Abstract
Objective
To assess the long-term effects of bilateral subthalamic nucleus deep brain stimulation (STN-DBS) on gait in a cohort of advanced Parkinson’s Disease (PD) patients.
Methods
This observational study included consecutive PD patients treated with bilateral STN-DBS. Different stimulation and drug treatment conditions were assessed: on-stimulation/off-medication, off-stimulation/off-medication, and on-stimulation/on-medication. Each patient performed the instrumented Timed Up and Go test (iTUG). The instrumental evaluation of walking ability was carried out with a wearable inertial sensor containing a three-dimensional (3D) accelerometer, gyroscope, and magnetometer. This device could provide 3D linear acceleration, angular velocity, and magnetic field vector. Disease motor severity was evaluated with the total score and subscores of the Unified Parkinson Disease Rating Scale part III.
Results
Twenty-five PD patients with a 5-years median follow-up after surgery (range 3–7) were included (18 men; mean disease duration at surgery 10.44 ± 4.62 years; mean age at surgery 58.40 ± 5.73 years). Both stimulation and medication reduced the total duration of the iTUG and most of its different phases, suggesting a long-term beneficial effect on gait after surgery. However, comparing the two treatments, dopaminergic therapy had a more marked effect in all test phases. STN-DBS alone reduced total iTUG duration, sit-to-stand, and second turn phases duration, while it had a lower effect on stand-to-sit, first turn, forward walking, and walking backward phases duration.
Conclusions
This study highlighted that in the long-term after surgery, STN-DBS may contribute to gait and postural control improvement when used together with dopamine replacement therapy, which still shows a substantial beneficial effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gait disturbances are one of the main manifestations of Parkinson’s disease (PD). They are characterised by slowness, difficulty in gait initiation, reduction or asymmetry of arm swing, gait festination, freezing of gait (FOG) [1], decrease of speed, stride length, and duration of the oscillation phase [2]. On the contrary, the cadence of steps may increase as a compensatory mechanism for the aforementioned alterations [2].
Bilateral subthalamic nucleus deep brain stimulation (STN-DBS) in advanced PD patients is an effective treatment in both short- and long-term follow-ups [3,4,5,6,7,8]. In the long term, STN-DBS allows a stable improvement of motor complications, tremor, and rigidity with a less relevant effect on axial symptoms (i.e. gait and balance symptoms, speech and swallowing troubles) and cognitive decline, which are the main causes of long-term impairment and disability [4, 9,10,11]. Current data about the effect of STN-DBS on gait are heterogeneous, with only a few studies objectively assessing gait alterations with an instrumental approach [12,13,14,15]. STN-DBS may improve postural instability and gait disturbances in the first year after surgery to the same extent as preoperative drug therapy [16]. However, other studies have reported that FOG may improve after surgery only if present in the med-off condition [11, 17]. Unfortunately, a deterioration in axial symptoms is reported from 5 to 8 years after surgery [18]. Data from long-term studies have shown that STN-DBS initially improves the Unified Parkinson Disease Rating Scale (UPDRS) compound subscores for postural and gait symptoms (PIGD subscore), albeit that the scores worsen over time and generally reach or exceed preoperative values 5 years after surgery [5, 18]. In addition, axial symptoms tend to worsen more rapidly than cardinal symptoms in the long term [5]. Therefore, walking and postural disorders seem to respond only initially to stimulation and then worsen in subsequent years, representing an important long-term issue for patients since it negatively affects their quality of life [4, 5, 10]. This study aimed to assess the long-term effects of bilateral STN-DBS on gait in a cohort of advanced PD patients through a standardised clinical-instrumental approach.
Methods
Participants
This longitudinal cohort study included consecutive patients treated with bilateral STN-DBS (electrodes: Medtronic 3389, Boston; M365DB220145DC0; IPG: Medtronic Activa PC, Boston Vercise Genus R16) from 2012 to 2017 at the Neurological Unit of the hospital Ospedale Civile di Baggiovara, Modena, Italy. All patients fulfilled the diagnosis of PD according to the UK Brain Bank criteria [19]. Exclusion criteria included: history of surgical complications related to STN-DBS that resulted in neurological deficits; history of ischaemic or haemorrhagic stroke, head trauma or other focussed brain injuries during the postoperative follow-up. The study was approved by the local ethics committee (protocol number: 2019/0056629), and written informed consent was obtained from participants according to the Declaration of Helsinki [20].
Clinical assessment
All subjects were assessed with a median 5-years follow-up after surgery (range 3–7 years). Neurological evaluation and instrumental analysis of gait were carried out on the same day and in the following conditions: on-stimulation/off-medication (at least 12-h washout of dopaminergic medications) [21]; off-stimulation/off-medication (stimulation was temporarily turned off for at least 1 h); on-stimulation/on-medication (stimulation was turned on and dopaminergic therapy was administered [early morning levodopa equivalent daily dose [LEDD] plus 30%]). In the on-stimulation/on-medication condition only, instrumental gait evaluation was also performed in the dual task condition (on-stimulation/on-medication dual task) in which the patient was asked to list as many possible consecutive words beginning with a specific letter while walking. Disease severity was assessed using the Hoehn and Yahr scale (H&Y) and UPDRS [22], while the risk of falls was assessed through the Hendrich Fall Risk Model II (HIIFRM) [23]. Akinesia, tremor, and PIGD subscores were calculated from the UPDRS. Patients were classified based on PD motor phenotype (tremor dominant [TD], indeterminate and PIGD) [24]. Furthermore, each patient was screened for the presence of mutations in the leucine-rich repeat kinase-2, glucocerebrosidase-1 (GBA1), α-synuclein and parkin genes [25]. The total amount of dopaminergic medications was calculated as LEDD milligrams [26]. Furthermore, STN-DBS was optimised in all patients within the 3 months prior to the assessment through a retest of all the contacts of the two electrodes choosing the most effective ones.
Instrumental analysis of gait
The instrumental evaluation of the overall walking ability was carried out with a wearable inertial sensor secured with an elastic belt at the sacrum level (S1). The commercial device G-WALK (BTS Bioengineering™, sampling frequency 100 Hz) was used. This device contains a three-dimensional (3D) accelerometer, gyroscope and magnetometer and measures the 3D linear acceleration, angular velocity, and magnetic field vector. Each patient performed the instrumented Timed Up and Go test (iTUG) [27] in the different stimulation and medication conditions reported above. In each condition, the patient was asked to perform the test three consecutive times, leading to a total of 12 acquisitions. All tests were performed barefoot under the supervision of an operator (without contact) to ensure patient safety. The execution of the entire series of tests and evaluations was documented through video recording. The iTUG test was performed using the standard test procedure, with a chair with armrests positioned 3 m away from a stool placed as a reference to turn around. Once the sensor was positioned, the patient was instructed to get up from the chair after the “go”, walk along a straight path of 3 m, turn 180°, return to the chair, rotate 180° again and sit down. The acquisition with the sensor was stopped once the subject had sat down, determining the end of the test. Patients were instructed to walk at spontaneous speed and without keeping their arms behind the back so as not to interfere with the sensor. In the on-stimulation/on-medication dual task condition, the patient was asked to list as many words as possible beginning with the same initial (F, A, S, different for each of the three acquisitions), indicated by the examiner just before the start of the iTUG test.
Data processing
Data from the inertial sensor were used to obtain the gait parameters described below. These were calculated through a Matlab script, purposely developed for the extraction of iTUG parameters by the Laboratory of Bioengineering and Neuromechanics of Movement of the University of Rome “Foro Italico”. The implemented methods are described in the literature introducing the instrumented analysis of the TUG test [28, 29], mainly referring to gait spatiotemporal parameters and dynamic stability of locomotion. The algorithms were adapted to the data format of the used sensors, equipped with a user interface, and transformed into executable software, usable only for research purposes, by our research group.
The analysis of the traces required the identification of specific events. Specifically, seven events were identified during the iTUG: start of the sit-to-stand phase, end of the sit-to-stand/start of the walk, end of the walk/start of the turn, end of the turn/start of the walk, end of the walk/start of the turn, end of the turn/start of the stand-to-sit, and end of the task. Based on the sequence of events, the following phases of the iTUG test were defined: sit-to-stand (Sit2St), forward walk, first turn, return walk, second turn, and stand-to-sit (St2Sit). The events were identified based on the trends of the accelerations and angular velocities, according to the indications of the literature [28] and with the support of the video. The rules set for identifying events based on inertial sensor data are reported in Supplementary Table 1. A preliminary automatic event identification was performed by the software. This was always visually checked by the operator and modified when necessary. Adjustments were necessary when analysing data from very compromised patients or patients presenting atypical movements, as in severe PD patients.
For each recorded test, in addition to data segmentation, the operator selected an initial time window where the participant kept the upright static posture. This was necessary to correct the sensor bias and to reorient its axes according to the anatomical antero-posterior (AP), medio-lateral (ML), and cranio-caudal (CC) axes.
Parameters
The following parameters were extracted: overall iTUG duration, duration of each phase, root mean square (RMS) amplitude of the trunk acceleration for each phase, and maximum trunk angular velocity, i.e. the speed of rotation, about the cranio-caudal axis. This is related to the subject’s ability to rotate quickly during the turn phases. Table 1 summarises the parameters obtained from the analysis of the inertial sensor data, with a brief explanation of their meaning. In addition, reference values for both the iTUG-derived parameters and the clinical scales used in this study were retrieved from the website rehabmeasures.org (S2) was consulted and are reported in Supplementary Table 2.
Statistical analysis
The primary outcome of the study was the changes in the sensor-based gait parameters in the different conditions tested. Since the sample size was limited and the variability within the sample was high compared to the effect due to the change in condition (see the results), the parameters have been normalised to the value in the on-stimulation/on-medication condition. This condition was chosen as a reference because it represents the daily condition of the patients. This has allowed quantifying, for each participant, the percentage variation of the different conditions compared to the on-stimulation/on-medication condition. Quade Nonparametric Analysis of Covariance was carried out for each normalised variable, with the condition as a factor and the years from surgery as a covariate. Pairwise comparisons were performed when appropriate. The analysis was conducted also using the patient’s age and the disease duration at the long-term evaluation as a covariate. This analysis was conducted to identify which parameter was most sensitive to the change in condition among those estimated. Secondary outcomes included the analysis of a possible correlation between clinical scales and sensor-based gait parameters, performed through Spearman correlation with corresponding Spearman rho values. Descriptive statistics were performed for each variable; continuous variables were expressed as mean (standard deviation) and median [range], while frequencies and percentages were calculated for categorical variables. The variables were tested for normal distribution using the Kolmogorov–Smirnov test of normality. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using the Jamovi software (version 2.2) and SPSS (IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp.).
Results
Patient population
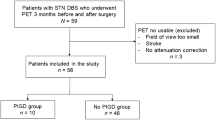
From 2012 to 2017, 40 PD patients underwent bilateral STN-DBS. Of these, 15 subjects were excluded from the analysis because of missing data (11 patients) or lack of consent to participate (4 patients). The remaining 25 PD patients were included (males: 18) and had a median follow-up of 5 years after surgery (range 3–7 years). Mean disease duration at surgery was 10.44 (4.62) years, mean age at surgery was 58.40 (5.73) years, and mean age at PD onset was 47.76 years (5.63). Nineteen patients were included in the PIGD subtype, five in the indeterminate, and one in the TD subtype. The genetic assessment revealed the presence of a heterozygous variant in the GBA1 gene in three patients (12%). The mean LEDD was 817.36 (358.50) mg. Motor scores and subscores in the different conditions are reported in Table 2, while the detailed description of stimulation parameters and settings is reported in Supplementary Table 3.
Changes of iTUG parameters in the different treatment conditions
Table 3 shows the mean values and SD for the duration of the iTUG subphases in the different clinical conditions. Table 4 summarises the results of the Quade test. Figure 1 allows observing the trend of the duration of the iTUG subphases in the different conditions tested, split by years after surgery. All sensor-based gait parameters included in the analysis significantly changed by comparing the different conditions tested. Post hoc analyses of specific gait parameters are discussed below. Details on statistical analyses are reported in the supplementary material. Similar results were obtained when age and disease duration were used as a covariate.
iTUG overall duration
A significant reduction in the normalised total iTUG duration was found in the on-stimulation/on-medication condition compared with the off-stimulation/off-medication (p < 0.001) and on-stimulation/off-medication (p < 0.001) conditions and between the on-stimulation/off-medication and off-stimulation/off-medication conditions (p < 0.001), suggesting that both stimulation and levodopa could improve motor ability. Furthermore, the dual task condition significantly increased the iTUG total duration compared with the on-stimulation/on-medication condition (p < 0.001), suggesting that the contemporary cognitive tasks interfered with gait inducing a slowdown.
Sit2St
A significant reduction in the normalised Sit2St duration was found in the on-stimulation/on-medication condition compared with the off-stimulation/off-medication (p < 0.001) and on-stimulation/off-medication (p = 0.006) conditions, and between the on-stimulation/off-medication and off-stimulation/off-medication conditions (p = 0.003). In addition, the dual task condition did not significantly affect phase duration when compared with the on-stimulation/on-medication condition, remaining significantly lower if compared with the on-stimulation/off-medication condition (p = 0.012).
Walking forward
A significant reduction in the normalised duration of the walking forward phase was found in the on-stimulation/on-medication condition compared with the off-stimulation/off-medication (p < 0.001), and the on-stimulation/off-medication conditions (p < 0.001) and between the on-stimulation/off-medication conditions and the off-stimulation/off-medication (p = 0.005) conditions. The dual task condition significantly increased the walking forward duration phase if compared with the on-stimulation/on-medication condition (p < 0.001).
First turn (to go back)
A significant reduction in the first turn phase normalised duration was found in the on-stimulation/on-medication condition compared with the off-stimulation/off-medication (p < 0.001) and on-stimulation/off-medication (p < 0.001) conditions, and between the on-stimulation/off-medication condition compared with the off-stimulation/off-medication (p = 0.010). The dual task condition significantly increased the first turn phase duration compared with the on-stimulation/on-medication condition (p < 0.001).
Walking backward
Similarly to the forward walking phase, a significant reduction in the normalised duration of the walking backward phase was found in the on-stimulation/on-medication condition compared with the off-stimulation/off-medication (p < 0.001) and the on-stimulation/off-medication conditions (p = 0.01). The dual task condition significantly increased the duration of the walking forward phase compared with the on-stimulation/on-medication condition (p < 0.001).
Second turn (to sit down)
A significant reduction in the second turn phase normalised duration was found in the on-stimulation/on-medication condition compared with the off-stimulation/off-medication (p < 0.001), and between the on-stimulation/off-medication and the off-stimulation/off-medication conditions (p < 0.001). The dual task condition significantly increased the second turn phase duration compared with the on-stimulation/on-medication condition (p = 0.003).
St2Sit
A significant reduction in the St2Sit normalised duration was found in the on-stimulation/on-medication condition compared with the off-stimulation/off-medication (p = 0.002) and the on-stimulation/off-medication conditions (p = 0.013), suggesting that only the synergistic effect of stimulation and medication significantly reduced the duration of the phase. As observed in the Sit2St phase, the cognitive task did not significantly influence the St2Sit duration if compared with the on-stimulation/on-medication condition, remaining significantly lower if compared with the on-stimulation/off-medication (p = 0.048) and off-stimulation/off-medication (p = 0.009) conditions.
Correlations between iTUG parameters and clinical scales
Table 5 shows the nonparametric correlation between the UPDRS-III, the HIIFRM and the iTUG-related parameters in the different conditions assessed.
In the on-stimulation/on-medication condition, there was a moderate correlation between the UPDRS-III total score and several iTUG parameters (i.e. iTUG duration [p < 0.01; rho-value 0.58]; Sit2St trunk vertical acceleration [p < 0.01; rho-value -0.53]; St2Sit duration [p < 0.01; rho-value 0.52]; Turn2 duration [p < 0.01; rho-value 0.60]; Turn2 angular velocity [p < 0.01; rho-value − 0.54]; walking forward trunk vertical acceleration [p < 0.01; rho-value − 0.57]; walking backward trunk vertical acceleration [p < 0.001; rho-value − 0.65]). Even in the dual task condition, we found a moderate correlation, stronger if compared with the on-stimulation/on-medication condition, between UPDRS-III total score and several iTUG parameters (i.e. iTUG total duration [p < 0.001; rho-value 0.64]; Sit2St duration [p < 0.05; rho-value 0.51]; Sit2St trunk vertical acceleration [p < 0.001; rho-value − 0.65]; forward walking duration [p < 0.01; rho-value 0.62]; turn 2 duration [p < 0.001; rho-value 0.69]), leading to assume that the increase in cognitive activity and the recall of attention towards a different goal negatively influences gait.
In the on-stimulation/off-medication condition, correlations between UPDRS-III total score and most of the iTUG parameters (i.e. iTUG total duration [p < 0.05; rho-value 0.45]; Sit2St duration [p < 0.05; rho-value 0.44]; Sit2St trunk vertical acceleration [p < 0.01; rho-value − 0.62]; turn 1 angular velocity [p < 0.01; rho-value − 0.58]; turn 2 angular velocity [p < 0.001; rho-value − 0.71]; forward walking duration [p < 0.05; rho-value 0.42]; turn 2 duration [p < 0.05; rho-value 0.46]) were also found.
In the off-stimulation/off-medication condition, the angular velocity of Turn 1 phase (p < 0.05; rho-value − 0.51) was the only gait parameter negatively correlated with UPDRS-III. Comparing on-stimulation/off-medication condition with off-stimulation/off-medication condition, the PIGD subscore worsened by only 1 point (from 8 to 9) compared to the 21 points of the UPDRS-III total score (from 26 to 47) (ratio 4.7%).
Concerning the HIIFRM scale, correlations with several iTUG parameters were found almost exclusively in the dual task condition, indicating that the risk of falling is mostly influenced by attention.
Discussion
In this study, we presented a clinical-instrumental long-term evaluation of the effects of both stimulation and medications on motor ability parameters in advanced PD patients treated with bilateral STN-DBS. The analysis of different stimulation and medication conditions allowed obtaining relevant information about the effects on motor ability of the two treatments both separately and in conjunction.
We found an increase in the duration of the iTUG test and a reduction of the acceleration and rotation of the trunk when comparing the on-stimulation/on-medication with the off-stimulation/off-medication conditions. Both stimulation and medication reduced the total duration of the iTUG and most of its different phases, suggesting that they can improve gait in the long-term after surgery. Overall, dopaminergic therapy had a more marked effect compared with stimulation. However, STN-DBS alone markedly reduced the total iTUG duration, the Sit2St phase, the first and second turn phases and forward walking, while a lower effect was observed on St2Sit phase and walking backward phases duration.
The progressive decline in gait and balance remains one of the major unmet needs in the long-term after surgery [30]; nevertheless, the effects of stimulation remain unclear. Most studies have reported non-significant or conflicting results [13, 31, 32], possibly related to methodological limitations and differences in gait evaluation (i.e. questionnaires, non-instrumented TUG, inertial sensors) [33]. A previous meta-analysis showed that STN-DBS has a mild positive effect on walking speed; however, outcomes varied substantially from large to negligible improvements [34]. Other studies have also shown an improvement in step length with bilateral STN-DBS [33, 35]. Our results support the hypothesis that stimulation maintains a significant effect even in the long-term, which adds up positively to levodopa therapy.
Data about the effects of STN-DBS on the Sit2St phase are scarce in the literature. PD patients have difficulties in initiating the Sit2St, showing slower angular displacements and smaller normalised hip flexion torque than healthy controls [36]. However, it has been reported that PD patients in the on-medication condition and healthy subjects have the same Sit2St duration, while patients are slower in the OFF-medication condition [37, 38]. This finding was also confirmed by Weiss et al., who found that the Sit2st duration was not significantly different between PD patients in the ON medication condition and age-matched healthy subjects [39]. However, in this study, the range Sit2St (amplitude range during the Sit2St time interval) and the jerk Sit2St (slope during Sit2St time interval) parameters were significantly lower in PD patients [39]. This was hypothesised to be related to axial bradykinesia [39, 40]. In our cohort, the Sit2St and the second turn phases were the parameters which benefitted more from the stimulation effect, suggesting that the Sit2St phase is positively influenced by both levodopa and stimulation.
The ability to turn during gait is impaired in PD and has been related to falls and FOG [41]. Compared to healthy older adults, patients with PD exhibit poorer balance and impaired segmental coordination while turning during walking. Moreover, they approach turns with a slower step and take slower and wider turns, with narrower and increased number of steps, and significantly decreased trunk rotation compared to healthy elderly adults [41]. However, data about the effects of STN-DBS on the first turn and the turn-to-sit phases of the iTUG are lacking in the literature. In our cohort, stimulation allowed reducing the duration of these phases leading to a more physiological turn, probably through its effects on axial bradykinesia and rigidity. Furthermore, we also found a moderate correlation between the first turn angular velocity of the iTUG and the HIIFRM scale, indicating that patients who perform worse on the turn can be at greater risk of falling. These subjects might be suitable for specific physiotherapy to improve their performance in the circular walk.
Another important finding in our study is represented by the negative influence of the dual task condition on both total iTUG duration and different phases, including the walking forward, the first and the second turn, and the walking backward phase. It is well known that dual tasks severely affect walking performances in people with PD regardless of the nature of the task [42]. Furthermore, adding a cognitive task to the iTUG enhances the identification of fall risk in people with PD [43]. However, only a few studies have assessed the impact of (cognitive) dual tasks on the iTUG. In particular, one study on 30 participants (15 with mild to moderate PD [Hoehn and Yahr I–II] and 15 age-matched controls) suggested that the dual task iTUG might be more sensitive as compared with the iTUG as a clinical biomarker for PD diagnosis [44]. Unfortunately, the authors did not address the sensitivity of the single iTUG subphases. In our cohort, the non-significant effect of dual task on both Sit2St and St2Sit phases could depend on the fact that the elevation and abasement movements are very short (< 2 s), monophasic and complex, so that a cognitive activity cannot be performed simultaneously. In this setting, cognitive activity may eventually inhibit and postpone the action but not slow it down in its execution. On the contrary, a cognitive task can be performed during a repetitive and automatic activity such as walking, with the result of prevailing on the hierarchical level according to the “cognitive/posture first” model and worsening motor performance.
We found several correlations between the UPDRS-III total score and some iTUG parameters. Indeed, previous studies have reported that higher UPDRS scores were associated with lower iTUG scores. In particular, the angular velocities of the turning phases were the most strongly correlated to PD severity suggesting that the turning velocity could be a good measure of disease progression for PD [45].
Interestingly, our data showed that the greatest correlation with iTUG was observed when patients were in the on-stimulation/on-medication or on-stimulation/off-medication conditions, compared to the off-stimulation/off-medication condition, when the test was worse performed. The underlying explanation could be that in the first two conditions the contribution of the PIGD subscore to the UPDRS-III total score is greater, respectively, 41.6% and 30.7%, compared to 19.1% on off/off state.
Globally, our results underline that in the long-term after surgery STN-DBS may maintain the improvement in gait in the acute setting when used in combination with dopamine replacement therapy, which has been shown to have a stronger effect on walking. Obviously, this improvement is less relevant if compared with the other cardinal symptoms of the disease (i.e. tremor, rigidity, and bradykinesia) as confirmed by the different worsening of PIGD subscore and UPDRS-III total score in the off-stimulation/off-medication condition. Indeed, when the patient, already deprived of the pharmacological effect switched off the stimulator, the UPDRS-III total score worsened by 21 points (from 26 to 47), while the PIGD subscore increased by only 1 point, meaning that the effect of STN-DBS is relevant but is mainly manifested in domains other than gait and postural control. In our cohort, levodopa administration not only improved gait parameters, but also significantly improved appendicular items on UPDRS-III. Total UPDRS-III was worse on-stimulation/off-medication compared to on-stimulation/on-medication with an improvement of > 50% with levodopa (26 vs 12). Both akinesia and tremor scores were substantially better on-stimulation/on-medication compared to on-stimulation/off-medication. These results highlight that levodopa has a significant positive effect on both gait and appendicular symptoms in the long-term after STN-DBS.
Many subphases of the iTUG are affected in different ways by the different conditions, allowing a pathophysiological analysis of specific aspects of motor behaviour and the assessment of the influence of the different therapeutic strategies on the specific subphases. Furthermore, the presence of the elevation/sitting tasks and the turns give additional sensitivity to the test, highlighting, through the dual task, how the hierarchical primacy of cognitive or postural functions can change depending on whether the motor task is ordinary, prolonged, and repetitive or on the contrary complex, short and monophasic.
This study has several limitations. First, the sample size (n = 25) is small, thus reducing the statistical power of our results. In addition, the instrumental gait analysis was not performed in the preoperative phase, not allowing the comparison of the postoperative data with the preoperative ones. In addition, the follow-up duration after surgery was variable among patients. The influence of this duration on the present findings remains to be assessed. In addition, the different conditions were not counter-balanced and, therefore, because of those limitations due to fatigue and learning effect should be also considered.
Moreover, only four patients were under bilateral low-frequency stimulation not allowing us to perform a statistical comparison between high- and low-frequency subgroups. In addition, we were not able to calculate the electrodes position and the volume of tissue activated thus representing another limitation of the study. In almost all patients, the first and second contacts were the ones activated as cathode and were also those found to be most effective at postoperative programming and those located at the depth where the greatest STN activity was recorded during intraoperative microrecording. Indeed, in each patient during STN-DBS surgery, we decided the depth of the definitive electrode by calculating to keep contacts 1 and 2 at the depths where we had recorded the maximum activity compatible with the STN. Another limitation is represented by the lack of the off-stimulation/on-medication condition which did not allow us to evaluate the single effect of dopaminergic treatment. However, this study has also several strengths including the objective gait measurements, the use of a validated protocol, produced by a centre with long expertise in gait instrumental analysis, and the reliability of clinical examination always performed by the same examiner who recorded and assessed all patients.
Conclusions
This study highlights the efficacy of bilateral STN-DBS on motor ability during locomotion in the long-term after surgery. Furthermore, using inertial sensors in a simple administration test such as the iTUG proved feasible in the clinical setting. This supports its use for quantifying changes in the overall motor ability of daily functional activities, such as linear walking, turning, lifting, and sitting, in day-to-day practice and clinical trials.
Availability of data and material (data transparency)
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.
Code availability
Not applicable.
References
Mirelman A, Bonato P, Camicioli R et al (2019) Gait impairments in Parkinson’s disease. Lancet Neurol 18:697–708
Kemoun G, Defebvre L (2001) Gait disorders in Parkinson disease. Clinical description, analysis of posture, initiation of stabilized gait. Presse Medicale Paris Fr 1983 30:452–459
Deuschl G, Schade-Brittinger C, Krack P et al (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355:896–908
Limousin P, Foltynie T (2019) Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol 15:234–242
Rodriguez-Oroz MC, Moro E, Krack P (2012) Long-term outcomes of surgical therapies for Parkinson’s disease. Mov Disord Off J Mov Disord Soc 27:1718–1728
Bove F, Mulas D, Cavallieri F et al (2021) Long-term outcomes (15 years) after subthalamic nucleus deep brain stimulation in patients with Parkinson disease. Neurology. https://doi.org/10.1212/WNL.0000000000012246. (Epub 2021 Jun 2)
Weaver FM, Follett K, Stern M et al (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301:63–73
Cavallieri F, Fraix V, Bove F et al (2021) Predictors of long-term outcome of subthalamic stimulation in Parkinson disease. Ann Neurol 89:587–597
Bove F, Fraix V, Cavallieri F et al (2020) Dementia and subthalamic deep brain stimulation in Parkinson disease: a long-term overview. Neurology 95:e384–e392
Zampogna A, Cavallieri F, Bove F et al (2022) Axial impairment and falls in Parkinson’s disease: 15 years of subthalamic deep brain stimulation. NPJ Park Dis 8:121
Di Rauso G, Cavallieri F, Campanini I et al (2022) Freezing of gait in Parkinson’s disease patients treated with bilateral subthalamic nucleus deep brain stimulation: a long-term overview. Biomedicines 10:2214
Rocchi L, Carlson-Kuhta P, Chiari L, Burchiel KJ, Hogarth P, Horak FB (2012) Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease: laboratory investigation. J Neurosurg 117:1141–1149
Collomb-Clerc A, Welter M-L (2015) Effects of deep brain stimulation on balance and gait in patients with Parkinson’s disease: a systematic neurophysiological review. Neurophysiol Clin Clin Neurophysiol 45:371–388
Hausdorff JM, Gruendlinger L, Scollins L, O’Herron S, Tarsy D (2009) Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 24:1688–1692
Vallabhajosula S, Haq IU, Hwynn N et al (2015) Low-frequency versus high-frequency subthalamic nucleus deep brain stimulation on postural control and gait in Parkinson’s disease: a quantitative study. Brain Stimulat 8:64–75
Bakker M, Esselink RAJ, Munneke M, Limousin-Dowsey P, Speelman HD, Bloem BR (2004) Effects of stereotactic neurosurgery on postural instability and gait in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 19:1092–1099
Krack P, Batir A, Van Blercom N et al (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349:1925–1934
Deuschl G, Paschen S, Witt K (2013) Clinical outcome of deep brain stimulation for Parkinson’s disease. Handb Clin Neurol 116:107–128
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
World Medical Association (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Defer GL, Widner H, Marié RM, Rémy P, Levivier M (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord Off J Mov Disord Soc 14:572–584
Fahn S, Marsden C, Calne D, Goldstein M (1987) Recent developments in Parkinson’s disease. Macmillan Health Care Information, Florham Park
Campanini I, Mastrangelo S, Bargellini A et al (2018) Feasibility and predictive performance of the Hendrich Fall Risk Model II in a rehabilitation department: a prospective study. BMC Health Serv Res 18:18
Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord Off J Mov Disord Soc 28:668–670
Grisanti S, Ferri L, Cavallieri F et al (2022) Increased stroke risk in patients with Parkinson’s disease with LRRK2 mutations. Mov Disord Off J Mov Disord Soc 37:1117–1118
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 25:2649–2653
Palmerini L, Mellone S, Avanzolini G, Valzania F, Chiari L (2013) Quantification of motor impairment in Parkinson’s disease using an instrumented timed up and go test. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc 21:664–673
van Lummel RC, Walgaard S, Hobert MA et al (2016) Intra-rater, inter-rater and test-retest reliability of an instrumented timed up and go (iTUG) test in patients with Parkinson’s disease. PLoS One 11:e0151881
Vervoort D, Vuillerme N, Kosse N, Hortobágyi T, Lamoth CJC (2016) Multivariate analyses and classification of inertial sensor data to identify aging effects on the timed-up-and-go test. PLoS One 11:e0155984
Hurt CP, Kuhman DJ, Guthrie BL, Lima CR, Wade M, Walker HC (2020) Walking speed reliably measures clinically significant changes in gait by directional deep brain stimulation. Front Hum Neurosci 14:618366
Pötter-Nerger M, Volkmann J (2013) Deep brain stimulation for gait and postural symptoms in Parkinson’s disease. Mov Disord Off J Mov Disord Soc 28:1609–1615
St George RJ, Nutt JG, Burchiel KJ, Horak FB (2010) A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology 75:1292–1299
Navratilova D, Krobot A, Otruba P et al (2020) Deep brain stimulation effects on gait pattern in advanced Parkinson’s disease patients. Front Neurosci 14:814
Roper JA, Kang N, Ben J, Cauraugh JH, Okun MS, Hass CJ (2016) Deep brain stimulation improves gait velocity in Parkinson’s disease: a systematic review and meta-analysis. J Neurol 263:1195–1203
Peterson DS, Mancini M, Fino PC, Horak F, Smulders K (2020) Speeding up gait in Parkinson’s disease. J Park Dis 10:245–253
Mak MKY, Levin O, Mizrahi J, Hui-Chan CWY (2003) Joint torques during sit-to-stand in healthy subjects and people with Parkinson’s disease. Clin Biomech Bristol Avon 18:197–206
Inkster LM, Eng JJ (2004) Postural control during a sit-to-stand task in individuals with mild Parkinson’s disease. Exp Brain Res 154:33–38
Mak MKY, Hui-Chan CWY (2005) The speed of sit-to-stand can be modulated in Parkinson’s disease. Clin Neurophysiol Off J Int Fed Clin Neurophysiol 116:780–789
Weiss A, Herman T, Plotnik M et al (2010) Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson’s disease? Med Eng Phys 32:119–125
Fatmehsari YR, Bahrami F (2011) Sit-to-stand or stand-to-sit: Which movement can classify better Parkinsonian patients from healthy elderly subjects? 2011 18th Iran Conf Biomed Eng ICBME. Tehran, Iran: IEEE; pp 48–53. http://ieeexplore.ieee.org/document/6168583/. Accessed Nov 13, 2022.
Weiss A, Herman T, Mirelman A et al (2019) The transition between turning and sitting in patients with Parkinson’s disease: a wearable device detects an unexpected sequence of events. Gait Posture 67:224–229
Raffegeau TE, Krehbiel LM, Kang N et al (2019) A meta-analysis: Parkinson’s disease and dual-task walking. Parkinsonism Relat Disord 62:28–35
Vance RC, Healy DG, Galvin R, French HP (2015) Dual tasking with the timed “up & go” test improves detection of risk of falls in people with Parkinson disease. Phys Ther 95:95–102
Byl N, Henry R, Rizzo R, Blum D (2018) Is the timed up and go (TUG) sensitive to differentiating patients with mild to moderate PD compared to age matched controls: a descriptive pilot study. Int Phys Med Rehabil J. 3. https://medcraveonline.com/IPMRJ/is-the-timed-up-and-go-tug-sensitive-to-differentiating-patients-with-mild-to-moderate-pd-compared-to-age-matched-controls-a-descriptive-pilot-study.html. Accessed Dec 5, 2022.
Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB (2010) The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:171–176
Dibilio V, Nicoletti A, Mostile G et al (1996) Dopaminergic and non-dopaminergic gait components assessed by instrumented timed up and go test in Parkinson’s disease. J Neural Transm Vienna Austria 2017(124):1539–1546
Acknowledgements
This study was partially supported by Italian Ministry of Health—Ricerca Corrente Annual Program 2023.
Funding
No funding reported.
Author information
Authors and Affiliations
Contributions
FC: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; IC: design of the study, data collection and analysis, manuscript preparation: writing of the first draft and review and critique; AG: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; CB: design of the study, data collection and analysis, manuscript preparation: writing of the first draft and review and critique; VFi: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; GDR: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; AF: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; BD: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; SS: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; NG: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; EB: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; MGC: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; JR: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; FA: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; FCa: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; MAM: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; SC: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; EM: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; AP: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; GV: design of the study, data collection and analysis, manuscript preparation: review and critique; EB: design of the study, data collection and analysis, manuscript preparation: review and critique; GP: design of the study, data collection and analysis, manuscript preparation: review and critique; SM: data collection and analysis, manuscript preparation: review and critique; VFr: data collection and analysis, manuscript preparation: review and critique; AF: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; AV: design of the study, data collection and analysis, manuscript preparation: writing of the first draft; ML: data collection and analysis, manuscript preparation: review and critique; GB: design of the study, data collection and analysis, manuscript preparation: review and critique; AM: design of the study, data collection and analysis, manuscript preparation: writing of the first draft and review and critique; EM: data collection and analysis, manuscript preparation: review and critique; FV: design of the study, data collection and analysis, manuscript preparation: writing of the first draft and review and critique. All the authors. All the authors approve the final version for publication.
Corresponding author
Ethics declarations
Conflicts of interest
E. Moro has received honoraria from Medtronic, Abbott and Kyowa for consulting services. She has also received grant support from Ipsen and Boston Medical. VF receiving honoraria for lecturing Boston Scientific and Medtronic. All the other authors declare no financial disclosures. The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local ethics committee (protocol number: 2019/0056629).
Consent for publication
Written informed consent was obtained from participants according to the Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cavallieri, F., Campanini, I., Gessani, A. et al. Long-term effects of bilateral subthalamic nucleus deep brain stimulation on gait disorders in Parkinson’s disease: a clinical-instrumental study. J Neurol 270, 4342–4353 (2023). https://doi.org/10.1007/s00415-023-11780-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11780-5