Abstract
Objective
We aimed to examine the longitudinal change of plasma neurofilament light chain (NFL) level and explore its diagnostic and prognostic implications in Parkinson’s disease (PD).
Methods
A total of 184 patients with early PD who completed 5-year annually repeated clinical assessments were included. Plasma NFL at baseline, 1 year, and 2 year were examined, which were quantified using the ultrasensitive Simoa technology. At baseline, blood from 86 sex- and age-matched healthy controls (HC) were obtained for comparison.
Results
Plasma NFL in PD patients at baseline was significantly higher than those in HC (P = 0.046), and significantly increased after 2 years (P = 0.046). Receiver operating characteristic curve indicated that a plasma NFL cut-off value of 10.79 pg/mL resulted in 39.7% sensitivity and 84.0% specificity, with an area under the curve of 0.635, to distinguish PD from HC (P < 0.001). Linear mixed-effect models indicated that baseline plasma NFL (> 9.24 pg/mL) correlated with a greater increase in the Unified Parkinson’s Disease Rating Scale III (estimate = 0.651, P = 0.001) and Hoehn & Yahr stage (estimate = 0.072, P < 0.001), and also correlated with a greater decrease in the Montreal Cognitive Assessment (estimate = − 0.387, P < 0.001) during follow-up visits.
Conclusions
Plasma NFL exhibits a tendency to increase with disease progression, and elevated baseline plasma NFL can serve as a predictor for accelerated motor deterioration and cognitive decline in PD. However, plasma NFL does not have high accuracy to distinguish individuals with early-stage PD from HC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a chronic neurodegenerative disease mainly characterized by motor and non-motor symptoms [24]. Although dopamine replacement treatment provides symptomatic benefits for most of the motor symptoms of PD, the disease course is still progressive [15]. High heterogeneity of clinical presentation, prognosis, and life expectancy among PD [4, 7, 27] contributes to the difficulty in determining the diagnosis and prognosis of the disease. In the management of PD, reliable diagnostic biomarkers that can distinguish patients with PD from healthy individuals, and prognostic biomarkers that can predict individual disease progression and be potential as an objective measurement for therapeutic response are urgently needed.
A promising biomarker for PD is the neurofilament light chain (NFL), which provides a sensitive measurement of neuroaxonal damage [13]. Compared with healthy controls (HC), no strong evidence for a difference in cerebrospinal fluid (CSF) or plasma NFL levels has been found in patients with PD [22]. Currently, technological advancement has allowed sensitive quantification of NFL in the plasma, which could allow accessible monitoring [13]. Previous studies have found that plasma NFL was elevated in some chronic neurodegenerative disorders, such as amyotrophic lateral sclerosis [8], Alzheimer’s disease [26], and multiple system atrophy [30].
A few studies have measured the longitudinal change of NFL [5, 17] as well as evaluated its association with motor progression and cognitive decline in PD [5, 14, 20, 28, 29]. Longitudinal comprehensive analyses of plasma NFL in a large well-designed cohort and validation of its effect on the progression of PD in an external cohort are needed. In the present study, we recruited a cohort of early PD patients who underwent a 3–5 year follow-up to determine the longitudinal changes of plasma NFL level, as well as to explore its diagnostic and prognostic implications in PD.
Methods
Ethics statement
This study was approved by the Ethics Committee of Sichuan University West China Hospital (NO: 2015-36), and written informed consent was obtained from all patients.
Study design
In the ongoing prospective longitudinal cohort study executed at the Department of Neurology, Sichuan University West China Hospital, a tertiary referral center in China, we aimed to investigate the biomarkers of diagnosis, prognosis, and progression of PD in the Chinese population. The present study included 184 PD patients with a disease duration < 5 years at baseline and 86 age- and sex-matched HC, which were recruited from February 2015 to November 2020. The last visits were scheduled for August 2023. All PD patients were diagnosed based on the Movement Disorders Society clinical diagnostic criteria for PD [23]. Patients with motor fluctuations and dyskinesia or those with Hoehn and Yahr (H&Y) stage ≥ 3 at baseline were excluded.
Evaluation protocol
Baseline demographic and clinical data including sex, age, age of PD onset, disease duration, and years of education were recorded. The therapeutic regimen at each visit was recorded. The levodopa-equivalent daily dose (LEDD) was calculated based on a previous report [25].
All recruited patients underwent a repeated series of neurological examinations and regular assessments at baseline and during follow-up. The severity of motor symptoms was evaluated using the Unified PD Rating Scale (UPDRS) Part III [18] and H&Y stage [12] at OFF-state after instructing patients to stop the anti-parkinsonian drugs for at least 12 h. The Hamilton Depression Rating Scale (HAMD) containing 24 items [16] was used to evaluate depression, while the Hamilton Anxiety Rating Scale (HAMA) [10] was used to evaluate anxiety. The Beijing version of the Montreal Cognitive Assessment (MoCA) screening tool was used to assess global cognitive function [19].
Measurement of NFL
All HC (n = 86) and patients with PD at baseline (n = 184), 1 year (n = 184), and 2 year (n = 124) were collected blood to examine the NFL levels. EDTA plasma NFL was quantified using an ultra-sensitive Simoa technology (Quanterix, MA, US) on the automated Simoa HD-X platform (GBIO, Hangzhou, China) per the manufacturer’s instruction. Plasma samples were diluted at a 1:4 ratio. Calibrators, quality controls, and all samples were measured in duplicate. The mean coefficient of variation (CVs) of duplicate measurement for concentration was 4.45% for NFL. Few samples with intra-assay CVs larger than 20% were re-measured. Assays were performed using kits with the same lot number. Operators were unaware of participants’ disease status and clinical information.
Statistical analyses
All statistical analyses were performed using the SPSS software (version 22.0). The statistical tests were two tailed, and P-value < 0.05 was considered statistically significant. Continuous variables were presented as means ± standard deviations (SD) and categorical variables as counts (percentages). The Student’s t-test was used for intergroup comparisons of clinical variables.
A longitudinal linear mixed analysis model was used to explore the association between plasma NFL and clinical variables (UPDRS III and MoCA) at each time. This model was used to explore the correlation between repeated measurements obtained in the same patient, which included patients with consecutive evaluations over the follow-up period. Repeated UPDRS III or MoCA score was set as the dependent variable (continuous variable) in the model. The primary fixed effect associated factors were repeated plasma NFL and follow-up time in years. The fixed covariables included the following repeated measures: sex, age, disease duration, and education.
An additional longitudinal linear mixed analysis model was further used to explore the prognostic effect of baseline plasma NFL on clinical outcomes (change in UPDRS III, H&Y stage, and MoCA). The repeated UPDRS III, H&Y stage, or MoCA score was set as the dependent variable (continuous variable) in the model. The primary fixed effect associated factors were dichotomous plasma NFL at baseline (> 9.24 pg/mL vs. ≤ 9.24 pg/mL, classified by the median value of NFL at baseline), follow-up time in years, and their interactions. The fixed covariables included the following repeated measures: sex, age at enrollment, disease duration at baseline, UPDRS III score at baseline, MoCA at baseline, and education.
Results
Baseline and follow-up data
In the present study, a total of 184 PD patients at baseline and 86 HC were included. No significant differences were observed in terms of mean age and sex between PD patients at baseline and HC (Supplementary Table 1). At baseline, the mean disease duration in patients with PD was 2.0 ± 1.2 years. The LEDD at baseline was 237.4 ± 235.1 mg/day (Table 1). All PD patients finished the 1-year visit, 177 patients finished the 2-year visit, 155 patients finished the 3-year visit, 110 patients finished the 4-year visit, and 61 patients finished the 5-year visit.
Cut-off value of NFL between PD and HC
Receiver operating characteristic curve (ROC) analysis showed that a plasma NFL cut-off value of 10.79 pg/mL resulted in 39.7% sensitivity and 84.0% specificity, with an area under the curve (AUC) of 0.635, to distinguish PD patients at baseline and HC. A plasma NFL cut-off value of 9.06 pg/mL resulted in 58.2% sensitivity and 71.0% specificity, with an AUC of 0.676, to distinguish PD patients at the 1-year visit and HC. In addition, a plasma NFL cut-off value of 10.87 pg/mL resulted in 45.6% sensitivity and 84.0% specificity, with an AUC of 0.665, to distinguish PD patients at the 2-year visit and HC (Fig. 1A).
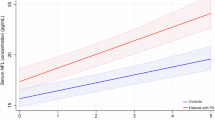
The diagnostic, prognostic implication and evolution of plasma NFL levels in PD. A Receiver operating characteristic curve analyses to differentiate PD and HC. B Comparison of plasma NFL levels between PD and HC as well as their evolutions in PD. C, D, E Linear mixed-effects models for the relationship between baseline plasma NFL levels and the motor and cognitive progression in PD. The interaction of higher baseline plasma NFL (> 9.24 pg/ml) × follow-up time in year was associated with a higher increase in the UPDRS III (estimate = 0.651, 95%CI = 0.264–1.039, P = 0.001) (C) and H&Y stage (estimate = 0.072, 95%CI = 0.042–0.103, P < 0.001) (D) with sex, baseline age, baseline disease duration, baseline plasma NFL, baseline UPDRS III score, and follow-up time in year as covariates, as well as associated with a higher decrease in the MoCA (estimate = − 0.387, 95%CI = − 0.544–0.231, P < 0.001) (E) with sex, education, baseline age, baseline disease duration, baseline plasma. NFL, baseline UPDRS III score, baseline MoCA score, and follow-up time in years as covariates
Longitudinal evolution of NFL
Plasma NFL of patients with PD at baseline was significantly higher than those of HC (P = 0.005) (Fig. 1B). The mean plasma NFL levels of PD increased from 10.42 ± 0.42 pg/mL at baseline to 11.19 ± 0.45 pg/mL after 1 year (P = 0.231) and 12.01 ± 0.72 pg/mL after 2 years (P = 0.046).
Association between NFL and UPDRS III or MoCA at each time
The linear mixed-effect model indicated that elevated plasma NFL was significantly associated with a higher UPDRS III score at each time (estimate = 0.278, 95%CI = 0.146–0.410, P < 0.001) after adjusting sex, age, disease duration, and follow-up time in years, but had no relationship with a lower MoCA score at each time (estimate = − 0.027, 95%CI = − 0.079–0.024, P = 0.296) after adjusting sex, age, disease duration, years of education, and follow-up time in years (Table 2).
Difference in the progression of PD between lower and higher NFL groups during follow-up
The linear mixed-effect models indicated that the interaction of higher baseline NFL levels (> 9.24 pg/mL) × follow-up time in year were associated with a greater increase in the UPDRS III (estimate = 0.651, 95%CI = 0.264–1.039, P = 0.001) (Fig. 1C) and H&Y stage (estimate = 0.072, 95%CI = 0.042–0.103, P < 0.001) (Fig. 1D) with sex, baseline age, baseline disease duration, baseline NFL, baseline UPDRS III score, and follow-up time in years as covariates, as well as associated with a greater decrease in the MoCA (estimate = − 0.387, 95%CI = − 0.544–0.231, P < 0.001) (Fig. 1E) with sex, education, baseline age, baseline disease duration, baseline NFL, baseline UPDRS III score, baseline MoCA score, and follow-up time in years as covariates.
Discussion
We reported the longitudinal evolution of plasma NFL levels and explored the diagnostic and prognostic implications of plasma NFL in a large sample of early Chinese PD cohort. We found that (1) plasma NFL is not a reliable biomarker for differentiating between PD patients and HC; (2) there is a tendency for an increase in plasma NFL as PD progresses; and (3) high plasma NFL is associated with faster progression in terms of both motor and cognitive impairments in PD after accounting for potential confounders. These findings indicated that plasma NFL may serve as a reliable biomarker of the severity and progression of PD. A plasma NFL measurement may help neurologists in identifying patients with PD at risk of rapid disease progression and may have the potential for recruiting target individuals and monitoring responses to therapy for future clinical trials.
Higher plasma NFL levels were observed in our patients with PD compared to HC, which is consistent with a previous report [17]. In the mixed British and Swedish cohorts, plasma NFL was not markedly elevated in patients with PD in general [11]. Numerous diffusion MRI studies have described white matter damage or reduced fractional anisotropy in axonal fiber connections in patients with PD compared to age- and sex-matched HC [6]. Thus, plasma NFL was considered to be used as an indicator for reflecting the white matter axonal damage in the brains in PD.
Although a previous study [20] reported that plasma NFL demonstrated excellent diagnostic accuracy (AUC = 0.833) in differentiating PD from HC, the current study revealed relatively weaker diagnostic accuracy for plasma NFL (all AUC less than 0.70). Age-specific reference values are crucial for accurately distinguishing PD from HC, as studies have demonstrated a correlation between CSF or plasma NFL and age [21]. Therefore, the lack of such reference values could compromise diagnostic accuracy. Our findings support that plasma NFL is not a reliable biomarker for differentiating between PD patients and HC.
In the present study, we observed a slight elevation in plasma NFL with the progression of PD (from 10.42 ± 0.42 pg/mL at baseline to 12.01 ± 0.72 pg/mL after a 2-year follow-up period). Our study supported that the pathological changes in PD at the early stage are characterized by mild alterations [9]. In the PPMI cohort, plasma NFL was also found to increase significantly over a 60-month monitoring period [17]. However, age could potentially exert a substantial influence on the long-term development of the NFL. Therefore, our finding that NFL demonstrates a tendency to increase with PD progression, which would require further investigation after an extended period for confirmation. Although the relatively small amplitude of plasma NFL was along with the duration at the early stage of PD, the association between plasma NFL and motor severity over time detected in PD patients in the present study demonstrated a close relationship between plasma NFL and the pathology of the disease. Our results also showed that baseline plasma NFL levels above a specific cut-off value (> 9.24 pg/mL) were associated with a higher risk for motor deterioration during 3–5-year follow-up visits. Our study was consistent with a few previous studies which found that plasma NFL correlated with disease severity and motor progression in PD [11, 14, 17, 20, 28]. Thus, plasma NFL measurements can be a candidate biomarker for identifying targeted patients to participate in future disease-modifying treatment clinical trials.
Another important finding of our study was that elevated baseline plasma NFL in early-stage PD patients can predict cognitive impairment with the disease progression. These findings further highlight the significance of plasma NFL as a biomarker for PD cognition decline, particularly at its initial stages. Our findings were consistent with three previous studies [1, 14, 20], but did not support a de novo PD cohort study [28]. In a previous postmortem pathology study, NFL protein was detected in senile plaque components in the hippocampus of patients with PD who had dementia but not in patients with PD who had normal cognition [2]. Studies with PD transgenic A53T-α-synuclein mouse model also showed that CSF and plasma NFL concentrations were 1000-fold higher in PD mice than in non-transgenic littermate control mice [3]. Notably, in that study, elevated CSF and plasma NFL levels were positively related to the number and size of neuronal α-synuclein inclusions. These findings corroborated the direct association between disease-pathognomonic α-synuclein protein deposition and fluid NFL in PD pathogenesis [3].
Following are the limitations of the study: (1) The study did not track the dynamics of NFL in healthy individuals. (2) The relatively short observation of disease progression in some patients is insufficient for determining the long-term effect of NFL on PD. (3) The lack of detailed cognitive testing reduced sensitivity in detecting any potential influence of plasma NFL on changes in cognitive function over time.
Conclusions
In conclusion, our study provides evidence indicating that plasma NFL exhibits a tendency to increase with disease progression, and elevated baseline plasma NFL at the early stage of PD can serve as a predictor for accelerated motor deterioration and cognitive decline in PD. However, plasma NFL does not possess sufficient accuracy to effectively differentiate individuals with early-stage PD from HC.
Data availability
The data for the present study are not available due to the data security policy in China.
References
Aamodt WW, Waligorska T, Shen J, Tropea TF, Siderowf A, Weintraub D, Grossman M, Irwin D, Wolk DA, Xie SX, Trojanowski JQ, Shaw LM, Chen-Plotkin AS (2021) Neurofilament light chain as a biomarker for cognitive decline in parkinson disease. Mov Disord 36:2945–2950
Arai H, Schmidt ML, Lee VM, Hurtig HI, Greenberg BD, Adler CH, Trojanowski JQ (1992) Epitope analysis of senile plaque components in the hippocampus of patients with Parkinson’s disease. Neurology 42:1315–1322
Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, Staufenbiel M, Neumann M, Maetzler W, Kuhle J, Jucker M (2016) Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91:56–66
Backstrom D, Granasen G, Domellof ME, Linder J, Jakobson Mo S, Riklund K, Zetterberg H, Blennow K, Forsgren L (2018) Early predictors of mortality in parkinsonism and Parkinson disease: a population-based study. Neurology 91:e2045–e2056
Backstrom DC, Eriksson Domellof M, Linder J, Olsson B, Ohrfelt A, Trupp M, Zetterberg H, Blennow K, Forsgren L (2015) Cerebrospinal fluid patterns and the risk of future dementia in early, incident parkinson disease. JAMA Neurol 72:1175–1182
Cochrane CJ, Ebmeier KP (2013) Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology 80:857–864
Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB (2017) Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain 140:1959–1976
Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, Toffanin E, Cagnin A, Briani C, Pegoraro E, Soraru G (2017) Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA Neurol 74:525–532
Halliday G, Hely M, Reid W, Morris J (2008) The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol 115:409–415
Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32:50–55
Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K, Fs SB (2017) Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88:930–937
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14:577–589
Lin CH, Li CH, Yang KC, Lin FJ, Wu CC, Chieh JJ, Chiu MJ (2019) Blood NfL: a biomarker for disease severity and progression in Parkinson disease. Neurology 93:e1104–e1111
Macleod AD, Taylor KS, Counsell CE (2014) Mortality in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29:1615–1622
Moberg PJ, Lazarus LW, Mesholam RI, Bilker W, Chuy IL, Neyman I, Markvart V (2001) Comparison of the standard and structured interview guide for the Hamilton depression rating scale in depressed geriatric inpatients. Am J Geriatr Psychiatry 9:35–40
Mollenhauer B, Dakna M, Kruse N, Galasko D, Foroud T, Zetterberg H, Schade S, Gera RG, Wang W, Gao F, Frasier M, Chahine LM, Coffey CS, Singleton AB, Simuni T, Weintraub D, Seibyl J, Toga AW, Tanner CM, Kieburtz K, Marek K, Siderowf A, Cedarbaum JM, Hutten SJ, Trenkwalder C, Graham D (2020) Validation of Serum neurofilament light chain as a biomarker of Parkinson’s disease progression. Mov Disord 35:1999–2008
Movement Disorder Society Task Force on Rating Scales for Parkinson’s D (2003) The Unified Parkinson’s disease rating scale (UPDRS): status and recommendations. Mov Disord 18:738–750
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699
Ng ASL, Tan YJ, Yong ACW, Saffari SE, Lu Z, Ng EY, Ng SYE, Chia NSY, Choi X, Heng D, Neo S, Xu Z, Keong NCH, Tay KY, Au WL, Tan LCS, Tan EK (2020) Utility of plasma Neurofilament light as a diagnostic and prognostic biomarker of the postural instability gait disorder motor subtype in early Parkinson’s disease. Mol Neurodegener 15:33
Oosterveld LP, Verberk IMW, Majbour NK, El-Agnaf OM, Weinstein HC, Berendse HW, Teunissen CE, van de Berg WDJ (2020) CSF or serum neurofilament light added to alpha-Synuclein panel discriminates Parkinson’s from controls. Mov Disord 35:288–295
Parnetti L, Gaetani L, Eusebi P, Paciotti S, Hansson O, El-Agnaf O, Mollenhauer B, Blennow K, Calabresi P (2019) CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol 18:573–586
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601
Schapira AHV, Chaudhuri KR, Jenner P (2017) Erratum: Non-motor features of Parkinson disease. Nat Rev Neurosci 18:509–509
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Weston PSJ, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, Druyeh R, Malone IB, Ahsan RL, Pemberton H, Klimova J, Mead S, Blennow K, Rossor MN, Schott JM, Zetterberg H, Fox NC (2017) Serum neurofilament light in familial Alzheimer disease: a marker of early neurodegeneration. Neurology 89:2167–2175
Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, Barker RA (2013) The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry 84:1258–1264
Ye R, Locascio JJ, Goodheart AE, Quan M, Zhang B, Gomperts SN (2021) Serum NFL levels predict progression of motor impairment and reduction in putamen dopamine transporter binding ratios in de novo Parkinson’s disease: an 8-year longitudinal study. Parkinsonism Relat Disord 85:11–16
Ygland Rodstrom E, Mattsson-Carlgren N, Janelidze S, Hansson O, Puschmann A (2022) Serum neurofilament light chain as a marker of progression in Parkinson’s disease: long-term observation and implications of clinical subtypes. J Parkinsons Dis 12:571–584
Zhang L, Cao B, Hou Y, Gu X, Wei Q, Ou R, Zhao B, Luo C, Shang H (2022) Neurofilament light chain predicts disease severity and progression in multiple system atrophy. Mov Disord 37:421–426
Acknowledgements
The authors thank all subjects for their participation in the study.
Funding
The present study was supported by the funding of the National Science Fund of China (Grant No. 82271272), the National Key Research and Development Program of China (Grant No. 2021YFC2501200), and the Sichuan Science and Technology Program (Grant No. 2022ZDZX0023).
Author information
Authors and Affiliations
Contributions
ORW: 1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: Design; 3) Manuscript: Writing of the draft. ORW, LKC, LJY, YTM, XY, WQQ, HYB, LCY, ZLY, JZ, ZB, CXP, SW, and WY: Patients enrollment and follow up. SHF: 1) Research project: Conception; 2) Statistical Analysis: Review and Critique; 3) Manuscript: Revision of the draft. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ou, R., Liu, K., Lin, J. et al. Relationship between plasma NFL and disease progression in Parkinson’s disease: a prospective cohort study. J Neurol 271, 1837–1843 (2024). https://doi.org/10.1007/s00415-023-12117-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12117-y