Abstract
Primary central nervous system lymphoma (PCNSL) is a rare type of non-Hodgkin lymphoma (NHL) manifesting in the brain, spinal cord, cerebrospinal fluid and/or eyes, in the absence of systemic manifestations. With an increasing incidence and a 30% 5-year overall survival if promptly treated, timely diagnosis and subsequent treatment is paramount. The typical MRI appearance for PCNSL is a solitary or multiple T2-hypointense, homogeneous gadolinium-enhancing lesion with restricted diffusion. Dexamethasone treatment might compromise and delay the diagnosis. Hallmark of treatment is induction with intravenous high-dose methotrexate consisting polychemotherapy followed by consolidation treatment. Consolidation treatment consists of either whole brain radiotherapy (WBRT) or autologous stem cell transplantation (ASCT). Given the (cognitive) side effects of WBRT, ASCT is increasingly being used as the first choice of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system lymphoma (PCNSL) is a B-cell non-Hodgkin lymphoma manifesting in the brain, spinal cord, cerebrospinal fluid and/or the eye, without systemic manifestations. An increase in incidence is observed, specifically among elderly. Though relapses are common, in most cases PCNSL is treatable which makes timely diagnosis and treatment essential for survival.
Epidemiology
PCNSL are rare tumours, comprising only 3–4% of intracranial tumours. In the United States, approximately 1500 new cases are diagnosed each year [1]. Although immunocompromised patients are at higher risk of developing a PCNSL, the incidence among the immunocompetent elderly (i.e., > 60-years-old) population is increasing without a clear understanding as to why. Currently, the median age of presentation is 65 years, in one third of cases presentation is at an age above 75 years. Men are slightly more often affected than women. Over the past three decades, the prognosis of PCNSL has improved in patients under the age of 70, whereas it has remained unchanged for elderly [2].
More uncommon presentations of PCNSL include those limited to the eye, the primary vitreoretinal lymphoma (PVRL), and those limited to the spinal cord. Leptomeningeal localisation is mostly seen in systemic lymphomas with central nervous system involvement and is rarely encountered as an isolated finding.
Diagnosis
Symptoms
As a result of the fast-growing nature of PCNSL, symptoms usually develop within days to weeks. Focal neurological deficits, cognitive or behavioural disturbances and/ or headache are among the most common signs. Seizures, which are very common in gliomas, are a less common presenting symptom [3, 4]. Table 1 provides an overview of presenting signs and symptoms.
Imaging
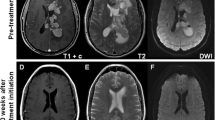
Most (65%) PCNSL are solitary lesions, however multifocal lesions are not uncommon. In immunocompetent patients, MRI images show T2-hypointens lesions with vasogenic edema, homogenous enhancement, and restricted diffusion (Fig. 1) [5]. Contrast enhancement may occasionally be more inhomogeneous or patchy, and in immunocompromised patients ring-enhancing lesions occur. In addition to the cerebral hemispheres, lymphomas are often localized in or around the midline structures, such as the periventricular region, thalamus and basal ganglia, or corpus callosum. In 10% of patients, additional non-contrast enhancing lesions are observed. Since these lesions respond to treatment, they are also regarded as tumorous [6]. Features such as calcifications, necrosis, and haemorrhages are very rare in central nervous system lymphomas.
PET-imaging
The distinction between PCNSL and secondary central nervous system involvement of a systemic lymphoma is important for the treatment protocol. CT imaging of neck, thorax and abdomen, bone marrow aspirate and testicular echography in men are required for making this distinction. Total body 18F fluorodeoxyglucose positron emission tomography (18F-FDG-PET) scan may replace these three modalities and may even be more sensitive [7, 8]. Although PET is sensitive for localisation of systemic activity, false-positive PET enhancement is common and may delay the diagnostic process. The International Primary CNS Lymphoma Collaborative Group (IPCG) currently recommends a systemic staging evaluation with a [18F]2-fluoro-2-deoxy-d-glucose-positron emission tomography (FDG-PET), preferably combined with contrast-enhanced CT scan [9].
The role of FDG-PET in cerebral lesions is under debate; MRI is the imaging modality of choice for initial diagnosis and follow-up [10]. If performed, FDG-PET of PCNSL shows an avid lesion with homogeneous uptake. Given the high cellular density and high glucose metabolism, lymphoma is 2–3 times more PET-avid than healthy grey matter [11].
Pathology
The pathophysiology of PCNSL is unknown. Although “primary” implies that the tumour originates in the central nervous system, the leading hypothesis is that the tumour homes to the nervous system after arising in other organs. This hypothesis is supported by the fact that the oncogenic protein Bcl-6 is found in virtually all PCNSL but is only expressed in lymphoid tissue which is normally not present within the nervous system. The Epstein-Barr-Virus (EBV) predisposes immunocompromised patients to developing a PCNSL, but this is not the case for immunocompetent patients. No genetic factors with an associated risk for developing PCNSL have been identified.
According to the WHO classification of tumours of haematopoietic and lymphoid tissues PCNSL are diffuse large B-cell lymphomas (DLBCL) [12]. In just 10%, lymphoma solely located within the CNS are Burkitt, low grade or T-cell lymphomas. Histopathologically, DLBCL are characterized by medium-to-large neoplastig cells with pleomorphic nuclei and prominent nucleoli, surrounded by a narrow rim of cytoplasm. Mitotic activity is often brisk. Tumour cells are characteristically centred around blood vessels, and necrosis is a common finding. (Fig. 2). Most cells express mature B-cell markers (CD20, CD79a, CD19, PAX5) and show a high Ki-67 proliferation index.
Both CSF and histological diagnostic accuracies are reduced after treatment with corticosteroids due to the inherent lysis of neoplastic cells. Multiple large series have found false-negative biopsies after dexamethasone treatment, causing a delay in both diagnosis and treatment [13, 14]. This signifies the relative contraindication for dexamethasone treatment—when suspecting PCNSL—until biopsy procured tissue.
Cerebrospinal fluid
A lumbar puncture is advised in case of an MRI-lesion suspect for lymphoma. Cerebrospinal fluid may provide the diagnosis, and is important for staging. A biopsy is strongly recommended for obtaining a definitive diagnosis. If this is not possible, the diagnosis of PCNSL can only be made through CSF in case of positive CSF results in combination with typical clinical and radiological characteristics. Cerebrospinal fluid typically shows a pleiocytosis with increased total protein and decreased glucose levels, but can also be without abnormalities. Cytology and immunophenotyping with flow cytometry provide additional information when lymphoma is suspected, providing positive results in up to 30% of cases. However, flow cytometry may also be false positive in case of an infectious problem, for instance with neuroborreliosis [15]. When the first CSF analysis shows no lymphoma localisation, a second lumbar puncture may be considered, yielding a diagnosis in an additional 8% of cases [16]. However, this small increase in diagnostic accuracy needs to be weighed against the delay it entails to analyse the CSF and thereby delaying the work-up for a biopsy [17].
Besides cytology and immunophenotyping, other CSF characteristics are helpful. A mutation in the MYD88-gene is often present in PCNSL and increased interleukin-10 concentrations (IL-10) may be observed. Testing for these items show promising sensitivity (94–98%) and specificity (98–99%) for both newly diagnosed and recurring PCNSL. However, the current literature is as of yet insufficient to justify a definitive diagnosis based on these factors alone [18]. As a result, brain biopsy remains the golden standard of diagnosis.
Further workup
As previously discussed, distinguishing PCNSL from a systemic lymphoma with CNS involvement is important for the treatment choice. Apart from imaging, ophthalmological examination is advised including fundoscopy and slit lamp examination to rule out vitreoretinal involvement. Only 4% of patients report ocular symptoms, whereas 15–20% of patients have asymptomatic ocular involvement [3, 19]. To prevent complications during chemotherapy, screening for HIV and hepatitis B and C is advised.
Primary diffuse large B-cell lymphoma of the vitreoretinal
When a PCNSL is localised exclusively in the vitreoretinal fluid, it is defined as a primary diffuse large B-cell lymphoma of the vitreoretina of which 90% develop cerebral localisation during the course of the disease. Therefore, the same treatment regimen as for PCNSL with cerebral localisation is advised. This includes high-dose intravenous methotrexate-based chemotherapy, possibly followed by consolidation therapy (see below). Since methotrexate might not penetrate the eye as well as other parts of the nervous system, some experts suggest intra-ocular chemotherapy or local radiation, albeit considering toxicity. Frail patients might benefit from local treatment only (i.e., omitting systemic treatment), retaining more quality of life in the subsequent years of survival [20]. Whether a PCNSL presents with or without ocular involvement does not seem to effect the prognosis. In those cases with ocular involvement (or PVRL) follow-up with fundoscopy and slit lamp examination is advised, in addition to traditional MRI follow-up [20, 21].
Differential diagnosis
The differential diagnosis of PCNSL includes other tumours (such as metastasis, gliomas or meningiomas), CNS infections (e.g. toxoplasmosis or brain abscess) and inflammatory/auto-immune disease (e.g. sarcoidosis, tumefactive multiple sclerosis). Table 2 provides an overview of the differential diagnosis with clinical and radiological features.
Treatment
Surgery
PCNSL are infiltrating tumours with a high sensitivity to chemotherapy and radiation, making surgical resection a less favourable option. Some retrospective studies and a post-hoc analysis of a prospective study have reported a positive effect of debulking, however these were selected cohorts with more favourable prognostic factors such as high Karnofsky performance score and solitary superficial lesions [20].
Systemic treatment
Randomized controlled trials (RCT) are scarce and the best systemic treatment has yet to be defined. Intravenous high-dose (> 3 g/m2 body surface) methotrexate is the hallmark of treatment, mostly combined with other blood–brain barrier penetrating chemotherapeutic agents. A common treatment plan is the MATRix regimen combining high-dose methotrexate (MTX), cytarabine, thiotepa and rituximab, based on a phase II study, in which three arms were compared and the MATRix regimen yielded best survival rates [22]. In a larger phase III RCT, patients were treated with MBVP (i.e., high-dose methotrexate, BCNU, teniposide, carmustine, and prednisolone) with or without rituximab, no survival benefit was found of adding rituximab. Responding patients received a cycle high-dose cytarabine after two cycles of (R)-MBVP [23]. Although different trials, the results of the MATRix treatment and MBVP regimen seem comparable. Complications of high-dose intravenous methotrexate treatment are renal insufficiency, transient hepatotoxicity, mucositis, and bone marrow depression.
Consolidation treatment: whole brain radiotherapy or autologous stem cell transplantation
When a good response is achieved with induction treatment using the aforementioned chemotherapeutic agents, “consolidation” treatment is advised for those patients in good clinical condition. A good response is defined as at least a 50% reduction of tumour mass when compared to baseline (partial response). Consolidation treatment may consist of whole brain radiotherapy (WBRT) or myeloablative chemotherapy followed by autologous stem cell transplantation (ASCT). WBRT is preferred over local radiation due to the infiltrative aspect of the tumour and frequent recurrence outside the irradiated area in historical series. Important side effects of WBRT are neurocognitive decline—which may occur even several years after treatment. Elderly (> 60 years-old) patients are more vulnerable to severe neurotoxicity. Nevertheless, even younger patients show reduced scores on several cognitive tests after WBRT when compared to those not receiving radiation. MRI can reveal white matter lesions and atrophy, associated with WBRT [24]. These findings were reported in patients receiving WBRT with 36–45 Gy, leading to protocols now prescribing WBRT with more modest 23–30 Gy doses, in which the cognitive decline may be less severe [23, 25]. Consolidation with myeloablastive chemotherapy followed with ASCT is a good alternative. The conditioning therapy should consist of high-dose thiotepa, in combination with other blood–brain-barrier penetrating drugs [26,27,28]. Two RCTs have studied the effect of ASCT when compared to WBRT [22, 29]: both studies did not identify differences in survival rates between the two treatment modalities. However, the WBRT group suffered more cognitive decline than the ASCT group, whereas ASCT was associated with a 10% treatment related mortality. Currently, patients below 70 years of age with acceptable clinical conditions (KPS > 70) are preferably treated with ASCT as consolidation treatment.
Intrathecal chemotherapy
No studies have specifically examined intrathecal chemotherapy as an add-on treatment besides systemic chemotherapy. In general it is not advised, as intravenous HD-MTX sufficiently penetrates the blood–brain-barrier. However, in the MBVP-regimen it is administered to those patients with CSF localisation after the first course of chemotherapy. The HOVON 105/ALLG NHL 24-study showed that this was only necessary for 8% of patients [23]. In those cases 15 mg methotrexate is administered twice weekly via lumbar puncture (or 10 mg via Ommaya reservoir) combined with a low dose corticosteroid to prevent arachnoiditis. Administration may be reduced over time after normalisation of CSF. Intrathecal methotrexate is potentially toxic, causing diffuse white matter lesions [30].
Treatment in elderly patients
Although the definition of “the elderly” differs among studies, they tend to have a worse prognosis than younger patients [31]. Older patients are also at a higher risk of developing neurotoxicity with cognitive decline, gait disturbance and urge incontinence mostly occurring from circa 6–12 months after treatment, with accompanying atrophy and white matter lesions on MRI. Treating the elderly patient is additionally challenging due to comorbidities such as reduced renal and/or cardiac function, although in most studies (with strictly selected cohorts) high-dose methotrexate was well tolerated. The preferred high-dose MTX protocol is unclear, but it is advised that older patients can be treated with the same dose intravenous methotrexate when provided with supportive care and monitoring of the renal function. In case of severe renal dysfunction (eGFR < 50 ml/min) lower dosage MTX is advisable [32]. Whole brain radiotherapy is ill-advised due to the cognitive side effects. However, when patients are unsuitable for chemotherapy, radiation may be a palliative option with a median survival of 8 months [31]. The cognitive side effects, especially when patients live longer than 6–12 months, should be discussed explicitly with patients. Large trials show that cognitive decline in the elderly population is mainly caused by the tumour itself, illustrated by the improvement of cognitive functions following induction therapy. However, the combination of chemotherapy and radiation causes leukoencephalopathy and atrophy, which may lead to dementia, ataxia and incontinence. The exact interval between treatment and these symptoms is unknown, but they may arise within 1 year after treatment.
Response evaluation
When assessing treatment response five categories are defined: complete response (no contrast enhancing lesion on MRI); complete response unconfirmed (a small—probably biopsy related—contrast enhanced lesion); partial response (decrease in contrast enhancement of at least 50%); stable disease (MRI contrast enhancement of < 50% decrease or < 25% increase); progression (more than 25% contrast enhancement or new lesions on MRI); and relapse (presence of tumour after complete response on previous MRI) [33].
A treatment response is important for prognosis, but analysis from a large RCT showed that in case of at least a partial response, the extent of response did not influence progression-free and overall survival [34].
Prognosis
If untreated, patients with PCNSL usually die within 1–3 months. Due to treatment options, prognosis has improved over the years with a 5-year survival ranging from 11% (95% confidence interval 8–15%) in 1989 to 30% (27–30% confidence interval) in 2015 [35]. This improvement in prognosis is only observed in younger (< 70 years) patients [2]. However, 50% of elderly patients who were able to receive MTX treatment survived over 2 years, thereby underlining the importance of adequate selection of patients eligible for chemotherapy.
Existing prognostic models for PCNSL are mainly used for stratifying patients for clinical trials [33, 36]. Age and clinical condition (KPS or WHO performance scores) are important parameters in both models. Other factors which may influence prognosis are: involvement of deep brain structures (periventricular, basal ganglia, brain stem, or cerebellum), increased serum lactate dehydrogenases (LDH), increased total protein in CSF and MMSE scores prior to treatment [37].
Refractory or relapsed PCNSL
PCNSL with a response rate of less than 50% or with persisting CSF localisation after induction treatment are defined as “refractory”. Treatment for both refractory and relapse PCNSL is unclear. Prognosis is poor, especially in case of persistent MRI abnormalities, with 50% of patients dying within months. Further treatment should be weighed against palliative care, considering cognitive performance, clinical condition, and comorbidity. When choosing further treatment in refractory or early (< 1 year) relapse, chemotherapy based on ifosfamide or etoposide is the treatment of choice. If the tumour relapses 1 year after completion of treatment, another course of HD-MTX is an option. If the patient did not receive earlier treatment with WBRT or ASCT, both can be considered (if the clinical condition permits it) [20].
Follow-up
Regular MRIs (every 3–4 months) are advised the first 2 years after treatment, after this period the best follow-up frequency is unclear. Only 6–25% of relapses are asymptomatic and most relapses occur in between imaging follow-up, therefore clinical follow-up after the first 2 years may be considered instead of regular MRIs. Five years after treatment, relapses are rare, and discontinuation of follow-up is therefore justified. Follow-up should not be aimed at tumour response only; the side effects of treatment regimens should also be considered. Quality of life and cognitive functioning are importance aspects, both of which are diminished among treated patients when compared to age and educational level matched peers [29].
PCNSL in immunocompromised patients
Immunocompromised patients, whether due to untreated HIV, use of immunosuppressant drugs, or organ transplant recipients, are at higher risk for developing a PCNSL. Since the introduction of antiretroviral therapy (ART), the incidence of PCNSL decreased in HIV patients. MRI features may be less typical in this specific group of patients and patchy or ring-enhancing lesions are more common, which may pose a diagnostic challenge. The pathophysiology is strongly related to reactivation of EBV in immunocompromised patients. Treatment is based on reducing immunosuppressant or properly treating HIV, combined with anti-tumour treatment as described above [20].
Future perspective
Clinical trials are studying the effect of less toxic treatments for frail patients with low-dose maintenance chemo-therapy or immunosuppressive drugs. The use of Chimeric antigenreceptor (CAR)-T cells is established in the treatment of relapsed systemic diffuse large B-cell lymphoma; the effect on PCNSL is currently studied in a phase 1 trial (Clinical trial numbers NCT05625594 and NCT04443829).
Conclusions
Primary central nervous system lymphomas are rare but treatable tumours. Early recognition and treatment are paramount. Chemotherapy (including high-dose methotrexate) and preferably followed by autologous stem cell transplantation are recommended treatments. Long-term cognitive decline may be caused by the tumour itself or as a side effect of treatment, warranting consideration during follow-up.
References
O’Neill BP, Decker PA, Tieu C, Cerhan JR (2013) The changing incidence of primary central nervous system lymphoma is driven primarily by the changing incidence in young and middle-aged men and differs from time trends in systemic diffuse large B-cell non-Hodgkin’s lymphoma. Am J Hematol 88:997–1000. https://doi.org/10.1002/AJH.23551
Mendez JS, Ostrom QT, Gittleman H et al (2018) The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 20:687–694. https://doi.org/10.1093/NEUONC/NOX187
Touitou V, Lehoang P, Bodaghi B (2015) Primary CNS lymphoma. Curr Opin Ophthalmol 26:526–533. https://doi.org/10.1097/ICU.0000000000000213
Bataille B, Delwail V, Menet E et al (2000) Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 92:261–266. https://doi.org/10.3171/jns.2000.92.2.0261
Küker W, Nägele T, Korfel A et al (2005) Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol 72:169–177. https://doi.org/10.1007/S11060-004-3390-7
Tabouret E, Houillier C, Martin-Duverneuil N et al (2017) Patterns of response and relapse in primary CNS lymphomas after first-line chemotherapy: Imaging analysis of the ANOCEF-GOELAMS prospective randomized trial. Neuro Oncol 19:422–429. https://doi.org/10.1093/neuonc/now238
Mohile NA, Deangelis LM, Abrey LE (2008) The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol. https://doi.org/10.1215/15228517-2007-061
Malani R, Bhatia A, Wolfe J, Grommes C (2019) Staging identifies non-CNS malignancies in a large cohort with newly diagnosed lymphomatous brain lesions. Leuk Lymphoma. https://doi.org/10.1080/10428194.2018.1563294
Barajas RF, Politi LS, Anzalone N et al (2021) Consensus recommendations for MRI and PET imaging of primary central nervous system lymphoma: guideline statement from the international primary CNS lymphoma collaborative group (IPCG). Neuro Oncol. https://doi.org/10.1093/neuonc/noab020
Makino K, Hirai T, Nakamura H et al (2011) Does adding FDG-PET to MRI improve the differentiation between primary cerebral lymphoma and glioblastoma? Observer performance study. Ann Nucl Med. https://doi.org/10.1007/s12149-011-0483-1
Rozenblum L, Houillier C, Soussain C et al (2022) Role of positron emission tomography in primary central nervous system lymphoma. Cancers (Basel). https://doi.org/10.3390/cancers14174071
Alaggio R, Amador C, Anagnostopoulos I et al (2023) Correction: “The 5th edition of The World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms”. Leukemia. 2022 Jul;36(7):1720–1748. Leukemia 37:1944–1951. https://doi.org/10.1038/s41375-023-01962-5
Brück W, Brunn A, Klapper W et al (2013) Differential diagnosis of lymphoid infiltrates in the central nervous system: experience of the network lymphomas and lymphomatoid lesions in the nervous system. Pathologe 34:186–197. https://doi.org/10.1007/S00292-013-1742-9
Corticosteroid pre-treated primary CNS lymphoma: a detailed analysis of stereotactic biopsy findings and consideration of interobserver variability—PubMed. https://pubmed.ncbi.nlm.nih.gov/26339344/. Accessed 26 Oct 2023
Saggese CE, Cecotti L, Lazzarino De Lorenzo LG (2013) Neuroborreliosis and CNS lymphoma: what is the nexus? Neurol Sci 34:2253–2254. https://doi.org/10.1007/s10072-013-1492-8
Bromberg JEC, Breems DA, Kraan J et al (2007) CSF flow cytometry greatly improves diagnostic accuracy in CNS hematologic malignancies. Neurology 68:1674–1679. https://doi.org/10.1212/01.WNL.0000261909.28915.83
Morell AA, Shah AH, Cavallo C et al (2019) Diagnosis of primary central nervous system lymphoma: a systematic review of the utility of CSF screening and the role of early brain biopsy. Neurooncol Pract 6:415–423. https://doi.org/10.1093/nop/npz015
Hiemcke-Jiwa LS, Leguit RJ, Snijders TJ et al (2019) MYD88 p. (L265P) detection on cell-free DNA in liquid biopsies of patients with primary central nervous system lymphoma. Br J Haematol 185:974–977. https://doi.org/10.1111/BJH.15674
Cassoux N, Merle-Beral H, Leblond V et al (2000) Ocular and central nervous system lymphoma: clinical features and diagnosis. Ocul Immunol Inflamm 8:243–250. https://doi.org/10.1076/OCII.8.4.243.6463
Hoang-Xuan K, Deckert M, Ferreri AJM et al (2023) European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro Oncol 25:37–53. https://doi.org/10.1093/neuonc/noac196
Grimm SA, Pulido JS, Jahnke K et al (2007) Primary intraocular lymphoma: an international primary central nervous system lymphoma collaborative group report. Ann Oncol 18:1851–1855. https://doi.org/10.1093/ANNONC/MDM340
Ferreri AJM, Cwynarski K, Pulczynski E et al (2017) Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the international extranodal lymphoma study group-32 phase 2 trial. Lancet Haematol 4:e510–e523. https://doi.org/10.1016/S2352-3026(17)30174-6
Bromberg JEC, Issa S, Bakunina K et al (2019) Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 20:216–228. https://doi.org/10.1016/S1470-2045(18)30747-2
Doolittle ND, Korfel A, Lubow MA et al (2013) Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 81:84–92. https://doi.org/10.1212/WNL.0B013E318297EEBA
Correa DD, Braun E, Kryza-Lacombe M et al (2019) Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol 144:553–562. https://doi.org/10.1007/s11060-019-03257-1
Scordo M, Wang TP, Ahn KW et al (2021) Outcomes associated with thiotepa-based conditioning in patients with primary central nervous system lymphoma after autologous hematopoietic cell transplant. JAMA Oncol 7:993–1003. https://doi.org/10.1001/JAMAONCOL.2021.1074
Alnahhas I, Jawish M, Alsawas M et al (2019) Autologous stem-cell transplantation for primary central nervous system lymphoma: systematic review and meta-analysis. Clin Lymphoma Myeloma Leuk 19:e129–e141. https://doi.org/10.1016/J.CLML.2018.11.018
Soussain C, Choquet S, Fourme E et al (2012) Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica 97:1751–1756. https://doi.org/10.3324/haematol.2011.060434
Houillier C, Taillandier L, Dureau S et al (2019) Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol 37:823–833. https://doi.org/10.1200/JCO.18.00306
de Faber S, Mutsaers P, van den Bent M, van der Meulen M (2021) Subacute neurological deficits and respiratory insufficiency due to intrathecal methotrexate. J Clin Transl Res. https://doi.org/10.18053/jctres.07.202106.013
Kasenda B, Ferreri AJM, Marturano E et al (2015) First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)—a systematic review and individual patient data meta-analysis. Ann Oncol 26:1305–1313. https://doi.org/10.1093/ANNONC/MDV076
Fritsch K, Kasenda B, Schorb E et al (2017) High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 31:846–852. https://doi.org/10.1038/LEU.2016.334
Abrey LE, Batchelor TT, Ferreri AJM et al (2005) Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 23:5034–5043. https://doi.org/10.1200/JCO.2005.13.524
van der Meulen M, Postma AA, Smits M et al (2021) Extent of radiological response does not reflect survival in primary central nervous system lymphoma. Neurooncol Adv 3:vda007. https://doi.org/10.1093/noajnl/vdab007
Van Der Meulen M, Dinmohamed AG, Visser O et al (2017) Improved survival in primary central nervous system lymphoma up to age 70 only: a population-based study on incidence, primary treatment and survival in the Netherlands, 1989–2015. Leukemia 31:1822–1825. https://doi.org/10.1038/leu.2017.128
Ferreri AJM, Blay JY, Reni M et al (2003) Prognostic scoring system for primary CNS lymphomas: the international extranodal lymphoma study group experience. J Clin Oncol 21:266–272. https://doi.org/10.1200/JCO.2003.09.139
van der Meulen M, Dirven L, Bakunina K et al (2021) MMSE is an independent prognostic factor for survival in primary central nervous system lymphoma. J Neurooncol 152:357–362. https://doi.org/10.1007/s11060-021-03708-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Koning, M.E., Hof, J.J., Jansen, C. et al. Primary central nervous system lymphoma. J Neurol 271, 2906–2913 (2024). https://doi.org/10.1007/s00415-023-12143-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12143-w