Abstract
Background
Persistent neuropsychiatric symptoms following acute COVID-19 infection are frequently reported. These include anxiety, depression, difficulty concentrating, fatigue, and insomnia. The longitudinal evolution of this neuropsychiatric burden is poorly understood and clinical guidelines concerning treatment are lacking.
Objective
We sought to describe the longitudinal evolution of neuropsychiatric symptoms in the post-acute sequelae of COVID-19 (PASC) syndrome and examine symptom treatment at a single center.
Methods
Consecutive participants experiencing persistent neurologic symptoms after acute COVID-19 infection were recruited from October 2020 to July 2022. Data collected included COVID-19 infection history, neurological exam and review of systems, Montreal Cognitive Assessment (MoCA), and self-reported surveys concerning neuropsychiatric symptoms and treatment. Data were collected at baseline and at 1-year follow-up.
Results
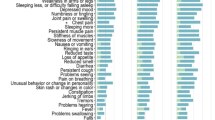
A total of 106 participants (mean age 48.6, females 67%) were included in the study. At 1-year follow-up, 72.5% of participants reported at least one neuropsychiatric symptom. Over half (52.5%) of participants reported persistent fatigue. At baseline, 38.8% of all participants had met the established MoCA cut-off score of < 26 for mild cognitive impairment; this decreased to 20.0% at 1 year. COVID-19 infection severity was associated with neuro-PASC symptoms (including fatigue and anxiety) at 1 year. Overall, 29% of participants started at least one new medication for COVID-19-associated neuropsychiatric symptoms. Of the participants who started new medications, fatigue was the most common indication (44.8%) followed by insomnia (27.6%).
Conclusions
Neuropsychiatric symptoms related to neuro-PASC improve over time but can persist for over a year post-recovery. Most treatment modalities targeted neuro-PASC fatigue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The COVID-19 pandemic has greatly impacted human health, with consequences expected over the next several decades. In addition to acute pulmonary and systemic symptoms from COVID-19 infection and mental health problems arising from quarantine and isolation, one area of significant concern is the increasingly recognized post-acute sequelae of COVID-19 (PASC) syndrome, which may be characterized by long-term pulmonary, cardiac, psychiatric, and/or neurological symptoms [1].
There is increasing evidence that a significant proportion of COVID-19 survivors experience persistent neuropsychiatric symptoms long after recovery from their acute infection [2,3,4]. A meta-analysis of 31 studies (n = 5153 participants) found that following COVID-19 infection, the pooled prevalence for depression was 45%, the pooled prevalence of anxiety was 47%, and the pooled prevalence of sleep disturbances was 34% [2]. A study of over 200,000 patients found that a third (33.62%) of COVID-19 patients had been diagnosed with neurological or psychiatric disorders, including anxiety, depression, post-traumatic stress disorder (PTSD), and psychosis, during the 6-month period following a COVID-19 infection [3]. In a psychiatric screening of 408 adult patients surveyed 1-month after hospitalization for COVID-19 infection, 56% scored in the pathological range in at least one clinical dimension for PTSD, depression, anxiety, obsessive–compulsive symptoms, and insomnia [4]. In addition to the prevalence of various neuropsychiatric symptoms following COVID-19 infection, potential predictors of long COVID include age, body mass index, and female sex [5].
Despite the high prevalence of neuropsychiatric symptoms in PASC syndrome, there is still much to understand regarding of the longitudinal evolution of these symptoms and the clinical management of these symptoms. We have previously described neurological symptoms and selected phenotypes from a prospective cohort of neuro-PASC patients at 6-month follow-up [6]. In this current study, we sought to evaluate the longitudinal pattern of neuropsychiatric burden in PASC patients at 1-year follow-up and examine current treatment patterns for patients with PASC symptoms.
Methods
Study design
This study leveraged a parent prospective cohort study of neurological complications of COVID-19 at the University of California San Diego [6]. Participants completed self-reported, previously validated surveys and interviews at several time points: baseline (i.e., after resolution of their acute COVID-19 infection), 6-month, and 1-year follow-up. The 6-month and 1-year time points were measured from the initial baseline visit. This study was approved by the UCSD Institutional Review Board, and participants provided written informed consent.
Participants
Consecutive patients meeting clinical diagnostic criteria for acute COVID-19 infection and reporting neurological or neuropsychiatric symptom post-infection were recruited from October 2020 to July 2022. Recruitment was conducted through the UCSD Neurology Clinic, Infectious Disease Clinic, Neuropsychology Clinic, and direct referrals to UCSD subspecialty clinics. Almost all participants had PCR confirmed infections, though a few were included from very early in the pandemic before testing was widely available. Participants were excluded from this study if they (1) did not have record of either a positive COVID-19 test result or clinically confirmed COVID-19 infection or (2) could not provide informed consent. Participants were divided into two cohorts. Cohort 1 consisted of participants who had been diagnosed with a neurological disease prior to COVID-19 infection. Cohort 2 consisted of participants who had never been diagnosed with a neurological disease.
Demographics and medical history
We collected general demographic and clinical data regarding our participants including gender, age, tobacco use, height, weight, BMI, employment status, and past medical history. Participants were queried on their medical history and medications including determination of prior neurological conditions such as Guillain–Barre syndrome, multiple sclerosis (MS), Alzheimer’s disease, migraine headaches, autoimmune encephalitis, narcolepsy, and Parkinson’s disease.
Instruments
We applied validated questionnaires to capture neuropsychiatric symptoms at baseline, 6 months, and 1 year. The PROMIS-29 form assessed seven health domains including pain interference, physical function, fatigue, sleep disturbance, depression, anxiety, and ability to participate in social roles in activities [7]. Domains were evaluated using four items per category and a 0–10 numeric rating for each item. The Impact of Events Scale—Revised (IES-R) measured participants’ subjective response to a traumatic event (COVID-19 infection) using three subscales: intrusion, avoidance, and hyperarousal [8]. This validated survey was used to assess participants’ level of distress following their COVID-19 infection with a maximum total score of 88. Score interpretation included— between 24 and 32: PTSD is a clinical concern; above 33: best cut-off for a probable diagnosis of PTSD [9, 10].
Modified Fatigue Impact Scale (MFIS) measured the impact of fatigue on physical, cognitive, and psychosocial functioning with a maximum total score of 84 [11]. The Epworth Sleepiness Scale (ESS) is a self-reported questionnaire used to assess daytime sleepiness with a maximum score of 24 [12]. The Montreal Cognitive Assessment (MoCA) is a screening test administered by healthcare providers to measure cognitive abilities including short-term memory, attention, language, and executive function; the MoCA has a maximum score of 30 points with a score of < 26 generally considered to be the threshold for mild cognitive impairment [13]. The MoCA score was adjusted for education level with one point added to the total score for individuals with 12 years or less of education. The Neurological Review of Systems (ROS) was adapted from standard clinical ROS templates used to assess the presence and severity of various neurological symptoms. In this study, only 1-year outcomes were reported as the 6-month results were previously published [6]. Cumulative baseline data (including participants from our previous study examining 6-month outcomes and all individuals who enrolled since) were reported for reference.
Data collection procedures
Data were collected through a secure REDCap electronic capture system. The PROMIS-29, IES-R, MFIS, and ESS questionnaires were sent electronically to participants via REDCap. The neurological ROS and MoCA were conducted during one-on-one visits with participants. To comprehensively analyze treatment received for COVID-19-related symptoms, chart review was conducted in addition to collecting self-reported medications from participants. Chart review included extraction of new medications started for neuropsychiatric symptoms that were considered related to an individual’s COVID-19 infection by his or her treating physician. Medications that individuals were taking prior to their COVID-19 infection for a previously diagnosed or unrelated neuropsychiatric condition were not included in analyses.
Statistical analysis
Descriptive analyses of frequencies, means, and standard deviation were reported as appropriate. Paired t-tests were used to compare differences in reported symptoms at baseline and at 1-year follow-up. Welch independent sample t tests were used to compare cohort 1 and cohort 2 at baseline and at 1-year follow-up. All statistical analyses were performed with R software version 4.2.1 (R Foundation for Statistical Computing), and p values < 0.05 were considered significant. To assess the relationship between MoCA scores and self-reported neurological symptoms at baseline, point-biserial correlations were employed using MoCA score as a continuous variable and the neurological ROS metrics of encephalopathy (altered mental status), memory difficulties, and trouble concentrating as dichotomous variables. We used a binary (“Yes” or “No”) classification for neurological ROS score and then used a point-biserial correlation to examine the relationship between each ROS score and each MOCA score for each patient across cohorts.
The association between initial COVID-19 infection severity (mild, moderate, severe) and clinical outcomes of interest utilized multivariable linear regression adjusting for age, sex, and time from acute infection. Pairwise differences between degrees of COVID-19 infection severity were compared using one-way ANOVA adjusting for multiple comparisons using the Tukey method.
Results
Participants
A total of 106 participants were included in the analysis, with 30 participants in cohort 1 (known prior neurologic condition) and 76 participants in cohort 2 (no known prior neurologic condition). Table 1 summarizes the demographic and clinical characteristics. The mean age at time of infection was 48.6 years (SD 12.6) and 67.0% of the participants were female. All participants reported experiencing acute COVID-19 infection symptoms; no participants had asymptomatic PCR testing. Of those recruited, 40 individuals completed their 1-year follow-up neurological ROS. Response rate varied for self-reported surveys (Tables 2, 3).
COVID-19 infection severity
Participants self-reported the severity of their acute COVID-19 infection based on the symptom severity and duration. Infection severity was reported as follows—asymptomatic: 0%, mild: 34.7%, moderate: 39.6%, and severe: 25.7%. The median duration of acute COVID-19 infection was 14 days (IQR 11). Overall, 12.5% of participants reported hospitalization for their acute COVID-19 infection.
Neurological review of systems and MoCA
Responses to the neurological ROS demonstrated a persistence of neuropsychiatric symptoms evaluated 1 year from baseline (Tables 2, 3). At baseline, fatigue was the most common symptom (69.9%). At 1 year, there was slight improvement in overall persistence and prevalence of individual symptoms but 72.5% of individuals still reported at least one neuropsychiatric symptom. Fatigue remained the most prevalent symptom (52.5%).
At baseline, 38.8% of all participants met the established MoCA cut-off score of < 26 for mild cognitive impairment. This percentage decreased to 20.0% at 1 year, and the mean MoCA score increased from 25.9 (± 3.1) to 27.6 (± 2.6) during this same time period. The point-biserial correlations for MoCA score and encephalopathy (altered mental status) was − 0.31 (p = 0.0058); for memory difficulties − 0.21 (p = 0.064); and for trouble concentrating − 0.13 (p = 0.24).
Validated instrument responses
The overall pooled percentage of participants meeting the best cut-off for a probable diagnosis of PTSD per the IES-R decreased from 21.9 to 12.5% from baseline to 1-year follow-up (Tables 2, 3). In an analysis of intra-participant changes from baseline to 1 year, we found a mean decrease in IES-R score of − 2.71 (p = 0.14, 95% CI [− 6.40, 0.97]).
The average total MFIS score for all participants decreased from 39.4 at baseline to 27.9 at 1-year (Tables 2, 3). Additionally, using the Flachenecker et al. recommended cut-off score of 38 to distinguish fatigued individuals from non-fatigued individuals, we observed a decrease in percentage of participants meeting this cut-off from 53.8 to 37.8% from baseline to 1 year [14]. When analyzing intra-participant changes, we filtered for patients who have completed both time points (baseline and 1 year) across both cohorts (n = 27). The mean MFIS score decreased by − 4.45 points (p = 0.12, 95% CI [− 10.17, 1.20]).
We compared scores from cohort 1 and cohort 2 to assess potential differences between participants with and without prior neurologic diseases. At baseline, there were no significant differences between the two cohorts in any of the neuropsychiatric PROMIS-29 metrics: anxiety, depression, fatigue, sleep disturbance, and ability to participate in social roles and activities (Table 2). In contrast, at 1-year, we found that cohort 2 (participants without prior neurological disease) reported a significantly greater ability to participate in social roles and activities (cohort 1: 14.3 ± 4.9, cohort 2: 18.2 ± 3.4, p = 0.033; Table 3). Additionally, at the 1-year time point, we observed a trend towards a lower severity of depression and anxiety symptoms in cohort 2 compared to cohort 1, although these results did not reach nominal statistical significance (cohort 1: 7.8 ± 3.9, cohort 2: 5.4 ± 2.5, p = 0.083 and cohort 1: 8.8 ± 4.1, cohort 2: 6.3 ± 3.0, p = 0.092, respectively).
Correlation between COVID-19 severity and PASC symptoms
In an adjusted multivariable regression model, COVID-19 infection severity (mild, moderate, or severe) was not associated with baseline MoCA or 1-year MoCA score (Table 4). On ANOVA analysis, there were no pairwise differences between the degrees of COVID-19 infection severity and baseline MoCA scores, but there was a pairwise difference between severe and moderate infections (− 3.54, 95% CI − 6.91, − 0.17). COVID-19 severity was associated with 1-year PROMIS scores for physical function, anxiety, depression, fatigue, sleep disturbance, and ability to participate in social roles and activities after adjusting for age, sex, and time from acute infection (Table 4). On ANOVA analysis, there were significant pairwise differences between severe vs. mild infections and severe vs. moderate infections for physical function; severe vs. mild infection and severe vs. moderate infection for physical function; all pairwise comparisons for fatigue; severe vs. mild infection for sleep disturbance, and all pairwise comparisons for ability to participate (Fig. 1).
Treatment
Out of 106 total study participants, 100 were evaluated for PASC treatment exposures with six excluded due to lack of electronic medical chart access. We found that out of these 100 participants, 29 individuals (29%) started treatment (medications, supplements, therapy, etc.) for neuropsychiatric symptoms attributable to neuro-PASC (Table 5). Of the 29 individuals who started new treatment, 12 were trialed on more than one medication for a single indication. Indications for starting new medications included anxiety, depression, trouble concentrating, insomnia, PTSD, migraine, neuropathy, mood disorder, and myoclonus. Out of the participants who started new medications, fatigue was the most common indication (44.8%) followed by insomnia (27.6%). The most common medication used for fatigue was amantadine (53.8% of prescriptions for fatigue). Overall, there was a large spectrum of medications and supplements used to treat the conditions listed. Generally, there was not a consistent pattern or drug-of-choice for neuro-PASC symptoms.
Discussion
Our study demonstrates that while neuro-PASC symptoms overall improve after 1-year post-infection, the majority of participants report persistent symptoms, the most common being fatigue, memory difficulties, trouble concentrating, and insomnia. At 1 year, approximately half of the participants reported experiencing fatigue; additionally, memory difficulties remained in approximately half of the participants at 1 year. We found that participants without prior neurological disease reported a significantly greater ability to participate in social roles and activities at 1 year, despite no difference at baseline. MoCA score was found to be correlated with self-reported ROS symptoms, specifically encephalopathy (altered mental status). Additionally, acute COVID-19 infection severity was associated with 1-year PROMIS scores for physical function, anxiety, fatigue, sleep disturbance, and ability to participate in social roles and activities. In an analysis of neuro-PASC treatment, we found that roughly a third of participants started at least one new medication for COVID-19-associated neuropsychiatric symptoms. A wide variety of medications were used and most of these were started for fatigue.
Although we observed both a decrease in the average total MFIS and a decrease in percentage of participants meeting the instrument-specific cut-off for fatigue from baseline to 1-year, fatigue was the still most commonly experienced symptom at 1 year, with over half of participants (52.5%) reporting persistent fatigue. When analyzing intra-participant changes, we found that there was clinically relevant improvement in MFIS score (− 4.45 points) at 1 year, but the result did not reach nominal statistical significance. Thus, although there is a promising trend towards improvement in the severity of fatigue over the year, the prevalence of fatigue remains high and is an important target for future research and treatment. In our analysis of treatments targeting fatigue, amantadine was the most prescribed medication for fatigue in our patients. Amantadine is a commonly used medication for MS-related fatigue and relatively accessible without controlled substance restrictions. The mechanism underlying fatigue in long COVID is still being evaluated, but it has been hypothesized to be related to an aberrant immune response to the virus and may have similarities to autoimmune CNS diseases [15]. Specific causal mechanisms proposed for MS-related fatigue include proinflammatory cytokines (including interferon-γ and TNF-α), alterations in the hypothalamic–pituitary–adrenal axis, loss of axons, and differential patterns of brain activation (including cingulate gyri and left primary sensory cortex) [16]. Alternatively, the fatigue may also be driven or exacerbated by increased respiratory effort from chronic pulmonary complications [17]. The prevalence of fatigue in PASC favors its selection as a primary symptom target for clinical trials. Two different studies seeking to treat Post-Viral or Post-COVID-19 Fatigue Syndrome are taking different approaches, one using Ruconest, a recombinant C1 esterase inhibitor indicated for the treatment of acute angioedema attacks, and the other using low-dose naltrexone (LDN) [18, 19]. Ruconest is a strong inhibitor of the complement system, kallikrein–kinin system, and the contact activation system, and is hypothesized to potentially correct immune dysfunction resulting from COVID-19 infection [20]. LDN has been demonstrated to exhibit immunomodulatory activity involving inhibition of B- and T-lymphocytes, antagonism of opioid growth factor receptor, and modulation of antigen-presenting cells such as microglia and macrophages; use of LDN has been shown to have some benefits in symptom control in conditions including multiple sclerosis and chronic fatigue syndrome [21, 22].
The approximate 50% decrease in the percentage of total participants meeting the best cut-off for a probable diagnosis of PTSD at 1 year compared to baseline illustrates that while acute COVID-19 infection and onset of PASC were initially viewed as a traumatic event, the impact of the events improve over time. However, the finding that a proportion of patients may still be categorized as meeting the best cut-off for a probable diagnosis of PTSD over a year after infection underscores the critical and lasting impact that COVID-19. For comparison, it has been estimated that approximately 20–30% of ICU survivors experience clinically relevant PTSD symptoms during their first year post-discharge [23]. This underscores the importance of evaluating the psychosocial impacts of COVID-19 infection in patients even long after their recovery from the acute infection. While there was an improvement in overall MoCA score and decrease in the percentage of participants meeting the score cut-off for mild cognitive impairment at the 1-year period, 20% of total participants still are characterized as mildly cognitively impaired at 1 year. Although MoCA score is only a screening tool, these findings illustrate the persistence of cognitive deficits in neuro-PASC. This finding is supported by anecdotal and self-reported statements by the participants. During follow-up study visits, many of these individuals reported an inability or difficulty to work at their old job, complete common activities of daily living (e.g., remember appointments and grocery lists and focus long enough to complete basic tasks), and interact with friends and family, which all have severe impacts on quality of life. These difficulties are likely multi-factorial, stemming from potential neurocognitive changes due to PTSD symptoms, other mood changes, and impacts from fatigue.
In addition to recognizing persistent long-term symptoms experienced in neuro-PASC, we found a wide spectrum of medications and supplements prescribed by physicians to treat neuropsychiatric symptoms in neuro-PASC patients. This highlights the need for evaluation of more standardized treatment guidelines for neuro-PASC. Additionally, approximately 40% patients of those who started new medication were tried on multiple medications for a single indication, suggesting that the current approach is more trial and error. Currently, treatment for neuro-PASC is focused on targeting individual symptoms as they arise, rather than treating the potential underlying mechanism or cause of neuro-PASC, which is still under investigation. Although we are only providing a descriptive look at medications used and not examining the efficacy of various treatments due to observational design and small sample size, we have highlighted current practice and this descriptive data may help inform clinicians treating similar patients and contribute to the ongoing larger effort to create efficacious treatment guidelines for neuro-PASC.
Emerging data identify immunological dysregulation and prolonged inflammation as underlying causes of many long-COVID symptoms, including neuro-PASC.[24, 25]. Current efforts in treating neuro-PASC include immunomodulatory therapies. For example, methylprednisolone and intravenous immunoglobulin (IVIG) are being explored as treatment for symptoms of long COVID with ongoing neurologic symptoms such as dizziness, trouble walking, or problems with strength [26]. Additionally, the efficacy and safety of temelimab, a recombinant humanized IgG4 monoclonal antibody that blocks the HERV-W protein, is currently being trialed as a treatment for PASC neuropsychiatric symptoms [27]. HERV activity has been hypothesized to influence PASC development; in addition to general activation in inflammation, in bronchial alveolar lavage samples of COVID-19 patients, upregulated expression of numerous HERV families were found [28, 29]. The HERV-W protein has also been correlated with the pathology of autoimmune disorders including type 1 diabetes and multiple sclerosis [30]. Specifically, MS-associated retrovirus (MSRV) and ERVWE1 are two members of the HERV-W family that have been identified as potential MS co-factors [31]. Activation of HERV-W/MSRV by Epstein–Barr virus (EBV) has been hypothesized as a mechanism underlying the association between EBV infections and autoimmune diseases such as MS [31, 32]. Results from the CHANGE-MS phase 2b trial of temelimab has demonstrated radiological indicators of potential anti-neurodegenerative effects in MS [33]. Other clinical trials for neuro-PASC include the use of vortioxetine, an antidepressant with established pro-cognitive properties, to potentially treat cognitive deficits developing during and/or after COVID-19 infection [34]. Vortioxetine is a receptor antagonist at the 5-HT receptor subtypes 3, 7, and 1D, a receptor agonist and partial agonist at the 5-HT 1A and 1B subtypes, respectively, and a serotonin transporter (SERT) inhibitor [35].
Limitations of this study include the modest sample size. During the peak of the pandemic, providers made strong efforts to advise and protect patients, including those with neurologic conditions that rendered them high risk for poor infection outcomes. In part because of these efforts and the cautiousness of high-risk patients, there was a lower rate of COVID-19 infections among chronic neurological disease patients compared to the overall population [36]. This may have contributed to the small sample size of cohort 1 individuals with prior neurological conditions. Although conclusions regarding differences in intra-group and intra-participant changes are limited by modest sample size, descriptive patterns and symptom evolution over time are important findings that may inform future studies. Furthermore, we acknowledge that there may be response bias such that participants who return for their 1-year follow-up may be the ones more likely to still be experiencing significant symptoms or conversely those with increased disability may not have been able to attend later visits. Additionally, there may be a potential recruitment bias, since participants were recruited at subspecialty referral centers where they are more likely to experience symptoms and seek medical care.
Strengths of this study include the longitudinal design and length of follow-up time, the extensive testing metrics and validated surveys used, and the capture of specific treatment modalities used to treat neuro-PASC. The longitudinal design allowed the demonstration of prolonged persistence of neuropsychiatric symptoms in PASC. Our findings are consistent with those of Rass et al. who examined neurological outcomes including fatigue, concentration difficulties, forgetfulness, hyposmia, headache, limb weakness, and myalgia [37]. They found that 1 year after COVID-19 infection, 59% of participants reported at least one neurological symptom and objective neurological examination revealed abnormalities in 64% of individuals.[37]. Additionally, our study includes a reference group of individuals who had pre-existing neurologic conditions prior to COVID-19 infection. This reference group allows us to observe whether there appears to be a difference in recovery trajectory between individuals with previous neurologic conditions and those without a prior neurological disease as well as compare the neuropsychiatric effects of COVID-19 to those with chronic neurologic illness. Furthermore, we have identified specific treatments strategies employed by our physicians to treat COVID-19-related neuropsychiatric symptoms.
Overall, our data highlight the persistent and severe nature of neuropsychiatric and cognitive symptoms after COVID-19 infection. These symptoms should be regarded as outcomes evaluated in future clinical trials targeting neuro-PASC. Multi-center studies with larger data sets may help with causal inference for the correlation between disease exposure and objective measures of cognition. In addition to strengthening this correlation, targeted treatments for neuro-PASC should continue to be trialed to provide meaningful improvements to patients’ symptoms and quality of life.
Conclusion
In conclusion, the high prevalence and severity of neuropsychiatric symptoms in PASC are an issue in need of treatment guidelines. Our data highlight that the treatment of neuro-PASC is symptomatic and highly variable. The underlying etiology of neuro-PASC is emerging as complex and will likely require a multi-modal treatment protocol, involving different targets to ameliorate symptoms and treat the underlying pathophysiology. Establishing effective therapies to treat PASC will not only improve quality of life for those directly impacted but will also have important implications for understanding the pathophysiology of neuro-PASC and future post-infectious neuroinflammatory disorders.
Data availability
Anonymized data and datasets not published within this article will be made available by request from any qualified investigator.
References
Levine RL (2022) Addressing the long-term effects of COVID-19. JAMA 328:823–824. https://doi.org/10.1001/jama.2022.14089
Deng J, Zhou F, Hou W et al (2021) The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci 1486:90–111. https://doi.org/10.1111/nyas.14506
Taquet M, Geddes JR, Husain M et al (2021) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 8:416–427. https://doi.org/10.1016/S2215-0366(21)00084-5
Mazza MG, De Lorenzo R, Conte C et al (2020) Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 89:594–600. https://doi.org/10.1016/j.bbi.2020.07.037
Sudre CH, Murray B, Varsavsky T et al (2021) Attributes and predictors of long COVID. Nat Med 27:626–631. https://doi.org/10.1038/s41591-021-01292-y
Shanley JE, Valenciano AF, Timmons G et al (2022) Longitudinal evaluation of neurologic-post acute sequelae SARS-CoV-2 infection symptoms. Ann Clin Transl Neurol 9:995–1010. https://doi.org/10.1002/acn3.51578
Hays RD, Spritzer KL, Schalet BD, Cella D (2018) PROMIS(®)-29 v2.0 profile physical and mental health summary scores. Qual Life Res Int J Qual Life Asp Treat Care Rehabil 27:1885–1891. https://doi.org/10.1007/s11136-018-1842-3
Weiss DS (2007) The Impact of event scale: revised. In: Wilson JP, Tang CS (eds) Cross-cultural assessment of psychological trauma and PTSD. Springer US, Boston, pp 219–238
Asukai N, Kato H, Kawamura N et al (2002) Reliability and validity of the Japanese-language version of the impact of event scale-revised (IES-R-J): four studies of different traumatic events. J Nerv Ment Dis 190:175–182. https://doi.org/10.1097/00005053-200203000-00006
Creamer M, Bell R, Failla S (2003) Psychometric properties of the impact of event scale-revised. Behav Res Ther 41:1489–1496. https://doi.org/10.1016/j.brat.2003.07.010
Larson RD (2013) Psychometric properties of the modified fatigue impact scale. Int J MS Care 15:15–20. https://doi.org/10.7224/1537-2073.2012-019
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540–545. https://doi.org/10.1093/sleep/14.6.540
Nasreddine ZS, Phillips NA, Bédirian V et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Flachenecker P, Kümpfel T, Kallmann B et al (2002) Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler Houndmills Basingstoke Engl 8:523–526. https://doi.org/10.1191/1352458502ms839oa
Sin DD (2023) Is long COVID an autoimmune disease? Eur Respir J 61:2202272. https://doi.org/10.1183/13993003.02272-2022
Braley TJ, Chervin RD (2010) Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep 33:1061–1067. https://doi.org/10.1093/sleep/33.8.1061
Azzolino D, Cesari M (2022) Fatigue in the COVID-19 pandemic. Lancet Healthy Longev 3:e128–e129. https://doi.org/10.1016/S2666-7568(22)00029-0
Melamed I (2021) Study to Evaluate the Benefit of RUCONEST in Improving Neurological Symptoms in Post COVID-19 Infection. In: Clin. Identifier NCT04705831. https://clinicaltrials.gov/ct2/show/NCT04705831?term=neurologic&type=Intr&cond=COVID-19&draw=2&rank=7. Accessed 28 Sept 2022
Nacul L (2022) Low-dose Naltrexone for Post-COVID Fatigue Syndrome. In: Clin. Identifier NCT05430152. https://clinicaltrials.gov/ct2/show/NCT05430152. Accessed 28 Sept 2022
Urwyler P, Moser S, Charitos P et al (2020) Treatment of COVID-19 with conestat alfa, a regulator of the complement, contact activation and Kallikrein-Kinin System. Front Immunol 11:2072. https://doi.org/10.3389/fimmu.2020.02072
Li Z, You Y, Griffin N et al (2018) Low-dose naltrexone (LDN): a promising treatment in immune-related diseases and cancer therapy. Int Immunopharmacol 61:178–184. https://doi.org/10.1016/j.intimp.2018.05.020
O’Kelly B, Vidal L, McHugh T et al (2022) Safety and efficacy of low dose naltrexone in a long covid cohort; an interventional pre-post study. Brain Behav Immun Health 24:100485. https://doi.org/10.1016/j.bbih.2022.100485
Jackson JC, Lassen-Greene C, Jutte JE, Stepanovic K (2020) PTSD after critical illness: current issues and future directions. In: Preiser J-C, Herridge M, Azoulay E (eds) Post-intensive care syndrome. Springer International Publishing, Cham, pp 177–188
Ortona E, Malorni W (2022) Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur Respir J. https://doi.org/10.1183/13993003.02245-2021
Castanares-Zapatero D, Chalon P, Kohn L et al (2022) Pathophysiology and mechanism of long COVID: a comprehensive review. Ann Med 54:1473–1487. https://doi.org/10.1080/07853890.2022.2076901
Nath A (2022) Immunotherapy for Neurological Post-Acute Sequelae of SARS-CoV-2. In: Clin. Identifier NCT05350774. https://clinicaltrials.gov/ct2/show/NCT05350774. Accessed 28 Sept 2022
Leppert D (2022) Temelimab as a Disease Modifying Therapy in PatientsWith Neuropsychiatric Symptoms in Post-COVID 19 or PASC Syndrome. In: Clin. Identifier NCT05497089. https://clinicaltrials.gov/ct2/show/NCT05497089?term=neurologic&type=Intr&cond=COVID-19&draw=3&rank=13. Accessed 28 Sept 2022
Kitsou K, Kotanidou A, Paraskevis D et al (2021) Upregulation of human endogenous retroviruses in bronchoalveolar lavage fluid of COVID-19 patients. Microbiol Spectr 9:e0126021. https://doi.org/10.1128/Spectrum.01260-21
Proal AD, VanElzakker MB (2021) Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 12:698169. https://doi.org/10.3389/fmicb.2021.698169
Curtin F, Bernard C, Levet S et al (2018) A new therapeutic approach for type 1 diabetes: rationale for GNbAC1, an anti-HERV-W-Env monoclonal antibody. Diabetes Obes Metab 20:2075–2084. https://doi.org/10.1111/dom.13357
Dolei A (2018) The aliens inside us: HERV-W endogenous retroviruses and multiple sclerosis. Mult Scler Houndmills Basingstoke Engl 24:42–47. https://doi.org/10.1177/1352458517737370
Mameli G, Poddighe L, Mei A et al (2012) Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS ONE 7:e44991. https://doi.org/10.1371/journal.pone.0044991
Hartung H-P, Derfuss T, Cree BA et al (2022) Efficacy and safety of temelimab in multiple sclerosis: results of a randomized phase 2b and extension study. Mult Scler Houndmills Basingstoke Engl 28:429–440. https://doi.org/10.1177/13524585211024997
McIntyre RS (2022) Vortioxetine for Post-COVID-19 Condition. In: Clin. Identifier NCT05047952. https://clinicaltrials.gov/ct2/show/NCT05047952. Accessed 28 Sep 2022
Sanchez C, Asin KE, Artigas F (2015) Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43–57. https://doi.org/10.1016/j.pharmthera.2014.07.001
Moreno-Torres I, Meca Lallana V, Costa-Frossard L et al (2021) Risk and outcomes of COVID-19 in patients with multiple sclerosis. Eur J Neurol 28:3712–3721. https://doi.org/10.1111/ene.14990
Rass V, Beer R, Schiefecker AJ et al (2022) Neurological outcomes 1 year after COVID-19 diagnosis: a prospective longitudinal cohort study. Eur J Neurol 29:1685–1696. https://doi.org/10.1111/ene.15307
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by EL, JY, LP, and JA. The first draft of the manuscript was written by EL and all authors commented on previous versions of the manuscript. AG was responsible for project concept co-development and referrals. RE contributed to referrals and general collaboration. JG is the PI of this project and is responsible for the project conception, study design, data collection, analysis plan, and drafting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Emilie Liu, Jennifer Yang, Lucas Patel, Jasmine Arora, Amanda Gooding, and Ronald Ellis report no disclosures relevant to the manuscript. Unrelated to the current work, Jennifer Graves has received grant or clinical trial funding from the NMSS, UCSD, Octave, Biogen, EMD-Serono and ABM. She serves on a steering committee for a clinical trial with Novartis and served on advisory boards for TG therapeutics, Genentech and Bayer.
Ethics statement
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Written informed consent was obtained from every participant.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, E.N., Yang, J.H., Patel, L. et al. Longitudinal analysis and treatment of neuropsychiatric symptoms in post-acute sequelae of COVID-19. J Neurol 270, 4661–4672 (2023). https://doi.org/10.1007/s00415-023-11885-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11885-x