Abstract
Cladribine tablets (Mavenclad®) were approved by the European Union in 2017 as high-efficacy therapy for highly active relapsing–remitting multiple sclerosis. In Israel, Mavenclad® was approved in 2018. Real-life experience has confirmed the efficacy of cladribine tablets over at least 4 years from the initial course. During the last years, several questions were raised concerning the management of people with MS who show disease activity during years 3 and 4 post-cladribine initiation and what treatment decisions are needed beyond year 4. A few expert boards have tried to provide insight based on research data and to suggest recommendations on the therapeutic dilemmas and treatment decisions with cladribine. However, there is currently no widely accepted consensus about these issues. The vast clinical experience gained in Israel in the past 5 years in several MS centers across the country allows for a broad perspective of the outcomes with long-term cladribine use. This article summarizes previously published recent recommendations and describes the insights of Israeli neurology key opinion leaders that convened for an advisory board meeting on January 29th, 2023, with the aim of reaching a consensus regarding cladribine long-term treatment and follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cladribine is an oral, deoxyadenosine analogue prodrug, with 2-chlorodeoxyadenosine triphosphate (2-Cd-ATP) serving as the active metabolite [1]. It specifically depletes B and T lymphocytes via DNA synthesis and repair inhibition, thus triggering apoptosis, along with minor influence on the innate immune system [1, 2]. Additionally, Cladribine decreases the levels of proinflammatory cytokines, chemokines, adhesion molecules, and migration of mononuclear cells into the cerebrospinal fluid (CSF) [1].
Cladribine tablets (Mavenclad®) were approved by the European Union in 2017 as high-efficacy therapy (HET) for highly active relapsing–remitting multiple sclerosis (RRMS). In Israel, Mavenclad® was approved in 2018, for the treatment of adult patients with highly active relapsing multiple sclerosis (RRMS) as defined by clinical or imaging features [at least two relapses in the year prior to treatment, or increase in neurological disability as assessed by the Expanded Disability Status Scale (EDSS), or one relapse and evidence of new brain MRI activity].
FDA approval of Mavenclad® took effect in 2019. Mavenclad is administered as a 2-dose/2-year protocol (3.5 mg/kg cumulative dose over 2 years). This short dosing regimen, along with a low need for monitoring, and high subject compliance, makes it an appealing therapeutic option for patients with MS (pwMS) [2, 3].
The pivotal Cladribine Tablets Treating Multiple Sclerosis Orally (CLARITY) study investigated the effect of treating pwMS with Cladribine versus matching placebo on relapse rate after 96 weeks [4] on 1326 subjects, and was published in 2010. The results demonstrated that Cladribine reduced the annual relapse rate (ARR) by 86%, the risk of sustained disability progression by 33%, and the number of gadolinium-enhancing (Gd+) lesions by 86% [2]. Four years after first dose, 91.1% of subjects were free of disability progression [3]. These findings were further supported by the prospective observational long-term safety registry (PREMIERE) [1] that collected long-term follow-up data of the patients who were previously enrolled in the CLARITY, CLARITY EXTENSION, ONWARD, and ORACLE clinical trials. This study revealed that 66% of 941 subjects did not receive any other DMT over 4.5 years after the last cladribine dose [1].
No specific serious adverse events (SAEs) or unexpected safety signals were observed [1].
Cladribine has a good safety and tolerability profile, with the most frequent adverse event (AE) (2.2%) being lymphopenia [3]. It typically occurs about 2–3 months after commencement of treatment, followed by a gradual recovery (immune reconstitution) [5]. Integrated safety analyses from the Cladribine clinical program showed no increased rates of malignancies or infections in subjects receiving additional courses of Cladribine during clinical studies, on top of the indicated dose [1].
The CLARITY-Extension study [6], investigated long-term safety, tolerability, and clinical benefits following two additional courses of Cladribine (years 3 and 4) vs. placebo in pwMS who completed the CLARITY trial. Patients with or without evidence of disease activity (DA) were included. Treatment with Cladribine tablets over 2 years, followed by 2 years of placebo, produced a durable clinical response. Administration of an additional two cycles of Cladribine in years 3 and 4 did not improve clinical or neuroimaging outcomes compared to placebo, in the whole group of patients. ARR and proportion of relapse-free subjects were broadly similar across treatment groups [6]. No significant differences in time to first qualifying relapse (relative to first dose) were seen between treatment groups. No Evidence Of Disease Activity-3 (NEDA-3) was observed in about 30% of subjects in both groups during years 3 and 4 [6], and 70% of subjects in both groups did not reach 3- or 6-month confirmed disability progression by the fifth year. AE incidence was comparable among groups, except for Lymphopenia Grade ≥ 3, which occurred at a higher incidence in the Cladribine treatment group than in the placebo group (yet eventually recovered to grade 0–1 in more than 90% of all subjects) [6].
Magalashvili et al. [2] retrospectively described the clinical outcomes and NEDA rates in 128 highly active RRMS patients, treated with Cladribine at years 3 and 4. The ARR decreased to 0.36 in the third year, and further decreased to 0.17 in the fourth year of Cladribine treatment. The percentage of relapse-free subjects in the third and fourth years was 68.9% and 82.9%, respectively. Mean EDSS at the third year was 3.1 ± 2.07; 83.6% of the patients remained neurologically stable (54.1%) or improved (29.5%). In the fourth year, EDSS was 3.2 ± 1.91, and 85.7% of the pwMS remained stable (57.1%) or improved (28.6%). NEDA-2 was reached for 59% of the patients in the third year, and 74.3% in the fourth year. Hence, Cladribine proved to be clinically effective in the third and fourth year of treatment in the majority of highly active RRMS patients [2].
Several expert opinion articles were published on the long-term use of Cladribine therapy for MS. Meca et al. [3] published practical recommendations regarding cladribine-candidate subject profiles, treatment replacement strategies, evaluation of response to treatment, and safety assessments. The experts recommended providing additional treatment with cladribine at years 3 and/or 4 in the event of relapse following completion on the indicated dose; thus, emphasizing the importance of ongoing monitoring of disease activity. Before to switching from Cladribine to another DMT [3], a baseline MRI taken up to 3 months prior to the change should be performed, along with the elimination of any possible infection. Absolute lymphocyte count (ALC) should be normalized, to minimize immunosuppression risk. Cladribine’s half-life is 24 h; therefore, pharmacokinetic interactions are not expected the week following the last dose of the drug. Switching to a new DMT is recommended 3–6 months after the last dose [3].
Oreja et al. [5] published an expert opinion on the long-term use of Cladribine tablets for MS subjects, based on a systematic literature review of real-world evidence (RWE). Oreja et al. reported the rates of disease activity (DA) at the end of years 2, 3, and 4 range from 12 to 18.7% [5]. In this article, six questions were raised, pertaining to Cladribine subject management. These questions were also discussed in the Israeli advisory board with the following recommendations: when managing a subject who completed the 2-year course of cladribine, yet experienced DA within years 3 or 4, and the ALC is > 800/mL, consider treating with a third course of cladribine. If a subject experiences equal/higher DA during year 3 or 4 compared to the baseline DA prior Cladribine treatment, consider switching to another DMT [5]. The CLARITY extension study evaluated the percentage of this group of subjects, experiencing this nature of DA during the last years of treatment, to be about 3% [3, 6]. When the patient is stable yet experiences DA in the fifth year of treatment or beyond, two options are valid: extension of the treatment-free period (there is no consensus regarding this approach; or continuation of treatment with Cladribine (Cladribine’s label has no contraindication for additional courses, nor a maximum number of courses) [3].
For the management of a pwMS who has completed the indicated two courses of Cladribine and remains stable with no evidence of DA in year 5 or later, Oreja et al. [5] recommended no further treatment with cladribine; rather, frequent MRI testing, patient-reported outcomes’ (PROs) examination, and biomarker assessments instead. Some experts supported continuation Cladribine treatment and this scenario was most controversial.
Habek et al. [7] published a position statement regarding Cladribine tablet therapy beyond 4 years. In this publication, eight MS experts used the Delphi method to establish a treatment algorithm for cladribine-treated subjects. Dependent on the status of DA, treatment options include an extension of the treatment-free period, retreatment with oral Cladribine or switching to a different HET DMT. Further cycles of Cladribine tablets should be considered in pwMS with minimal (no relapses, one-to-two new lesions) or moderate (one relapse, three-to-four new lesions) DA, whereas significant DA (> 1 relapse, > 3 new lesions) or progression justify switching to another HET [7]. Patterns of treatment response to Cladribine were also defined by Habek et al. [7], with “temporary responders” and “sustained responders” being likely to benefit from one to two additional Cladribine courses. The fourth treatment cycle could be delayed until the fifth year if there is a relapse. Mid-term responders should be treated with additional Cladribine courses or switched to another DMT.
Meuth et al. [1] published an expert opinion focusing on Cladribine treatment beyond 4 years.
Factors, such as time from last treatment, severity of DA compared to baseline, and radiological findings, all played a role in the decision whether to continue Cladribine treatment or not [1]. The DA inflammatory level being a major factor for deciding whether to switch to an alternative therapy or maintain Cladribine therapy. Surveillance should include clinical appointments with EDSS and cognitive assessments and PROs, every 3–6 months. Neurofilament light chain (NfL) levels are an optional parameter for surveillance as well [1].
Despite the above described insights from few groups, further (and more conclusive) recommendations concerning the treatment of pwMS beyond 4 years are substantially needed. The vast clinical experience gained in Israel in the past 5 years in several medical centers across the country using Cladribine therapy allows for a broad perspective of the outcomes with long-term Cladribine use. We describe here the insights and recommendations of Israeli neurology key opinion leaders (KOLs) that convened for an advisory board held on January 29th, 2023, with the aim of reaching a consensus regarding Cladribine tablet long-term treatment and follow-up.
Methods
Delphi method is a method used for creating consensus on a specific topic or question, by a group of experts. Answers are given anonymously, and participants are asked to answer same question in more than one round. After every round, there is a discussion with relevant data presented to the participants, and then, they are asked to answer again. In this way, the range of responses is reduced and participants can create a consensus.
The Israeli KOLs were invited by the Israel MS society, based on their expertise in the field and representing the major MS centers in Israel.
In preparation for the meeting, four recent expert opinion articles, pertaining to long-term Cladribine treatment (and described in the background of this article) [1, 3, 5, 7] were sent a-prior to the participants along with a questionnaire containing nine questions, previously asked by Habek et al. [7]. Voting on each question/recommendation was performed using the Delphi method—explain more? (minimizing bias by anonymous voting, facilitated discussion, group feedback, and statistical analysis of responses [7]). Voting on the questionnaire was performed twice: once at the beginning of the meeting (round one), and once at the end of the meeting (round two, after a presentation and a productive discussion). Each question was rated on a scale between one (1) and seven (7), while a consensus agreement was set at 75% or above of participants voting in agreement (scores between five and seven):
-
1.
Strongly Disagree
-
2.
Disagree
-
3.
Somewhat Disagree
-
4.
Neither Agree nor Disagree
-
5.
Somewhat Agree
-
6.
Agree
-
7.
Strongly Agree.
The following questions/statements were set to the votes during the advisory board [7]:
-
1.
It is of utmost importance to follow MS subjects who start treatment with Cladribine tablets at least annually. Minimum follow-up should include relapse, EDSS, brain MRI, and lymphocyte count assessment at 2 and 6 months after each cycle. If feasible, additional tests such as the symbol digit modality test, 9-Hole Peg Test (9-HPT), and Timed 25-Foot Walk (T25-FW) test, should be performed annually.
-
2.
In case of DA between the first and the second cycle of treatment with Cladribine tablets, it is recommended to continue with the second cycle unless significant DA or progression occurs, in which case switching to another HET with a different mode of action (MOA) should be considered. Factors, such as pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision. Furthermore, re-baselining of the MRI (3–6 months after the first cycle) should be performed when considering MRI activity.
-
3.
During years 2–4, for MS subjects who completed two cycles of Cladribine tablets, if there is no DA and progression, we recommend annual clinical and MRI follow-up, without additional treatment with Cladribine tablets or other DMTs.
-
4.
During years 2–4, if minimal DA is present, we recommend continuing annual follow-up or considering additional cycle(s) of Cladribine tablets.
-
5.
During years 2–4, in the case of significant DA or disease progression, continuing treatment with Cladribine tablets should be considered, or switching to another HET with a different MOA. Other factors, such as the number of new lesions on MRI, the severity of relapse, pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision.
-
6.
Beyond the fourth year, if there is no DA and progression, we recommend annual clinical and MRI follow-up, without additional treatment with Cladribine tablets or other DMTs. If feasible, additional tests, such as the symbol digit modality test, 9-HPT, and T25-FW test, should be performed annually.
-
7.
Beyond the fourth year, if there is minimal DA (defined as one-to-two new T2 lesions), we recommend continuing annual follow-up or considering additional cycle(s) of Cladribine tablets. Factors, such as pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision.
-
8.
Beyond the fourth year, if there is moderate DA (defined as one relapse or three-to-four new T2 lesions), we recommend administering additional cycle(s) of Cladribine tablets or considering a switch to another HET. Other factors, such as the site of lesions on MRI, the severity of relapse, pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision.
-
9.
Beyond the fourth year, if there is significant DA defined as one relapse or four new T2 lesions and/or disease progression, switching to another HET with a different MOA should be considered. Other factors, such as pregnancy planning, comorbidities, and previous DMTs, should be considered in the final decision.
During the advisory board, real-world data, expert opinion articles [1, 3, 5, 7], and results from the CLARITY EXTENSION trial [6] were presented. A discussion regarding each of the nine questions in the questionnaire ensued. The following topics were also discussed:
-
Which cases should be considered for continued treatment with Cladribine, and when it is appropriate to switch to another DMT?
-
What are the proper monitoring parameters for subjects receiving Cladribine?
-
How to define minimal/moderate/significant DA, which warrants further treatment cycles with Cladribine? Which clinical parameters should be monitored for treatment with Cladribine?
-
What is the proper definition of DA for subjects on Cladribine therapy?
-
Some clinical parameters are included in NEDA-3; however, some are not routinely tested while assessing DA (NfL as a biomarker, volumetry, and cognitive tests)—should they be included?
-
The importance of yearly brain and spinal cord MRI testing for assessing DA of Cladribine therapy.
The second round of voting was compared to the first baseline votes to determine if the extent of consensus changed as a result of the information exchange during the advisory board.
Results
This section summarizes the outcomes of the questionnaire presented before and during the advisory board meeting, along with additional discussion that was held during the meeting.
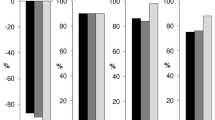
Figure 1 illustrates the results of the first voting round, at baseline, prior to meeting commencement. It is evident that even at baseline, a consensus was reached for each question, meaning that the Israeli experts are aligned with their outlook on Cladribine therapy.
The results of the first voting round. For each question (Y axis), a representation of the voting percentage is displayed. Purple represents mark (7), green represents mark (6), and blue represents mark (5). For each question, if the votes for marks 5–7 combined are > 75%, then a consensus is reached
Figure 2 illustrates the strength of agreement (power of consensus) for the first voting round. It is evident that at baseline, not only that a consensus was reached for all questions (all questions achieved voting of 75% and above for marks five to seven), but three out of nine questions also reached a mark of six and above (questions 3, 6, and 7), indicating a high level of agreement among participants at baseline.
Towards the end of the advisory board, a second round of voting took place, referring to the same questions as previously, with the aim of checking whether a higher degree of consensus was reached among participants. Figures 3 and 4 represent the results of the second voting accordingly.
The results of the second voting round. For each question (Y axis), a representation of the voting percentage is displayed. Purple represents mark (7), green represents mark (6), and blue represents mark (5). For each question, if the votes for marks 5–7 combined are > 75%, then a consensus is reached
When comparing Fig. 3 to Fig. 1, the results in both voting rounds were quite similar. The following remarkable differences were noted:
-
Question number 1, focusing on the importance of follow-up and monitoring of pwMS, received almost twofold voting for mark 7 in the second round (~ 82% vs 45%); indicating the acknowledgement of surveillance at the end of the advisory board.
-
Question number 4, focusing on additional Cladribine treatment during years 2–4 upon minimal DA, received a higher mark (six and above) by all participants in the second round, as opposed to the first round of voting- where 18% of participants voted for marks two to three.
-
Question number 5, focusing on additional Cladribine treatment during years 2–4 upon significant DA, received a higher mark (five and above) by all participants in the second round, as opposed to the first round of voting—where 18% of participants voted for marks two to three.
-
Question number 7, focusing on additional Cladribine treatment beyond the fourth year upon minimal DA, voting for marks six and seven increased by ~ 9% each in the second round of voting—indicating a higher agreement among participants.
Figure 4 illustrates the strength of agreement (power of consensus) for the second voting round. It is evident that the consensus from baseline improved at the end of the advisory board meeting. Not only did all questions reach consensus, but seven out of nine questions reached a mark of six and above (questions 1, 2, 3, 4, 6, 7, and 9), indicating an increased level of agreement among participants when the advisory board adjourned.
Discussion and conclusions
The Israeli advisory board meeting yielded meaningful insights regarding long-term cladribine therapy for pwMS beyond the second year of treatment.
A consensus was reached for all questions already at baseline (prior to the meeting commencement, during the first round of voting). This is a clear demonstration of agreement regarding the long-term treatment with Cladribine based on clinical experience and local medical community knowledge.
The advisory board assisted in promoting a higher consensus rate (“strength of agreement”) among the participants regarding the nine questions raised. The discussion concluded with the following recommendations:
Disease activity indicators
-
The following definitions of major criteria are indicative of DA, and the presence of at least one of the below would justify an additional treatment with cladribine beyond year 2 (supported by the findings of Meuth et al. [1]):
-
≥ 1 relapse
-
EDSS worsening by ≥ 1 EDSS point confirmed over 3 months in subjects with a baseline EDSS score ≤ 4.0, ≥ 0.5 EDSS points confirmed over 3 months in subjects with a baseline EDSS > 4.5
-
The emergence of ≥ 3 T2 lesions or ≥ 2 Gd and T1 lesions within 1 year from a reference MRI.
-
These disease activity variables indicate that for pwMS who achieved NEDA-3 at Year 2 and on, there is no need for additional treatment; these patients should continue clinical and radiological monitoring.
-
For pwMS that achieved only NEDA-2 criteria and/or experienced worsening symptomatology suggestive of mild-to-moderate disease activity such as increased fatigue, decreased cognition or 1–3 new T2 brain MRI lesions, at Year 2 and on, recommendation is made for one additional cladribine course, at the related timeframe.
-
For pwMS patients that experienced an acute severe relapse, disability worsening (increased EDSS > 1.5), or > 3 new T2 lesions, at Year 2 and on, recommendation is made to complete an additional full 2-year-2-dose cladribine course, at the related timeframe or to or consider a switch to another HET.
-
-
The following parameters are suggested as additional (minor) indicators of DA, to be considered:
-
Cognitive decline
-
The emergence of ≥ 2 T2 lesions or ≥ 1 Gd and T1 lesions within 1 year from a reference MRI
-
Significant progression in brain atrophy
-
Worsening of fatigue or decrease in a quality-of-life assessment.
-
Significant increase in serum neurofilament levels.
-
The advisors propose to follow up cognitive function using a cognitive battery that covers various aspects of cognition, and especially information processing speed and executive functions that are known to be impaired in MS. It is suggested to perform cognitive assessment before initiation of treatment and at yearly follow-ups. A battery consisting of the three tests of BICAM: (SDMT, CVLT, and BVMT) is widely accepted and used by most MS neurologists.
Considering evaluation of brain atrophy, the advisors commented that in a single patient (and at a single time point), WBV cannot be measured accurately. It was suggested to follow brain atrophy annually, and only if there is a consistent reduction of WBV, to consider this as a possible additional marker of disease progression.
Fatigue and quality of life should be assessed at annual follow-ups, using the accepted questionnaires (Short Form heath survey SF12, Modified Fatigue Impact Scale).
There was a post-meeting agreement between the members of the advisory board about recommending re-dosing with oral cladribine when at least one of the above major indicators of DA or two of the minor criteria are present. However, this recommendation is not based on hard data and represents only a suggestion for the development of future, evidence-based algorithms.
For DA beyond year 3 and 4, the advisors agreed that it is preferable to administer an additional full 2-year-2-dose cladribine course or to consider switching therapy (depending on the initial response to the first courses of cladribine).
Subject monitoring and long-term use of cladribine
-
Subject monitoring is essential for the decision of whether to switch therapy, treat with an additional course of Cladribine, or only monitor the disease state. Additionally, subject reaction to treatment must be taken into account.
-
Lymphocyte count decrease is not considered a marker for a response, but consistently low absolute counts of lymphocytes (< 500) should advocate against repeated treatment.
-
Subject response during the initial 2 year courses of treatment is important, where the default option is to continue Cladribine, rather than to switch therapy, when there was a satisfactory initial response.
-
Patients should continue treatment with Cladribine during the second year, completing the recommended dosing (including subjects with mild-to-moderate disease activity), unless a subject is a clear “non-responder” (i.e., fulfilling the above criteria for DA). Re-treatments with Cladribine during years 3 and 4 (or beyond fourth year) should also be considered when no other treatment alternatives are available or the subject received prior therapies without the option for further HET.
-
For subjects who experience new DA beyond 4 years, Cladribine re-dosing is indicated with a full treatment course or switching to another DMT. The decision will be based on the response to the initial two courses of Cladribine.
The participants expressed their concerns about the lack of safety data on long-term effects of repeated Cladribine courses.
To summarize, RWE addressing the long-term use of Cladribine is increasing, yet some outcomes are still inconclusive. Expert opinions and experience may provide guidance on questions that occur in clinical practice.
Additional clinical studies are required to explore if further Cladribine cycles are effective and well tolerated. Studies for the characterization of patient profiles who are likely to benefit from additional treatment are also warranted. Finally, it is crucial to identify subjects with a high risk of MS relapse to offer them further Cladribine treatment.
This Israeli advisory board on the use of Cladribine tablet therapy for pwMS beyond the second year of cladribine treatment has provided important insights. The participating panel of KOLs concluded that pwMS who responded well after 2 years of therapy should continue monitoring of disease activity. For pwMS that developed DA following the full 2-year-2-dose course, additional one or two treatment courses with cladribine are recommended, depending on the magnitude of disease activity at the related timeframe. The possibility of switching to another HET should also be considered as an alternative option (especially for those who did not show a satisfactory response to the initial scheme to cladribine).
In addition, a proactive approach for responding subjects beyond the fourth year is suggested. The “wait and see” (close monitoring only) is controversial as some disease parameters are not monitored, and therefore, the disease may not be detected.
The participants underlined the importance of the introduction of additional parameters for the detection of “silent” DA, such as the testing of cognitive functions, the computerized evaluation of the total T2 lesion load and brain and grey matter volumes in MRI, and the testing of serum neurofilaments (NFL) levels. When there is no DA, the decision for retreatment should be based on the subject’s personal characteristics and risk factors. The conclusions of this committee may contribute to the development of future long-term cladribine-treatment protocols.
Data availability
No data available.
References
Meuth SG, Bayas A, Kallmann B, Linker R, Rieckmann P, Wattjes MP, Mäurer M, Kleinschnitz C (2022) Long-term management of multiple sclerosis patients treated with cladribine tablets beyond year 4. Expert Opin Pharmacother 23:1503–1510. https://doi.org/10.1080/14656566.2022.2106783
Magalashvili D, Mandel M, Dreyer-Alster S, Didikin M, Harari G, Flechter S, Achiron A (2022) Cladribine treatment for highly active multiple sclerosis: real-world clinical outcomes for years 3 and 4. J Neuroimmunol 372:577966. https://doi.org/10.1016/j.jneuroim.2022.577966
Meca-Lallana V, García Domínguez JM, López Ruiz R, Martín-Martínez J, Arés Luque A, Hernández Pérez MA, Prieto González JM, Landete Pascual L, Sastre-Garriga J (2022) Expert-agreed practical recommendations on the use of cladribine. Neurol Ther 11:1475–1488. https://doi.org/10.1007/s40120-022-00394-0
Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, Vermersch P, Chang P, Hamlett A, Musch B et al (2010) A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 362:416–426. https://doi.org/10.1056/NEJMoa0902533
Oreja-Guevara C, Brownlee W, Celius EG, Centonze D, Giovannoni G, Hodgkinson S, Kleinschnitz C, Havrdova EK, Magyari M, Selchen D et al (2023) Expert opinion on the long-term use of cladribine tablets for multiple sclerosis: systematic literature review of real-world evidence. Mult Scler Relat Disord 69:104459. https://doi.org/10.1016/j.msard.2022.104459
Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, Dangond F, Adeniji AK, Vermersch P (2018) Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler 24:1594–1604. https://doi.org/10.1177/1352458517727603
Habek M, Drulovic J, Brecl Jakob G, Barbov I, Radulovic L, Rajda C, Rejdak K, Turčáni P (2023) Treatment with cladribine tablets beyond year 4: a position statement by southeast european multiple sclerosis centers. Neurol Ther 12:25–37. https://doi.org/10.1007/s40120-022-00422-z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical standard statement
None of the authors of this manuscript, participants of this committee, has any conflict of interest, for any possible contribution of the conclusions presented in this article, in the development of future protocols for the long term use of Cladribine.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Petrou, P., Achiron, A., Cohen, E.G. et al. Practical recommendations on treatment of multiple sclerosis with Cladribine: an Israeli Experts Group Viewpoint. J Neurol 270, 5188–5195 (2023). https://doi.org/10.1007/s00415-023-11846-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11846-4