizure remission and remain seizure free without taking any anti-seizure medications (ASMs) after early immunotherapy [3]. However, some patients still develop persistent seizures despite receiving aggressive immunotherapy [4]. The International League Against Epilepsy (ILAE) proposed the term “autoimmune-associated epilepsy”, which is usually resistant to both ASMs and immunotherapy, to refer to this chronic disease [5]. Therefore, most cases of autoimmune-associated epilepsy are drug-resistant epilepsy (DRE), which is a major contributor to the disorder burden among AE survivors.
DRE after AE is a challenge in clinical practice, but little is known about what specific risk factors may influence the development of this sequela. Previous studies have shown that several clinical characteristics contribute to epilepsy after AE, including status epilepticus, a large number of ASMs, and delayed immunotherapy [4, 6]. Nevertheless, the findings of these studies are controversial due to a lack of a unified definition of AE and different follow-up periods. In this context, we aimed to analyze the clinical and paraclinical findings of adult AE patients and identify potential prognostic factors that may predict the development of DRE.
Materials and methods
Patients
This retrospective observational study enrolled adult AE patients (aged ≥ 18 years) from the Department of Neurology, the First Affiliated Hospital, Sun Yat-sen University, between January 2016 and December 2022. The diagnosis of AE was made according to the consensus criteria established by Graus in 2016 [7]. The exclusion criteria were as follows: (1) patients with a previous diagnosis of epilepsy; (2) patients with other identified etiologies that may cause seizures, including structural, genetic, infectious, or metabolic factors; (3) patients who died or were lost to follow up and those with incomplete clinical data.
The study data of each eligible patient were collected from the medical records database, including demographic and clinical characteristics such as the type of seizure; the presence of status epilepticus, including refractory status epilepticus; disease severity, including intensive care unit (ICU) admission and mRS (modified Rankin Scale) score at acute stage; diagnostic test results, including cerebrospinal fluid (CSF), electroencephalography (EEG) features, and brain magnetic resonance imaging (MRI) findings; and treatment strategies, including ASMs and immunotherapy. Seizure outcomes, brain MRI data, and mRS score at last follow-up were collected via telephone interviews or outpatient electronic data with a minimum follow-up of 12 months after the onset of AE.
Informed consent was obtained from all individual participants included in the study. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University.
Definition of variables
DRE was defined as the failure of adequate trials of two tolerated, appropriately chosen, and used ASM schedules to achieve sustained seizure freedom [8]. First-line immunotherapy included steroids, intravenous immunoglobulin, and plasma exchange/immunoadsorption alone or in combination. Second-line immunotherapy included the use of rituximab, mycophenolate mofetil, cyclophosphamide, and azathioprine. Delayed immunotherapy was defined as immunotherapy that started 28 days after disease onset [9].
Antibody evaluation
All paired serum and CSF samples were screened for antibodies. Cell-based assays (CBAs) were used for detecting Abs targeting neuronal surface antigens, such as N-methyl-d-aspartate receptor (NMDAR), α-amino-3‑hydroxy-5-methyl-4-iso-xazolepropionic acid receptor (AMPAR), leucine-rich glioma-inactivated 1 (LGI-1), contactin-associated protein-2 (CASPR2), gamma-aminobutyric acid B receptor (GABABR), metabotropic glutamate receptor 5 (mGLUR5), etc. Immunoblots and tissue-based assays (TBAs) were used for screening Abs targeting intracellular neuronal antigens, including Hu, Yo, Ri, collapsin response mediator protein 5 (CV2/CRMP5), Ma2/Ta, glutamic acid decarboxylase 65 (GAD65), amphiphysin, etc. Additionally, CBAs or TBAs were used for detecting rare Abs targeting gamma-aminobutyric acid type A receptor (GABAAR) and glycine receptor (GlyR) in a few laboratories.
Investigation panel for the other causes
CSF sample were tested using culture for bacteria, Ab assays for major viruses, and next-generation sequencing (NGS) for various pathogens. CNS neoplasm, demyelinating diseases associated with anti-aquaporin-4 (AQP4) antibody or myelin oligodendrocyte glycoprotein (MOG) antibody, toxic/metabolic aetiologies, systemic or primary CNS vasculitis, and prion disease were carefully excluded based on the clinical and laboratory profiles.
Statistical analysis
Statistical analyses were performed with SPSS, version 26. Continuous variables were defined as the median (range), and categorical variables were described as counts and percentages. The statistical significance of intergroup differences was assessed with the Mann–Whitney U test for quantitative variables and the Chi-squared test for categorical variables. Multiple logistic regression analysis was performed to investigate the independent predictors of DRE if multiple variables were found to be associated with DRE in the univariate analyses (p < 0.05). A p value < 0.05 was considered to indicate statistical significance.
Results
Demographic and clinical characteristics of patients with Ab-positive AEs and Ab-negative AEs
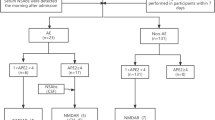
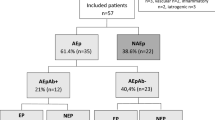
We identified 238 patients who met the diagnostic criteria for AE. A total of 117 patients were excluded based on the exclusion criteria. More detailed information on the excluded patients is shown in Fig. 1. A total of 121 patients were ultimately enrolled, 40.5% (n = 49) of whom were female. The median age of the participants was 33 (24.0–44.5) years. The median follow-up duration for the entire cohort was 44.7 months (range 12–57).
Antibodies, including anti-NMDAR (n = 35), anti-LGI1 (n = 6), anti-GAD65 (n = 1), anti-GlyR (n = 1), anti-AMPAR2 (n = 1), anti-mGLUR5 (n = 1), and anti-amphiphysin (n = 1), were detected in 46 (38%) patients. There were 75 individuals who met the consensus criteria for Ab-negative AE. Table 1 shows the comparison of clinical features between Ab-positive and Ab-negative patients. Ab-positive AEs were more common in females (p = 0.04), younger age of onset (p = 0.033), and clinical presentations, such as involuntary movement (p < 0.001), autonomic dysfunction (p < 0.001), and comorbid tumors (p < 0.001), were significantly more common among those with Ab-positive AEs. Ab-negative patients were more likely to have bilateral FLAIR lesions (p = 0.014), especially in the limbic system (p = 0.003) (Fig. 2). More patients received second-line immunotherapy in the Ab-positive group (p < 0.001). No significant differences were detected in the seizure semiology between Ab-positive and Ab-negative patients.
Brain MRI T2/FLAIR of patients with Ab-negative AE (A–C) and anti-LGI1 encephalitis (D–F). The T2/FLAIR sequence of a 31-year-old female with Ab-negative AE show bilateral hyperintensity in hippocampus and insular lobes (A–C, white arrows). The T2/FLAIR sequence shows hyperintensity in the left hippocampus of a 72-year-old man patient with anti-LGI1 encephalitis (D–F, white arrow)
In our cohort, 75.2% (n = 91) of patients experienced acute symptomatic seizures. Focal to bilateral tonic–clonic seizures or bilateral tonic–clonic seizures were the most common seizure types. Among patients with seizures, status epilepticus was observed in 41 individuals, and refractory status epilepticus was observed in 28 individuals, which was the main reason for requiring ICU admission (n = 42). At the last follow-up, DRE was found in 29.8% (n = 36) of patients. Among 35 patients with anti-NMDAR encephalitis, 82.9% (n = 29) experienced acute symptomatic seizures and the incidence of DRE was 11.4% (n = 4), which was lower than that in Ab-negative AEs (11.4% vs 38.7%, p = 0.004). There are no significant differences in mRS score at follow-up between Ab-positive and Ab-negative patients. Follow-up brain MRI were obtained for 41 patients, and hippocampal sclerosis was found in 17 individuals (41.5%).
Predictors of DRE after AE according to univariate analysis
There were significantly greater incidences of DRE in patients with antibody negativity (p = 0.006), focal seizures (p = 0.001), focal to bilateral tonic–clonic seizures or bilateral tonic–clonic seizures (p = 0.001), status epilepticus (p < 0.001), refractory status epilepticus (p < 0.001), and ICU admission (p < 0.001) (see Table 2). 85.1% (n = 103) of patients presented with abnormal EEG findings, including focal slowing waves (53.7%), diffuse slowing background (42.1%) and interictal epileptiform discharges (38.8%). Among them, only the presence of interictal epileptiform discharges was significantly related to the development of DRE (p < 0.001). Brain MRI abnormalities on T2/FLAIR imaging were present in 61.2% of patients. We found that bilateral T2/FLAIR hyperintensity (p = 0.002), cerebral cortical involvement (p = 0.041), and limbic system involvement (p < 0.001) were related to the development of DRE. Eighty-nine patients were treated with ASMs, and the median number of ASMs used was 3 (ranging from 2 to 6). A significant association between an ASM number ≥ 3 and the development of DRE was found (p < 0.001). However, we did not find differences in immunotherapy strategies or immunotherapy initiation times between DRE patients and non-DRE patients.
Predictors of DRE after AE according to multivariate regression analysis
Multivariate analysis revealed five independent factors that were significantly related to DRE after AE: antibody negativity (OR 3.628, 95% CI 1.092–12.050, p = 0.035), focal seizure (OR 6.431, 95% CI 1.838–22.508, p = 0.004), refractory status epilepticus (OR 8.802, 95% CI 2.445–31.689, p = 0.001), interictal epileptiform discharges on EEG (OR 6.773, 95% CI 2.206–20.790, p = 0.001), and T2/FLAIR hyperintensity in the limbic system (OR 3.286, 95% CI 1.060–10.183, p = 0.039). Table 3 shows the multivariable logistic regression results for the predictors of DRE in patients with AE.
Discussion
In this retrospective study, we provide detailed information on DRE after AE in our center. The overall prevalence of DRE was 29.8%, which is significantly greater than that reported in prior studies [10,11,12]. These inconsistent results may be due to differences in ethnicity, sample sizes, antibody types, and follow-up periods. The independent factors that predicted the development of DRE after the acute phase of AE included antibody negativity, focal seizures, refractory status epilepticus, interictal epileptiform discharges on EEG, and T2/FLAIR hyperintensity in the limbic system.
In our center, the incidence of drug-resistant epilepsy in patients with anti-NMDAR encephalitis was significantly lower than that in Ab-negative AE. This is in line with the previous studies that patients with anti-NMDAR encephalitis have a low rate of chronic seizures and long-term ASMs treatment may be unnecessary [13, 14]. Furthermore, improved seizure outcome was time-dependent in patients with anti-NMDAR encephalitis, as a longer follow-up period was associated with lower risk of seizure relapse [13]. Long-term follow-up data are needed in the future to clarify the changes in seizure rates of different subtypes of AE over the follow-up period, so as to help develop individualized treatment regimens. Furthermore, accumulating evidence has shown that patients with neural surface antibodies have a greater rate of seizure remission than do those with intracellular antibodies [13, 15, 16]. However, we did not demonstrate this issue because of the small sample size. Consistent with these prior findings, our result show that antibody negativity is a greater risk factor for developing enduring seizures. This result may be due to the existence of other unexplored pathophysiological mechanisms that lead to poor response to immunotherapy in patients with Ab-negative AE, such as unknown pathogenic antibodies or T-cell-mediated neuronal cytotoxicity [4, 17,18,19]. Of noted, the proportion of receiving the second-line immunotherapy in Ab-negative AEs was lower than that in Ab-positive AEs, although the baseline severity was similar between these two groups. The high incidence of DRE in Ab-negative AEs may, therefore, be partly explained by inadequate immunotherapy. Future studies on larger samples are warranted to further investigate this issue.
It is commonly considered that acute symptomatic seizures secondary to AE are often multifocal or generalized due to neuroinflammation. We found that focal seizures can predict the development of DRE. A previous meta-analysis showed that the prevalence of DRE was greater in patients with focal epilepsy [20]. One possible mechanism is that recurrent focal seizures may cause progressive connectivity reorganization and thus increase the propensity for seizure generation [21]. Status epilepticus is an important independent risk factor for developing DRE [10, 11, 22]. Our study revealed that refractory status epilepticus was a predictor of DRE. Recently, a series of new-onset refractory status epilepticus (NORSE) studies showed that patients with NORSE were more likely to develop DRE than all causes of status epilepticus [23,24,25]. According to previous clinical research and experimental animal model studies, prolonged seizure activity causes cytotoxic edema and activation of microglia and astrocytes followed by neuronal death and network reorganization, ultimately leading to a lower epilepsy threshold and refractory seizures [26,27,28,29]. From this perspective, early individualized refractory status epilepticus management should be applied to limit the extent of the damage and thus prevent irreversible sequelae.
EEG is a useful tool for predicting the development of DRE after an AE. Our study revealed an association between interictal epileptiform discharges and the development of DRE. Previous studies have reported that focal or generalized epileptiform discharges are independent risk factors for developing chronic epilepsy [3, 30,31,32]. The researchers found that a high seizure burden does not indicate frequent episodes of interictal epileptiform discharges, while the presence of interictal epileptiform discharges was associated with the persistence of seizures after an AE over the follow-up period [33]. The exact mechanism has not been elucidated. Seizures and neuroplasticity are closely related, as they can positively affect each other [34]. We hypothesized that interictal epileptiform discharges may reflect frequent epileptic activity resulting from underlying autoimmune pathology, while repeated seizures that alter neuronal networks may in turn increase the possibility of interictal epileptiform discharges.
Hyperintensity in the limbic system is a risk factor for developing DRE. Notably, we found that more than half of the DRE patients had hippocampal sclerosis during the follow-up period. The reorganization of the neuronal network in the hippocampus may contribute to vulnerability to enduring seizures in the chronic stage. It has been reported that the hippocampus is highly susceptible to damage, and the progressive cellular and molecular changes caused by acute inflammation could lead to neuroplastic changes in this epileptogenic brain region [34]. Thus, hyperintensity in the limbic system might become an imaging biomarker as a target for neuroprotective treatments to prevent irreversible sequelae [32, 35, 36]. Long-term follow-up MRI may reveal the pathophysiology of DRE after an AE. However, potential selection bias should be considered, as patients with DRE might have a greater chance of undergoing repetitive MRI evaluations. Similar results have been reported by Steriade et al. who reported that the presence of mesial temporal hyperintensity is an independent predictor of ongoing seizures after AE [37]; however, they did not find a correlation between hippocampal atrophy and chronic epilepsy in a longitudinal MRI analysis. Additional factors beyond structural changes, such as ongoing neuroinflammation, may also account for the development of enduring seizures [32, 38]. Future studies with larger samples are needed to uncover the pathological mechanisms involved and guide treatment.
In clinical practice, the combination of ASMs and immunotherapy is an optimal strategy for achieving seizure-free outcomes. Several studies have reported that a greater number of ASMs are an independent risk factor for epilepsy after AE [4, 31]. Most patients in our cohort required a large number of ASMs due to the high proportion of status epilepticus. However, we did not observe differences in the number of ASMs between the DRE and non-DRE groups. The timing of immunotherapy initiation is crucial, as unfavorable seizure outcomes may be attributed to delayed treatment [39]. Most of our patients received timely immunotherapy, and we did not find significant differences between delayed immunotherapy and DRE. At present, there is no standardized management guideline for immunotherapy strategy of Ab-negative AE. In our cohort, the timing and regimen of second-line immunotherapy were based on disease severity, drug responsiveness and acceptance of the treatment by the patients and their families. Previous studies have showed that aggressive immunotherapy with the second-line immunotherapy may have the potential to achieve better seizure outcomes [40] However, the immunotherapy regimen did not appear to be associated with epilepsy outcomes in this cohort. We speculate that this result may be related to fewer patients receiving the second-line immunotherapy, especially those with Ab-negative AEs. The effect of long-term immunotherapy in patients with DRE remains unclear. Clinical trials are needed to determine the benefits and optimal regimens of long-term immunotherapy in these patients.
Limitations
Our study has limitations. First, the retrospective and tertiary nature of the study implies the risk of inaccurate records and selection bias. Second, nonmotor seizures may have been overlooked based on telephone follow-up. Third, the sample size of this study was small, and the conclusions need to be verified in future cohort studies with larger samples. Fourth, retrospective analysis may not be able to finely distinguish seizure types, and focal seizures may be underestimated.
Conclusions
DRE after AE is a challenge in clinical practice. The independent factors that predicted the development of DRE after the acute phase of AE included antibody negativity, focal seizures, refractory status epilepticus, interictal epileptiform discharges on EEG, and T2/FLAIR hyperintensity in the limbic system. For AE patients with an increased risk of developing DRE, long-term ASMs treatment should be recommended.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Britton JW, Dalmau J (2019) Recognizing autoimmune encephalitis as a cause of seizures: treating cause and not effect. Neurology 92:877–878. https://doi.org/10.1212/WNL.0000000000007444
Yeshokumar AK, Coughlin A, Fastman J et al (2021) Seizures in autoimmune encephalitis—a systematic review and quantitative synthesis. Epilepsia 62:397–407. https://doi.org/10.1111/epi.16807
Liu X, Guo K, Lin J et al (2022) Long-term seizure outcomes in patients with autoimmune encephalitis: a prospective observational registry study update. Epilepsia 63:1812–1821. https://doi.org/10.1111/epi.17245
Matricardi S, Casciato S, Bozzetti S et al (2022) Epileptic phenotypes in autoimmune encephalitis: from acute symptomatic seizures to autoimmune-associated epilepsy. J Neurol Neurosur PS. https://doi.org/10.1136/jnnp-2022-329195
Steriade C, Britton J, Dale RC et al (2020) Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia 61:1341–1351. https://doi.org/10.1111/epi.16571
Chen SS, Zhang YF, Di Q et al (2021) Predictors and prognoses of epilepsy after anti-neuronal antibody-positive autoimmune encephalitis. Seiz Eur J Epilep 92:189–194. https://doi.org/10.1016/j.seizure.2021.09.007
Graus F, Titulaer MJ, Balu R et al (2016) A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 15:391–404. https://doi.org/10.1016/S1474-4422(15)00401-9
Kwan P, Arzimanoglou A, Berg AT et al (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51:1069–1077. https://doi.org/10.1111/j.1528-1167.2009.02397.x
Shen CH, Fang GL, Yang F et al (2020) Seizures and risk of epilepsy in anti-NMDAR, anti-LGI1, and anti-GABA(B) R encephalitis. Ann Clin Transl Neur 7:1392–1399. https://doi.org/10.1002/acn3.51137
Pillai SC, Mohammad SS, Hacohen Y et al (2016) Postencephalitic epilepsy and drug-resistant epilepsy after infectious and antibody-associated encephalitis in childhood: clinical and etiologic risk factors. Epilepsia 57:e7–e11. https://doi.org/10.1111/epi.13253
Guery D, Cousyn L, Navarro V et al (2022) Long-term evolution and prognostic factors of epilepsy in limbic encephalitis with LGI1 antibodies. J Neurol 269:5061–5069. https://doi.org/10.1007/s00415-022-11162-3
Wesselingh R, Broadley J, Buzzard K et al (2022) Prevalence, risk factors, and prognosis of drug-resistant epilepsy in autoimmune encephalitis. Epilep Behav 132:108729. https://doi.org/10.1016/j.yebeh.2022.108729
Liu X, Yan B, Wang R et al (2017) Seizure outcomes in patients with anti-NMDAR encephalitis: a follow-up study. Epilepsia 58:2104–2111. https://doi.org/10.1111/epi.13929
Ni G, Lin W, Cai X et al (2020) Associations between seizures and MRI in patients with anti-NMDAR encephalitis. Acta Neurol Scand 142:460–465. https://doi.org/10.1111/ane.13298
Ilyas-Feldmann M, Pruss H, Holtkamp M (2021) Long-term seizure outcome and antiseizure medication use in autoimmune encephalitis. Seiz Eur J Epilep 86:138–143. https://doi.org/10.1016/j.seizure.2021.02.010
Smith KM, Britton JW, Thakolwiboon S et al (2023) Seizure characteristics and outcomes in patients with neurological conditions related to high-risk paraneoplastic antibodies. Epilepsia 64:2385–2398. https://doi.org/10.1111/epi.17695
Graus F, Escudero D, Oleaga L et al (2018) Syndrome and outcome of antibody-negative limbic encephalitis. Eur J Neurol 25:1011–1016. https://doi.org/10.1111/ene.13661
Lee WJ, Lee HS, Kim DY et al (2022) Seronegative autoimmune encephalitis: clinical characteristics and factors associated with outcomes. Brain 145:3509–3521. https://doi.org/10.1093/brain/awac166
Mojzisova H, Krysl D, Hanzalova J et al (2023) Antibody-negative autoimmune encephalitis: a single-center retrospective analysis. Neurol-Neuroimmunol. https://doi.org/10.1212/NXI.0000000000200170
Sultana B, Panzini MA, Veilleux CA et al (2021) Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology 96:805–817. https://doi.org/10.1212/WNL.0000000000011839
Englot DJ, Konrad PE, Morgan VL (2016) Regional and global connectivity disturbances in focal epilepsy, related neurocognitive sequelae, and potential mechanistic underpinnings. Epilepsia 57:1546–1557. https://doi.org/10.1111/epi.13510
Huang Q, Ma M, Wei X et al (2018) Characteristics of seizure and antiepileptic drug utilization in outpatients with autoimmune encephalitis. Front Neurol 9:1136. https://doi.org/10.3389/fneur.2018.01136
Hocker S, Nagarajan E, Rabinstein AA et al (2016) Progressive brain atrophy in super-refractory status epilepticus. Jama Neurol 73:1201–1207. https://doi.org/10.1001/jamaneurol.2016.1572
Kim HJ, Lee SA, Kim HW et al (2020) The timelines of MRI findings related to outcomes in adult patients with new-onset refractory status epilepticus. Epilepsia 61:1735–1748. https://doi.org/10.1111/epi.16620
Specchio N, Pietrafusa N (2020) New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev Med Child Neurol 62:897–905. https://doi.org/10.1111/dmcn.14553
Cross DJ, Cavazos JE (2007) Synaptic reorganization in subiculum and CA3 after early-life status epilepticus in the kainic acid rat model. Epilepsy Res 73:156–165. https://doi.org/10.1016/j.eplepsyres.2006.09.004
Rami A, Niquet J, Konoplew A (2019) Early aberrant growth of mossy fibers after status epilepticus in the immature rat brain. Mol Neurobiol 56:5025–5031. https://doi.org/10.1007/s12035-018-1432-y
Mariajoseph FP, Sagar P, Muthusamy S et al (2021) Seizure-induced reversible MRI abnormalities in status epilepticus: a systematic review. Seiz Eur J Epilep 92:166–173. https://doi.org/10.1016/j.seizure.2021.09.002
Butler CR, Westbrook GL, Schnell E (2022) Adaptive mossy cell circuit plasticity after status epilepticus. J Neurosci 42:3025–3036. https://doi.org/10.1523/JNEUROSCI.1008-21.2022
Steriade C, Moosa A, Hantus S et al (2018) Electroclinical features of seizures associated with autoimmune encephalitis. Seiz Eur J Epilep 60:198–204. https://doi.org/10.1016/j.seizure.2018.06.021
Gifreu A, Falip M, Sala-Padro J et al (2021) Risk of developing epilepsy after autoimmune encephalitis. Brain Sci. https://doi.org/10.3390/brainsci11091182
Zhong R, Zhang X, Chen Q et al (2022) Acute symptomatic seizures and risk of epilepsy in autoimmune encephalitis: a retrospective cohort study. Front Immunol 13:813174. https://doi.org/10.3389/fimmu.2022.813174
Gifreu A, Falip M, Sala-Padro J et al (2021) Risk of developing epilepsy after autoimmune encephalitis. Brain Sci. https://doi.org/10.3390/brainsci11091182
Jarero-Basulto JJ, Gasca-Martinez Y, Rivera-Cervantes MC et al (2018) Interactions between epilepsy and plasticity. Pharm Base. https://doi.org/10.3390/ph11010017
Malter MP, Widman G, Galldiks N et al (2016) Suspected new-onset autoimmune temporal lobe epilepsy with amygdala enlargement. Epilepsia 57:1485–1494. https://doi.org/10.1111/epi.13471
Yokoi S, Kidokoro H, Yamamoto H et al (2019) Hippocampal diffusion abnormality after febrile status epilepticus is related to subsequent epilepsy. Epilepsia 60:1306–1316. https://doi.org/10.1111/epi.16059
Steriade C, Patel PS, Haynes J et al (2023) Predictors of seizure outcomes of autoimmune encephalitis: a clinical and morphometric quantitative analysis study. Clin Neurol Neurosurg 231:107854. https://doi.org/10.1016/j.clineuro.2023.107854
Carreno M, Bien CG, Asadi-Pooya AA et al (2017) Epilepsy surgery in drug resistant temporal lobe epilepsy associated with neuronal antibodies. Epilep Res 129:101–105. https://doi.org/10.1016/j.eplepsyres.2016.12.010
Casciato S, Morano A, Fattouch J et al (2019) Factors underlying the development of chronic temporal lobe epilepsy in autoimmune encephalitis. J Neurol Sci 396:102–107. https://doi.org/10.1016/j.jns.2018.10.026
Byun JI, Lee ST, Jung KH et al (2016) Effect of immunotherapy on seizure outcome in patients with autoimmune encephalitis: a prospective observational registry study. PLoS ONE 11:e0146455. https://doi.org/10.1371/journal.pone.0146455
Funding
This study is funded by National Key Program of China (no. 2022YFC2503804), National Natural Science Foundation of China (no. 82271489, no. 81971203, no. 81801287) and Science and Technology Program of Guangzhou, China (no. 2024A04J4564), Guangdong Provincial Clinical Research Center for Neurological Diseases (no. 2020B1111170002), Guangdong Province International Cooperation Base for Early Intervention and Functional Rehabilitation of Neurological Diseases (no. 2020A0505020004), Guangdong Provincial Engineering Center for Major Neurological Disease Treatment, Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease, and Guangzhou Clinical Research and Translational Center for Major Neurological Diseases.
Author information
Authors and Affiliations
Contributions
WH, XL, JZ, and JC: collected data at the First Affiliated Hospital, Sun Yat-sen University. HZ, ZC, and GN: extracted and analyzed data. WH: drafted the manuscript, which then was critically revised by all the authors. All the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
There is no conflict of interest related to the study.
Ethical standard
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University and conforms to World Medical Association Declaration of Helsinki.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, W., Zhang, H., Li, X. et al. Prognostic factors underlying the development of drug-resistant epilepsy in patients with autoimmune encephalitis: a retrospective cohort study. J Neurol 271, 5046–5054 (2024). https://doi.org/10.1007/s00415-024-12432-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-024-12432-y