Abstract
Background
Neuropsychiatric manifestations (NP) are common in systemic lupus erythematosus (SLE). However, the pathophysiological mechanisms are not completely understood. Neurofilament light protein (NfL) is part of the neuronal cytoskeleton. Increased NfL concentrations, reflecting neurodegeneration, is observed in cerebrospinal fluid (CSF) in several neurodegenerative and neuroinflammatory conditions. We aimed to explore if plasma NfL could serve as a biomarker for central nervous system (CNS) involvement in SLE.
Methods
Sixty-seven patients with SLE underwent neurological examination; 52 underwent lumbar puncture, while 62 underwent cerebral magnetic resonance imaging (MRI). We measured selected auto-antibodies and other laboratory variables postulated to have roles in NP pathophysiology in the blood and/or CSF. We used SPM12 software for MRI voxel-based morphometry.
Results
Age-adjusted linear regression analyses revealed increased plasma NfL concentrations with increasing creatinine (β = 0.01, p < 0.001) and Q-albumin (β = 0.07, p = 0.008). We observed higher plasma NfL concentrations in patients with a history of seizures (β = 0.57, p = 0.014), impaired motor function (β = 0.36, p = 0.008), increasing disease activity (β = 0.04, p = 0.008), and organ damage (β = 0.10, p = 0.002). Voxel-based morphometry suggested an association between increasing plasma NfL concentrations and the loss of cerebral white matter in the corpus callosum and hippocampal gray matter.
Conclusion
Increased plasma NfL concentrations were associated with some abnormal neurological, cognitive, and neuroimaging findings. However, plasma NfL was also influenced by other factors, such as damage accrual, creatinine, and Q-albumin, thereby obscuring the interpretation of how plasma NfL reflects CNS involvement. Taken together, NfL in CSF seems a better marker of neuronal injury than plasma NfL in patients with SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurological involvement in patients with systemic lupus erythematosus (SLE) is common and significantly contribute to morbidity [1]. The pathophysiological mechanisms are unclear and while some phenomena are the result of an injury to the neuronal cells because of vascular disturbances or immune attack, others are caused by an inhibition or excitation of neuronal receptors [1]. The latter is exemplified in murine SLE models by anti-NR2 antibodies which in low levels can impair neuronal function, and cause excitotoxic neuronal death at higher concentrations [2]. It is critical to investigate if the cerebral manifestations are potentially reversible or static because of neuronal death. Therefore, biomarkers reflecting neuronal damage, possibly predicting clinical outcomes, and allowing insights into prevailing mechanisms are long awaited.
Neurofilament light protein (NfL) is part of the neuronal cytoskeleton. Increased NfL levels in the cerebrospinal fluid (CSF) are observed in several neurodegenerative disorders and predict future rates of cognitive decline [3]. In addition, NfL levels in the CSF (CSF NfL) correlate with central nervous system (CNS) involvement in autoimmune inflammatory diseases, such as multiple sclerosis, SLE, and primary Sjøgren’s syndrome [3, 4].
Lumbar puncture is a more invasive procedure than blood sampling, and it would have been advantageous if the blood-based measures of NfL provided equivalent and adequate clinical information as CSF measures. A study of elderly subjects advocated the aforementioned fact, suggesting that plasma NfL levels could be an equally good biomarker as CSF NfL. Moreover, plasma NfL can predict cognitive decline and changes in hippocampal volumes and fractional anisotropy in the corpus callosum [5]. It has been demonstrated that a reduction in the hippocampal and corpus callosum volumes occurs in SLE patients. Therefore, we aimed to explore the association between plasma NfL concentrations in patients with SLE and past and present neurological or psychiatric (neuropsychiatric- NP) manifestations, cerebral magnetic resonance imaging (MRI) abnormalities, blood–brain barrier function, CSF NfL, and other selected biomarkers, including kidney function [5,6,7,8].
Patients and methods
Patients
Aiming to perform a population-based study, all (86) SLE patients who fulfilled the American College of Rheumatology (ACR) revised classification criteria for SLE [9] followed at Stavanger University Hospital were invited to participate in the study. Sixty-eight (79%) of these patients gave written informed consent to participate in the study and were for research purposes only admitted to the hospital for 2 days, and underwent an extensive standardized examination by specialists in internal medicine (EH and LG) and neurology (ABT). One patient was later excluded because of a brain tumor, thus resulting in 67 patients for analysis. Fifty-two patients underwent lumbar puncture; however, some of the CSF analyses were not performed in five of them because of insufficient CSF volumes. Five of the 67 patients did not undergo an MRI examination because of contraindications (claustrophobia or cochlear implants).
Neuropsychological testing was performed by trained psychometricians and supervised by a neuropsychologist (SSM) who also evaluated the results and mapped the findings to the following domains: simple and complex attention, memory, visual–spatial processing, language, reasoning/problem solving, psychomotor speed, and motor function. Table 3 outlines the neuropsychological test battery, and a detailed description of the procedures has been previously reported [20]. We defined the cut-off for an abnormality as a standardized score ≥ 2SD from the reference mean, based on normative data for each test. For depression, we applied Beck Depression Inventory and used a score ≥ 13 as the cut-off value. Past and present neuropsychiatric manifestations were evaluated and established according to recommendations from ACR by a group of specialists in rheumatology, internal medicine, neurology, radiology and neuropsychology [15]. We have previously investigated the role of NfL in CSF in the same SLE cohort [4].
The study was approved by the Regional Committee for Medical and Health Research Ethics (2010/1455/REK vest), and was conducted in compliance with the tenets of the Declaration of Helsinki.
Laboratory analyses
Routine hematological, biochemical and immunological tests were analyzed in the hospital’s laboratory. Anti-nuclear antibodies (ANA) were detected by a HEp-2000 assay (Immunoconcepts, Sacramento, California, USA), and antibodies to double-stranded DNA (dsDNA) were verified by a Nova Lite dsDNA Crithidia luciliae 708200 indirect immunofluorescence assay (Nova Diagnostics, San Diego, California, USA). We calculated the IgG index as a measure of intrathecal IgG production and Q-albumin, reflecting blood–brain barrier integrity, using standard methods [16]. The presence of anti-phospholipid antibodies (aPL) was defined by positive results for either anti-cardiolipin or beta2-glycoprotein1 IgM or IgG antibodies or a positive lupus anticoagulant test [17]. We analyzed anti-NR2 antibodies in the CSF by electrochemiluminescence method on a SECTOR Imager 2400 platform (MSD, Gaithersburg, MD, USA). The synthetic DWEYSVWLSN decapeptide was applied as antigen. The cut-off value for anti-NR2 antibodies was based on CSF from 24 subjects who underwent lumbar puncture as part of a neurological examination. None of these subjects later turned out to have any inflammatory, autoimmune, or malignant diseases. The CSF sample with the highest signal out of all these subjects was chosen as the cut-off value/internal calibrator and measured together with the samples on each plate. For each sample, a ratio against the internal calibrator was calculated. Samples with ratio ≥ 1.0 were considered positive, and < 1.0 were considered negative. The procedure is previously described in details [18]. Anti-ribosomal P protein antibodies in CSF was analyzed by enzyme-linked immunosorbent assay (ELISA) using synthetic C-terminal 22-amino acid ribosomal P peptide conjugated to human serum albumin as an antigen, the procedure is previously described in details [19]. Protein S100B was analyzed in the CSF by ELISA (Abnova, Jhongli City, Taiwan). Moreover, we measured CSF NfL concentrations using an in-house ELISA [3]. Plasma NfL concentrations were measured using Single molecule array (Simoa) technology and the NF-light Advantage kit, based on the manufacturer’s instructions (Quanterix, Billerica, MA).
Statistical analyses
While continuous data are summarized as medians and ranges, categorical data are presented as numbers and percentages. We calculated correlations between plasma NfL and the age, urine albumin/creatinine ratio, and CSF NfL concentrations using Spearman rank correlation.
Plasma NfL measures were log-transformed to achieve the normality of data for more appropriate use in regression analyses. To examine possible associations between plasma NfL and laboratory and clinical variables corrected for age, we conducted linear regression analyses with log-transformed plasma NfL as a dependent variable. We applied Bonferroni correction for multiple testing. Moreover, we examined laboratory and clinical variables with p-value < 0.2 in age-adjusted linear regression, in multivariable linear regression analyses. One model was created for each clinical phenomenon, with log-transformed plasma NfL as the dependent variable. We excluded variables that lacked significant effects from the final models, with the exception of the age, serum creatinine, and Q-albumin, which were considered important variables regardless of their significance level.
We performed identical procedures to analyze the effect of similar laboratory and clinical variables on the NfL plasma/CSF ratio.
All analyses were performed with IBM SPSS Statistic 26.
MRI and voxel-based morphometry
Image acquisition and white matter hyperintensity (WMH) evaluation procedures have been previously described. However, the images were captured in a 1.5 Tesla system (Philips). Two neuroradiology specialists (OJG and MKB) performed WMH assessment according to the Scheltens’ visual rating scale [20, 21].
MRI images were preprocessed using default settings by the CAT12 extension (Gaser, http://dbm.neuro.uni-jena.de/wordpress/vbm/download/) of SPM12 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Moreover, the images were smoothed using an 8 mm full-width-half-maximum Gaussian kernel [22].
We performed multiple regression tests in SPM12 to explore the association between plasma NfL concentrations and gray matter (GM) or white matter (WM) volume changes. We created two models. The age, sex, and total intracranial volume were applied as nuisance variables to correct for potential influence in one model. In contrast, the age, sex, total intracranial, creatinine, and Q-albumin were applied as nuisance variables in the other model. Analyses were performed over all GM and WM voxels (whole-brain analysis) and, hypothesis-driven, within a hippocampus (GM) or corpus callosum (WM) mask, respectively. The masks were defined by applying WFU_pickatlas version 3.0.5 (http://www.nitrc.org/projects/wfu_pickatlas/) [23, 24].
We applied family-wise error correction for multiple testing, using p < 0.05 as the significance threshold [25].
Results
Table 1 summarizes the selected demographic and clinical data.
Three patients (5%) had biopsy-confirmed, active glomerulonephritis, and one had underwent renal transplant due to end-stage renal disease. One patient had moderate renal failure with a serum creatinine level of 187 mmol/L (reference interval 60–125 mmol/L).
Plasma NfL concentrations increased with increasing age (Spearman ρ = 0.62, p < 0.001) but were not correlated with sex (Spearman ρ = 0.18, p < 0.138).
We observed a moderate correlation between NfL concentrations in CSF and plasma (Spearman ρ = 0.51, p < 0.001).
While creatinine was moderately correlated with plasma NfL (Spearman ρ = 0.41, p = 0.001), it was weakly correlated with CSF NfL (Spearman ρ = 0.30, p = 0.04).
We then performed linear regression analyses with log-transformed plasma NfL as a response variable and the selected laboratory variables as explanatory variables, corrected for age (Table 2). The aforementioned model demonstrated a weak association between plasma NfL and creatinine and between plasma NfL and estimated glomerular filtration rate.
We constructed a linear regression model with log-transformed plasma NfL as the dependent variable, and the age, creatinine, and age multiplied by creatinine as explanatory variables to determine if the effect of creatinine on plasma NfL was age-dependent. However, we did not observe an effect of the interaction variable age multiplied by creatinine (p = 0.69).
There was no correlation between plasma NfL and the albumin/creatinine ratio in urine (Spearman ρ = 0.22, p = 0.08).
We observed a small but significant association between increasing levels of plasma NfL and Q-albumin (Table 2). The data of one patient were censored in this analysis because of standardized residuals > 3. There were no other associations between plasma NfL concentrations and the selected laboratory variables (Table 2). No association between plasma NfL and anti-DNA-antibodies or complement C3 or C4 levels were found (data not shown).
The ratio of NfL plasma/CSF was not correlated to the variables in Table 2 (data not shown).
No association was found between current prednisolone dose and NfL in plasma (β = 0.02 [95%CI − 0.02 to 0.07], p = 0.3). We have no data on cumulative prednisolone dose in our cohort.
Plasma NfL and clinical variables
Disease activity and accrued damage
Plasma NfL levels increased with the disease activity, as measured by the SLE disease activity index (SLEDAI) score (Table 3). None of the patients had onset of the NP manifestations included in the SLEDAI within the last 10 days of examinations. Plasma NfL levels increased with disease-related organ damage, as assessed by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) score (Table 3).
NP manifestations
In this close-to population based cohort, 63 patients (94%) had experienced at least one past or present NP syndrome (25 had one, 18 had two, 10 had three, 7 had four, one had five and one had six NP manifestations). The number of patients with the various past or present NP manifestations are given in Table 3. Cognitive dysfunction, mood disorder and polyneuropathy were diagnosed based on results of testing at inclusion, all other manifestations included occurrence in the past, for headache limited to the last year. Plasma NfL concentration was higher in patients with active NP manifestations, (including only cognitive dysfunction, mood disorders and polyneuropathy) compared to patients with previous NP manifestations (β = 0.35, [95%CI 0.06–0.64], p = 0.02).
Patients with lacunar infarcts on cerebral MRI had borderline significant higher plasma NfL levels than those without (Table 3). This was not observed in cortical infarcts.
In addition, patients with a history of seizures had higher plasma NfL levels (Table 3). We observed a borderline, but not significant association between total cognitive dysfunction (all domains) and plasma NfL (Table 3). Only motor impairment was associated with higher plasma NfL levels in the subgroups. To test for potential additive effect of multiple NP manifestations in the same patient, we added total number of NPSLE manifestations as a correcting variable in these analyses, but no effect of this was seen in the model (data not shown). None of the associations between NP manifestations and plasma NfL remained significant after applying the conservative Bonferroni correction for multiple testing (Table 3).
There were no other associations between plasma NfL concentrations and NPSLE. We investigated possible associations with subtypes of headache and polyneuropathy (i.e., tension-type headache and migraine, and sensory, motor, and sensorimotor polyneuropathy) in subanalyses, without revealing any association with plasma NfL levels (data not shown).
The ratio of NfL plasma/CSF concentrations was not influenced by any reported CNS manifestation (β = 0.006 [95%CI − 0.02 to 0.03], p = 0.6) peripheral nervous system manifestation (β = − 0.003 [95% CI − 0.02 to 0.009], p = 0.6), or total number of NPSLE manifestation (β 0.04 [95% CI − 0.05 to 0.14], p = 0.4), all analyses corrected for age, sex, creatinine, and Q-albumin.
Multivariable regression analyses with both laboratory and clinical variables
Based on the results of initial regression analyses, we included creatinine, Q-albumin, and aPL in multivariable regression analyses. In the above-mentioned models, the SLEDAI NP items, motor function, and a history of seizures were found to exert an effect on plasma NfL concentrations (Table 4). However, SDI scores, lacunar infarcts, cognitive (all domains), or specifically memory or language dysfunction in multivariable regression analyses did not exert an effect on the plasma NfL concentrations (data not shown).
Plasma NfL and MRI findings
White matter hyperintensities
There were no associations between WMHs (Scheltens’ scores) and plasma NfL concentrations (β = 0.005 (95%CI − 0.016 to 0.026), p = 0.6)), when corrected for age, sex, creatinine, and hypertension.
Voxel-wise analyses
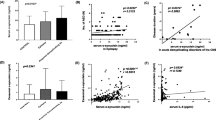
No areas appeared in whole-brain analyses corrected for age, sex, and total intracranial volume, in which GM or WM volumes were associated with plasma NfL concentrations. However, small clusters of hippocampal GM and corpus callosum WM volume loss were associated with increased plasma NfL in regions of interest (ROI) analysis (Fig. 1; Table 5).
Clusters of voxels with GM- or WM loss with increasing plasma NfL-concentration (p < 0.05, FWE corrected) overlaid on modulated and warped mean images of all patients. The cluster color represents the statistical significance of GM or WM decrease according to the gradation of the color bar. a Hippocampus GM analyses corrected for age, sex and total intracranial volume. b Corpus callosum WM analyses corrected for age, sex and total intracranial volume. c Whole brain WM analyses corrected for age, sex, total intracranial volume, serum creatinine and Q-albumin. d Corpus callosum WM analyses corrected for age, sex, total intracranial volume, serum creatinine and Q-albumin
We then corrected for creatinine and Q-albumin, and observed a considerable increase in cluster sizes of volume loss within the corpus callosum mask, while all clusters within the hippocampus mask disappeared, except for one (Fig. 1; Table 5). In whole-brain analyses, GM volumes were not influenced by plasma NfL. However, several clusters appeared for smaller WM volumes with increasing plasma NfL concentrations (Fig. 1; Table 5). We observed no effects of creatinine or Q-albumin in the whole-brain or ROI analyses of GM and WM, respectively (data not shown).
Discussion
In the present study, we found that some abnormal neurological, cognitive, and neuroimaging findings were associated with increased plasma NfL levels. However, we also show that numerous factors may influence the plasma NfL concentration and thereby obscure the understanding of how the aforementioned levels reflect the pathophysiology and clinical and laboratory findings. Moreover, we did not observe an association between brain-reactive autoantibodies and plasma NfL, compared to our previous observation that anti-NR2 antibodies exert a strong influence on the CSF NfL levels [4].
We observed moderate correlation between CSF and plasma NfL concentrations; and that blood–brain barrier permeability influenced plasma NfL concentrations. In addition, creatinine rather than age exerts a strong influence on plasma NfL levels. The latter observation remains to be explained since NfL has a relatively large molecular weight (64 kDa) which is comparable to albumin (61 kDa), and thus only minute amounts should be excreted into the urine, however, as far as we know, this has not been investigated yet.
Increasing plasma NfL concentrations were associated with reduced GM and WM volumes in the hippocampus and corpus callosum, respectively. These are brain regions where loss of cerebral matter has been observed in SLE patients. Our findings were also in accordance with a recent study that indicated plasma NfL as a prognostic marker of degeneration in the above-mentioned specific regions during aging [5].
To obtain a more comprehensive understanding of the potential usefulness of plasma NfL measures for NPSLE, we performed analyses corrected first for conventional factors (age), and secondly also for kidney function (creatinine) and blood–brain barrier function (Q-albumin). We hypothesized that the latter model generates a more accurate picture of how plasma NfL levels might reflect harmful neuronal processes. Furthermore, we revealed an association between increasing plasma NfL with a widespread reduction of WM volumes in the corpus callosum and other parts of the brain, while the association with GM volume reduction in the hippocampus disappeared.
Regarding cognitive performance, we observed higher plasma NfL concentrations in patients with impaired motor function. It is uncertain if the observation is because of WM loss in the anterior part of the corpus callosum. However, impaired motor function has been associated with decreased fractional anisotropy in the anterior parts of the corpus callosum in elderly subjects, similar to our results [26].
Regarding clinical neurological findings, we only observed an association between a history of seizures and increased plasma NfL. Notably, the seizures were absent during the examination and blood collection. A potential hypothesis could be that these patients manifested chronic neuronal harmful processes that would predispose a susceptible brain to seizures over time. Neither the association between impaired motor function and plasma NfL nor between history of seizures and plasma NfL remained significant after the conservative correction for multiple testing, the findings should therefore be cautiously interpreted.
We observed a borderline significant association between lacunar infarcts and plasma NfL. This association, independent of the time from insult, could reflect microvasculopathy, a common finding in SLE [1]. Nonetheless, the association disappeared upon correcting creatinine and Q-albumin levels. Stroke diagnosis was based on the presence of cortical infarcts on MRI. High NfL levels in plasma and CSF reportedly appear in stroke patients in the acute stage and return to baseline levels following 3–5 months [27].
The disease activity and chronic organ damage were low in the SLE cohort. Nevertheless, we observed strong associations between plasma NfL concentrations and both disease activity and damage accrual. Items related to current or present CNS manifestations are heavily weighed in SLEDAI, but none of our patients had new-onset NP manifestations included in this index. Although we found an association between plasma NfL concentration and active NP manifestations, this cannot explain the association between SLEDAI score and plasma NFL as the active NP manifestations in our patient cohort are not among those manifestations included in the SLEDAI. This finding underscores the difficulties of interpretation the association between plasma NfL and neuronal damage in patients with other organ involvement as SLE patients. The association between damage accrual and plasma NfL persists while deleting CNS items, but disappears while correcting for creatinine and Q-albumin, thus indicating that the aforementioned association was because of non-CNS factors.
The limitations of this study include its cross-sectional design and the use of both past and present NPSLE phenomena. We applied the original ACR recommendation for NPSLE syndromes. However, the use of strict attribution criteria limited to the closeness in time would have been an optimal approach [1]. Headache, polyneuropathy without electrophysiological pathology, mild depression, anxiety, and mild cognitive dysfunction do not exclusively represent NPSLE because they have a high prevalence in the general population [28]. An attribution model only including ‘lupus headache’ as defined by the SLEDAI scoring guidelines could potentially revealed association between plasma NfL and headache, however, as none of our patients had such headache the last 10 days upon inclusion, this could not be explored in our cohort. Including a control group of healthy subjects would have been desirable.
Diffusion tensor imaging is the preferred modality over voxel-based morphometry for WM analyses. Brain volume changes and plasma NfL must be the dependent and explanatory variables, respectively, in voxel-based morphometry. This might have influenced the results, particularly in the model including Q-albumin and creatinine and necessitates interpreting the findings with caution. We performed a very conservative correction for multiple testing in the voxel-based morphometry, with the possibility of our failure to identify other areas of plasma NfL-associated true volume loss.
The strengths of our study were the unselected patients with well-classified disease and comprehensive and systematic clinical examinations.
In conclusion, increased plasma NfL levels are associated with the loss of WM and GM in the corpus callosum and hippocampus, respectively, thereby reflecting SLE processes in the brain. To some extent, neurological findings and cognitive impairment are also associated with elevated plasma NfL levels. However, plasma NfL levels are influenced by several other factors, such as damage accrual, creatinine, and Q-albumin. We have explored a small group of SLE patients with combined past and present NP manifestations, our findings should be further explored in larger cohorts including patients with active, major NP manifestations. Taken together, CSF NfL level seems to be a better marker of neuronal injury than plasma NfL in patients with SLE.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
23 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00415-024-12238-y
References
Hanly JG, Kozora E, Beyea SD, Birnbaum J (2019) Review: nervous system disease in systemic lupus erythematosus: current status and future directions. Arthritis Rheumatol (Hoboken, NJ) 71(1):33–42. https://doi.org/10.1002/art.40591
Faust TW, Chang EH, Kowal C, Berlin R, Gazaryan IG, Bertini E, Zhang J, Sanchez-Guerrero J, Fragoso-Loyo HE, Volpe BT, Diamond B, Huerta PT (2010) Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc Natl Acad Sci USA 107(43):18569–18574. https://doi.org/10.1073/pnas.1006980107
Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90(8):870–881. https://doi.org/10.1136/jnnp-2018-320106
Tjensvoll AB, Lauvsnes MB, Zetterberg H, Kvaloy JT, Kvivik I, Maroni SS, Greve OJ, Beyer MK, Hirohata S, Putterman C, Alves G, Harboe E, Blennow K, Goransson LG, Omdal R (2021) Neurofilament light is a biomarker of brain involvement in lupus and primary Sjogren’s syndrome. J Neurol 268(4):1385–1394. https://doi.org/10.1007/s00415-020-10290-y
Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I, Machulda MM, Kremers WK, Knopman DS, Jack C Jr, Petersen RC, Kern S (2019) Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 93(3):e252–e260. https://doi.org/10.1212/WNL.0000000000007767
Appenzeller S, Carnevalle AD, Li LM, Costallat LT, Cendes F (2006) Hippocampal atrophy in systemic lupus erythematosus. Ann Rheum Dis 65(12):1585–1589. https://doi.org/10.1136/ard.2005.049486
Appenzeller S, Rondina JM, Li LM, Costallat LT, Cendes F (2005) Cerebral and corpus callosum atrophy in systemic lupus erythematosus. Arthritis Rheum 52(9):2783–2789. https://doi.org/10.1002/art.21271
Akamine S, Marutani N, Kanayama D, Gotoh S, Maruyama R, Yanagida K, Sakagami Y, Mori K, Adachi H, Kozawa J, Maeda N, Otsuki M, Matsuoka T, Iwahashi H, Shimomura I, Ikeda M, Kudo T (2020) Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep 10(1):20350. https://doi.org/10.1038/s41598-020-76990-7
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40(9):1725. https://doi.org/10.1002/1529-0131(199709)40:9%3c1725::AID-ART29%3e3.0.CO;2-Y
Wechsler D (1955) Manual for the Wechsler Adult Intelligence Scale. Manual for the Wechsler Adult Intelligence Scale. Psychological Corp., Oxford
Wechsler D (1987) WMS-R: Wechsler Memory Scale-revised: manual. Harcourt Brace Jovanovich, New York
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G (1993) Wisconsin card sorting test manual: revised and expanded. Psychological Assessment Resources Inc, Odessa
Stroop JR (1935) Studies of interference in serial verbal reactions. J Exp Psychol 18:643–662
Reitan RM, Wolfson D (1985) The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. Neuropsychology Press, Tucson
ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature (1999) The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum 42(4):599–608. https://doi.org/10.1002/1529-0131(199904)42:4%3c599::AID-ANR2%3e3.0.CO;2-F
Tibbling G, Link H, Ohman S (1977) Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Investig 37(5):385–390. https://doi.org/10.1080/00365517709091496
Harboe E, Tjensvoll AB, Maroni S, Goransson LG, Greve OJ, Beyer MK, Herigstad A, Kvaloy JT, Omdal R (2009) Neuropsychiatric syndromes in patients with systemic lupus erythematosus and primary Sjogren syndrome: a comparative population-based study. Ann Rheum Dis 68(10):1541–1546. https://doi.org/10.1136/ard.2008.098301
Lauvsnes MB, Maroni SS, Appenzeller S, Beyer MK, Greve OJ, Kvaloy JT, Harboe E, Goransson LG, Tjensvoll AB, Omdal R (2013) Memory dysfunction in primary Sjogren’s syndrome is associated with anti-NR2 antibodies. Arthritis Rheum 65(12):3209–3217. https://doi.org/10.1002/art.38127
Isshi K, Hirohata S (1996) Association of anti-ribosomal P protein antibodies with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum 39(9):1483–1490
Harboe E, Beyer MK, Greve OJ, Goransson LG, Tjensvoll AB, Kvaloy JT, Omdal R (2009) Cerebral white matter hyperintensities are not increased in patients with primary Sjogren’s syndrome. Eur J Neurol 16(5):576–581. https://doi.org/10.1111/j.1468-1331.2009.02537.x
Lauvsnes MB, Beyer MK, Kvaloy JT, Greve OJ, Appenzeller S, Kvivik I, Harboe E, Tjensvoll AB, Goransson LG, Omdal R (2014) Association of hippocampal atrophy with cerebrospinal fluid antibodies against the NR2 subtype of the N-methyl-d-aspartate receptor in patients with systemic lupus erythematosus and patients with primary Sjogren’s syndrome. Arthritis Rheumatol (Hoboken, NJ) 66(12):3387–3394. https://doi.org/10.1002/art.38852
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11(6 Pt 1):805–821. https://doi.org/10.1006/nimg.2000.0582
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3):1233–1239
Maldjian JA, Laurienti PJ, Burdette JH (2004) Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 21(1):450–455
Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC (1993) Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1(3):210–220. https://doi.org/10.1002/hbm.460010306
Serbruyns L, Gooijers J, Caeyenberghs K, Meesen RL, Cuypers K, Sisti HM, Leemans A, Swinnen SP (2015) Bimanual motor deficits in older adults predicted by diffusion tensor imaging metrics of corpus callosum subregions. Brain Struct Funct 220(1):273–290. https://doi.org/10.1007/s00429-013-0654-z
Pujol-Calderon F, Portelius E, Zetterberg H, Blennow K, Rosengren LE, Hoglund K (2019) Neurofilament changes in serum and cerebrospinal fluid after acute ischemic stroke. Neurosci Lett 698:58–63. https://doi.org/10.1016/j.neulet.2018.12.042
Ainiala H, Loukkola J, Peltola J, Korpela M, Hietaharju A (2001) The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology 57(3):496–500
Funding
Dr. Maria Boge Lauvsnes is supported by grants from the Western Norway Regional Health Authority (WNRHA), (#F-12525). Dr. Henrik Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (SRC) (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (SSSCR) (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), and the UK Dementia Research Institute at UCL. Dr. Kaj Blennow is supported by SRC (#2017-00915), ADDF (#RDAPB-201809-2016615), the Swedish Alzheimer Foundation(#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), SSSCR (#ALFGBG-715986) and the European Union Joint Program for Neurodegenerative Disorders (#JPND2019-466-236). Mrs Ingeborg Kvivik is supported by grants from WNRHA (#912129). Dr. Guido Alves is supported by grants from the Norwegian Research council (#287842), the Norwegian Parkinson Research Foundation, and Reberg’s Legacy. Dr. Shunsei Hirohata is supported by a grant from the Japan Agency for Medical Research and Development (#15eK0410022h0001). Dr. Roald Omdal is supported by grants from WNRHA (#911807).
Author information
Authors and Affiliations
Contributions
All authors meet the ICMJE Uniform Requirements for Manuscripts Submitted to Biomedical Journals criteria for authorship.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Henrik Zetterberg reports that he served on scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. Dr. Kaj Blennow has served as a consultant or on advisory boards for Abcam, Axon, Biogen, Lilly, MagQu, Novartis, and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. Dr. Mona Beyer has received Honoria for lecturing from Novartis and Biogen Idec, Merck AB, Roche Norge, and Sanofi Genzyme. Drs. Lasse G. Gøransson and Roald Omdal has received speaker honoraria from several pharmaceutical companies. None of the other authors have any conflict of interest with regard to the work.
Ethical approval
This study complied with the Helsinki Declaration and was approved by the Regional Research Ethics Committee (REKvest 2010/1455).
Informed consent
Written informed consent was obtained from all patients.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lauvsnes, M.B., Zetterberg, H., Blennow, K. et al. Neurofilament light in plasma is a potential biomarker of central nervous system involvement in systemic lupus erythematosus. J Neurol 269, 3064–3074 (2022). https://doi.org/10.1007/s00415-021-10893-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10893-z