Abstract
Introduction
Myotonic dystrophy type 1 (DM1) is a progressive, multisystemic, and autosomal dominant disease. Muscle wasting and weakness have been associated with impaired functional capacity and restricted social participation in affected individuals. The disease’s presentation is very heterogenous and its progression is still under-documented.
Objective
The aim of the study was to document the progression of muscular strength and functional capacity in the DM1 population over a 3-year period.
Methods
Twenty-three individuals with juvenile or adult phenotypes of DM1 were recruited to complete clinical assessments in 2016 and 2019. Maximal isometric muscle strength (MIMS) was evaluated with quantified muscle testing and functional capacity was evaluated with the Mini-BESTest, the 10-m walk test at comfortable and maximal speeds, the Timed Up and Go and the 6-min walk test. Participants also completed three questionnaires: DM1-Activ, Upper Extremity Functional Index and Lower Extremity Functional Scale (LEFS). Subgroup analyses were evaluated for sex, phenotype, and type of physical activity practiced during the 3-year period.
Results
For the whole group, there was a significant decline in the scores of the Mini-BESTest and the LEFS. Also, MIMS significantly declined for prehension, lateral pinch as well as for hip abductors, knee extensors and ankle dorsiflexors muscle groups. Subgroups analyses revealed that men lost more MIMS than women, and that adult phenotype lost more MIMS than juvenile phenotype.
Conclusion
Quantified muscle testing is a better indicator of disease progression over a 3-year period than functional tests. Phenotype and sex are important factors that influence the progression of DM1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myotonic dystrophy type 1 (DM1) is an autosomal dominant disease and represents the most common form of adult dystrophy [1]. The worldwide prevalence is 1:20,000 [2] but reaches 1:475 in the Saguenay—Lac-St-Jean (SLSJ) region of Canada [3]. DM1 is caused by an abnormal expansion of the cytosine-thymine-guanine (CTG) triplet repeat located on the myotonic dystrophy protein kinase (DMPK) gene [4]. DM1 is slowly progressive and multisystemic where myotonia along with muscular wasting and weakness are among the cardinal symptoms [1]. Muscle weakness has important consequences in the daily life of DM1 individuals. The decrease of muscle strength is correlated with mobility limitations, [5] and lower-limb muscle strength is an explanatory factor of disrupted participation in daily activities and social roles [6]. DM1 is classically categorized into five phenotypes based on the age of onset and the number of CTG repeats: congenital, infantile, juvenile, adult, and late-onset [7]. The presence and severity of signs and symptoms, as well as their progression, vary greatly not only between but also within the different phenotypes [8]. Previous studies have also shown that the adult and late phenotypes present different profiles of upper- and lower-limb muscle impairments and should then not be pooled together to assess muscle strength [9,10,11]. To the best of our knowledge, these differences in phenotypes have never been examined between adult versus juvenile phenotypes. Sex is another important factor that contributes to the heterogeneity of the disease, as the disease presents itself differently in men and women [10,11,12]. Men tend to present more often with myotonia and severe muscle deficiencies while women tend to present more with cataracts and digestive tract dysfunctions [10].
To counter the impacts of DM1 on muscle deficiencies and physical limitations, the development of any intervention meant to decelerate or stop its progression, such as rehabilitation interventions, is needed. Especially given that physical activity and strength training have been shown to be safe in the DM1 population [13] and that a study on DM1 lifestyle risk factors has shown that 82.3% of respondents exercised less than three times a week, while 75.9% of them wished they did more exercise [14]. Furthermore, it has been shown that starting habitual exercise can have a protective effect on muscle strength [15] and that strength training can result in maximal strength gains in DM1 [16]. To assess the effect of a given intervention on physical deficiencies progression, a thorough understanding of the evolution of the disease is essential. A first transversal study has shown that maximal muscular strength and functional capacities are decreased in DM1 compared to healthy people [17]. It has been reported in this study that people with DM1 have 48.9% of maximal muscle strength of the knee extensors compared to healthy subjects and took more time to ascend and descend stairs [17]. Another transversal study has compared muscle strength loss to the time of duration of the disease [5] and has estimated that maximal strength loss varies between 1.2 and 3.0% a year, depending on the muscle group [5]. However, considering their transversal design, these two studies do not provide information on the actual decline of muscular and functional capacities over time. Our group recently demonstrated that, over a 9-year period, individuals with DM1 presented a significant decline in: (1) maximal muscle strength (24.5–52.8% of depending on the muscle group evaluated) [11] (2) hand strength and dexterity [12] and (3) social participation [18]. In all of these three studies, sex and phenotype (adult vs late-onset) were important explanatory factors in the differences of observed decline [11, 12, 18]. However, from these longitudinal studies, with two-time points 9 years apart, one cannot extrapolate the loss of maximal muscle strength over a shorter period. Another work from a different group studied muscle strength, gait, and balance progression over a 5-year period [19]. A significant decline in four muscle groups was observed after five years and was influenced by sex. However, in this study, muscle strength was measured by performing “break tests” which is known to be less reliable than “make tests” [20, 21] and results were reported in newtons without considering the lever arm length. There is a clinical need to document the progression of muscle impairment over a shorter period to guide clinical conduct and inform therapeutic trial design. The aim of the study was therefore to document the progression of muscle strength and functional capacity over a 3-year period, in the juvenile and adult DM1 population.
Methods
Study setting and participants
This present study is part of a larger ongoing longitudinal study that started in 2002 which has had 4 phases until now (Fig. 1). The present study used the data collected from the phases 3 (P3) and 4 (P4) since outcome measures were exactly the same between these two phases. From the 91 participants recruited at P3, 32 volunteers agreed to undertake a muscular biopsy procedure for the purposes of a study relying on fundamental analyses. The recruitment at P4 was first conducted among this group of participants since larger objectives related to muscle biopsy analyses are pursued with this study. Out of these 32 participants, 29 were still alive in 2019 and were invited to participate in P4. To counter attrition and maintain our longitudinal cohort at 32 participants, other participants were recruited from those who did not agree to a muscle biopsy but completed a clinical assessment at P3 (n = 59), according to the same inclusion/exclusion criteria given below. All participants were recruited from the neuromuscular clinic of the Centre intégré universitaire de santé et de services sociaux (CIUSSS) du Saguenay‒Lac-St-Jean (SLSJ), site Jonquière. The inclusion criteria were (1) to have a genetically confirmed diagnosis of the adult or juvenile phenotype of DM1, (2) to be between 18 and 70 years old and (3) to be able to give informed consent. Exclusion criteria were (1) to have any other neuromuscular disease and, (2) to have any contraindication to a physical evaluation. A written informed consent was obtained from all participants and the project was approved by the committee of ethics of research of the CIUSSS of Saguenay‒Lac-St-Jean.
Procedures
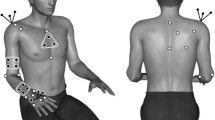
Sociodemographic characteristics were obtained through a general questionnaire (age, sex) or the participant's medical record (phenotype, CTG repeat length in blood). All evaluations (muscle strength assessment, functional evaluation and questionnaires) were done in 2 separate visits at both P3 and P4 to limit fatigue. Participant’s anthropometric measurements were taken at P3 and P4. At P4, participants were asked if they had practiced strength training, other types of physical activity (including physically active job or regular physical activity without doing strength training) or no physical activity in the last 3 years. This question was essential to be able to take into account the interference of the practice of physical activity with the natural progression of the disease considering that some participants (n = 6) have participated in a 12-week supervised strengthtraining program during the 3-year period carried out by our research group [16]. Only the patients that have completed the strengthtraining program have been classified into the strengthtraining category. Maximal isometric muscle strength (MIMS) was evaluated by quantified muscle testing (QMT) for the following muscle groups: prehension, lateral pinch, shoulder flexors, elbow flexors, hip flexors, hip extensors, hip abductors, knee extensors, knee flexors and ankle dorsiflexors. Functional tests were the 6-min Walk Test (6MWT), the Timed Up and Go (TUG), the 10-m Walk Test (10mWT) at comfortable and maximal speed and the Mini-BESTest. The questionnaires were the DM1-Activ, the Upper Extremity Functional Index (UEFI) and the Lower Extremity Functional Scale (LEFS). All evaluations were performed by the same physical therapist (M-P R) at both P3 and P4 except for the DM1-Activ and the UEFI which were administered by a social worker at P3. All tests were chosen for their metrological properties and based on the conclusions of the Outcome Measures in Myotonic Dystrophy type 1 (OMMYD) report [22].
Quantified muscle testing
All MIMS evaluations were done with at least two trials: if the two initial trials had more than 10% difference, another trial was made until there were two trials within 10% (to a maximum of 6 trials per muscle group to limit fatigue). All muscle groups were evaluated on both sides except if the participant had an injury preventing the use of maximal strength. MIMS contractions lasted at least 10 s while the evaluator gave a vigorous standardized encouragement. Prehension strength was evaluated using the Jamar hand dynamometer (JLW instruments, Chicago, USA) with the participant sitting and his elbow at 90-degree flexion, forearm in mid-pronation. Lateral pinch was evaluated in the same position as prehension with the Jamar Plus digital pinch gauge (JLW instruments, Chicago, USA). All other muscle groups were evaluated with the Medup® linear handheld electronic dynamometer (Atlas medic, Québec, Canada). Participants were positioned according to standardized procedures, developed by Hébert et al. [23], to eliminate the effect of gravity, minimize compensatory mechanisms and keep the evaluated muscles at optimal length. The lever arm was measured to report the results in newton meters.
6-min walk test
Walking endurance was assessed by the 6MWT. Before the test, the participants had a mandatory 5-min sitting rest to limit fatigue. Participants were then instructed to walk the most laps they were safely able to, in a 30-m corridor, within 6 min. They were allowed to take standing or sitting rests as needed; however, the stopwatch would not be paused while they rested. For this one-trial test, participants could use their usual walking aids and were asked to wear comfortable walking shoes.
10-m walk test
Comfortable and maximal walking speeds were assessed by the 10mWT. Participants were instructed to walk at a comfortable pace at a 14-m distance. The stopwatch would be started on the 2-m mark and stopped on the 12-m mark. A 2-m acceleration and deceleration zone were used to ensure the accuracy of the measurement. Participants could use their usual walking aids during the test and had to wear comfortable walking shoes. The same procedure was repeated at the participant’s maximal walking speed with the appropriate instructions. Only one trial was performed for both tests [24].
Mini-BESTest
To assess their balance, participants performed the 14 tasks following standardized instructions [25]. Each task was graded from 0 to 2 points, with a total possibility of 28 points with a higher score representing a better performance. Shoes and orthoses were allowed. For walking tasks, participants were allowed to use their walking aid, however, they would automatically lose a point in each specific task the walking aid was used.
Timed Up and Go
The TUG is a test that provides information about balance, gait speed and functional mobility [26]. The TUG was done with 3 trials where the participants had to get up from a chair, walk 3 m and come back to sit on the chair. Participants were allowed to use their usual walking aid for these tests.
Questionnaires
The participants answered all questionnaires with an evaluator who read the questions and provided clarifications if needed. The DM1-Activ is a 20-item questionnaire designed to evaluate activities and participation in individuals with DM1. Its maximal score is 40 points, where a lower score represents a bigger impact of the disease [27]. The UEFI and the LEFS are both 20-item questionnaires designed to evaluate disabilities of the upper and lower limbs, respectively. Their maximal score is 80 points each, where a lower score represents more severe disabilities.
Data analysis
The participant’s age was described as mean, minimum, maximum, and standard deviation at P3 and P4. Other participants’ characteristics (phenotype, sex, type of physical activity, and the number of CTG repetitions at P3) were described as the frequencies and the percentage. All evaluation results are presented as the means with the standard deviation. The results of QMT for each muscle group were calculated from the average of the two closest trials on each side, meaning that the result is an average of 4 measurements. The TUG results were calculated from an average of the 3 trials. A linear mixed model was used to compare measures for all tests and questionnaires between the baseline (P3) and follow-up (P4) for the whole group of participants and for subgroups of participants separated by sex, phenotype and type of physical activity practiced during the 3-year period between the evaluations (strength training, physical activity and sedentary). The same linear mixed model was used to determine the within- and between-participant interactions where the within interaction represents time and the between interaction represents the different subgroups. A significant within-participant interaction indicates that the subgroups progressed at a different rate during the 3 years of the study. A significant between-participant interaction means that the subgroup averages were different. A significant within-participant interaction with the absence of a between-participant interaction, therefore, means the slope of progression between the groups are different but the subgroup averages are not different. Then, significant within and between interactions for the same variable do not allow to discriminate if the effect observed is a true difference in progression or solely a difference between the subgroups. To further the analysis, the baseline results at P3 were compared between subgroups. These results were expressed as the percentage of the means at P3 for one subgroup compared to another. The Mann–Whitney U test for non-parametric unmatched data was used to assess the statistical difference between subgroups at P3. In every analysis, a p value of < 0.05 was considered significant.
Due to a delay in material acquisition, twelve participants did not complete one item of the mini-BESTest (item 8) at P3. Some data were also missing for an item in the UEFI at P3. In these situations, the P4 score was used to impute the missing data to minimize the influence on the results. All data were analyzed using IBM SPSS Statistics for Windows, Version 23 (IBM, North Castle, USA).
Results
Demographics
Of the 91 participants of P3, it was aimed to recruit the 32 who volunteered for the muscle biopsy. From them, 3 participants died, 7 participants did not have juvenile or adult phenotypes and 3 refused to participate in P4 for personal reasons. To counteract attrition at P4, 4 people from those who completed the clinical assessment only at P3 (n = 59) were recruited, for a total of 23 participants (Fig. 2). Patient’s characteristics are given in Table 1.
Functional assessment and questionnaires
All results from the functional assessments and questionnaires can be found in Table 2. For the whole group, there was a significant decrease in the score of the Mini-BESTest and the LEFS over the 3-year period. These significant differences were present in the subgroup of men, but not in women. The 6MWT, Mini-BESTest and LEFS scores decreased significantly in the adult phenotype subgroup. In the juvenile phenotype and strength training subgroups, only the LEFS score has decreased significantly. In the sedentary subgroup, participants significantly decreased in their Mini-BESTest and LEFS scores, but they also presented a significant decrease in the 6MWT and TUG tests. In the subgroup analysis, significant within interactions without significant between interactions were only observed for LEFS (sex, phenotype, strength training vs physical activity and physical activity vs sedentary subgroups) and Mini-BESTest scores (phenotype and strength training vs physical activity subgroups).
Maximal muscle strength
Maximal muscle strength results can be found in Table 3. For the whole group, there was a significant decrease in prehension, lateral pinch, hip abduction, knee extension, and ankle dorsiflexion. In the women subgroup, there was a significant decline in two muscle groups while for men, the decline was significant in seven muscle groups. In the adult phenotype subgroup, there was a significant decline for five muscle groups while for the juvenile phenotype subgroup three muscle groups had decreased significantly. In the strength training group, there was a significant decline in four muscle groups, the physical activity group had a significant decline in four different muscle groups and the sedentary group had no significant MIMS decline. The strength training subgroup had more significant loss in upper limb muscle groups (three out of four) while the physical activity subgroup had more significant losses in the lower limb (three out of four). In the subgroup analysis, significant within interactions without significant between interactions were observed for prehension, hip abduction, knee extension and ankle dorsiflexion for phenotype and physical activity vs sedentary subgroups, and lateral pinch for all subgroups except strength training vs sedentary.
Baseline comparisons
Results for baseline comparisons can be found in Table 4 for functional assessments and questionnaires and in Table 5 for maximal muscle strength. At P3, men performed significantly better than women for almost every evaluation except the TUG, DM1-Activ, UEFI, and lateral pinch. The juvenile and adult phenotypes had no significant differences at baseline. Physical activity and strength training were only significantly different for the 10mWT at maximal speed for functional tests (strength training subgroup walked faster than physical activity subgroup) while they were significantly different for maximal strength in every muscle group (strength training group was stronger) except for prehension and lateral pinch. Sedentary and strength training were significantly different in almost every evaluation (strength training subgroup was more functional and stronger) except DM1-Activ, UEFI, and prehension. Finally, the sedentary and physical activity subgroups were only different for knee extension muscle strength evaluation at P3.
Discussion
This study is the first to assess DM1 disease progression on muscle strength and functional capacities over a 3-year period by taking sex, phenotype, and the practice of physical activity into consideration. This study reinforced the concept that DM1 individuals should not be pooled together by showing that: (1) evolution of the disease is different between juvenile and adult phenotypes, (2) sex influences disease progression [11, 19] and (3) physical activity type can have a protective effect on the loss of functional capacities such as walking endurance and balance.
Among the whole group, Mini-BESTest, was the only functional test which showed significant decline over the 3-year period and this change was above the standard error of the measurement (SEM) of 1.26 points measured in adults with balance impairments [28]. The Mini-BESTest has been shown to be valid in DM1 population [29] and our results showed that the impact of the disease progression on balance can be captured by this test on a 3-year period as the change in three years is below the standard error of the measure. However, more studies are needed to confirm if this effect has not been driven only by one of the subcategories evaluated that also had a significant change (men, adult phenotype and/or sedentary). Clinically speaking, this result is highly important since individuals with DM1 stumble or fall up to 10 times more often than healthy volunteers [30, 31]. Furthermore, since balance requires many complex systems, such as sensory orientation and motor control, the decline in performance in the Mini-BESTest could be explained by other factors than the observed MIMS losses in this study [29]. The TUG, the 6MWT and the 10mWT tests do not seem sufficiently sensitive to measure a significant difference on a 3-year period in the whole group. The decline of the TUG was only significant for the sedentary subgroup. The subjective dimension associated with the standardized instructions asked the participants to walk at a self-selected speed might have influenced the results by affecting participant motivation and subsequently increase intra-individual variability [32]. This element is especially important in DM1, where 40% of the population presents apathy [33]. Even if not statistically significant, the increase in time to complete the TUG (P4 vs P3) exceeds the SEM calculated in a DM1 population of 0.7 s [34] for women and adult subgroups. Despite that most of the results for the 6MWT were not significant, a significant decline was seen for the adult and sedentary subgroups. Also, the difference between P3 and P4 for men was superior to the SEM established in the elderly (22 m) [35], a population similar to DM1, which is described as a model of premature aging [36]. More importantly, while all 23 participants were able to complete the 6MWT at P3, four of them were unable or refused (due to perceived difficulty) to complete the test at P4. These four participants have thus significantly lost walking capabilities but had not been included in the results. Furthermore, three of the four who did not complete the 6MWT at P4 were juvenile, which might explain why the difference in the 6MWT is not significantly different between P3 and P4 for the juvenile phenotype. Nevertheless, we can hypothesize that strength training and physical activity can have a protective effect. This might explain why only sedentary participants showed a significant decline of the 6MWT result. Likewise, the difference of time needed to execute the 10mWT comfortably between P3 and P4 is superior to the SEM, which is of 0.6 s for a DM1 population [34], for the whole group, women, adult and juvenile phenotypes as well as sedentary subgroups (10mWT data in seconds not shown). This is also true for the 10mWT at a maximum speed, where the SEM is of 0.4 s for a DM1 population [34], for the whole group, men, women, adult phenotype, juvenile phenotype, physically active and sedentary subgroups. This shows a certain walking speed decline over time, even though it was not statistically significant.
The absence of change in the DM1-Activ and the UEFI scores could be explained by the slowly progressive nature of DM1 as well as compensatory mechanisms developed by participants overtime to counter the observed maximal strength loss. The LEFS was the only questionnaire that showed a significant decline for the whole group and many subgroups. Conceivably, perceived changes may be more evident when they involve lower limbs in the DM1 population. Interestingly, there was a significant change for the strength training subgroup although they were the group that declined the least in general for functional capacities. Perhaps strength training allows for better awareness of their physical capabilities. Overall, the sedentary subgroup has presented the worst functional portrait, which suggests that strength training and physical activity have a positive impact on the preservation of function in DM1 over time.
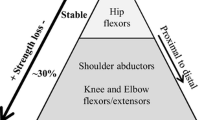
Regarding the upper limb muscle strength, distal muscle groups lost more strength than proximal muscle groups during the 3 years between the evaluations. This agrees with previously reported patterns of distal to proximal strength loss [11, 12]. When comparing progression between men and women, the former significantly lost MIMS in all upper limb muscle groups while the latter only had significant loss in the lateral pinch grip. Greater MIMS loss in men compared to women has also been observed over a 9-year period [12]. In the phenotype subgroup analysis, the only significant MIMS loss in the juvenile phenotype was lateral pinch, while both lateral pinch and prehension were significantly declined in the adult subgroup. More progression of MIMS loss for the adult phenotype may seem surprising, however, it is to be noted that on average, the juvenile subgroup was 9 years younger. The duration of the disease could therefore be another hypothesis that explains differences in the progression of the disease. When comparing upper limb strength between physical activity types, it is surprising to note that the strength training subgroup has more significant MIMS losses than the two other subgroups. It is important to note that all 6 participants in the strength training subgroup participated in a program that focused on lower limb strength only, which could explain a lack of protective effects in upper limb muscle groups. Moreover, all participants in the strength training subgroup were men, which could be another confounding factor that explains their greater loss in maximal strength. Furthermore, we hypothesize that in DM1, stronger muscles tend to lose more. The strength training subgroup was indeed significantly stronger for the shoulder and elbow flexors at P3. Although the difference was not significant for prehension and lateral pinch, the maximal strength of the physical activity and sedentary subgroups ranged between 48.6 and 75.2% of the strength training subgroup. Thus, a floor effect could be seen in weaker muscle groups. This would also explain why the sedentary subgroup, which had the lowest strength scores at P3, showed no significant change over time in upper limb MIMS.
For lower limb muscle strength, the proximal/distal maximal strength loss pattern was not so clear. As it may seem surprising that the juvenile phenotype had no significant loss in ankle dorsiflexion, while the adult phenotype did significantly lose strength, as with the upper limb, the difference in age between the subgroups should be noted once again. As with the upper limb, the sedentary subgroup also showed no significant MIMS decline. However, when considering the percentage of strength loss, even if the results were not statistically significant, the sedentary subgroup has almost always lost more than the other two subgroups. The absence of statistically significant results in the sedentary subgroup could therefore be explained by a decreased statistical power of this study due to a limited number of participants. Furthermore, even if they were significantly stronger at P3, the strength training subgroup only significantly lost strength in hip abduction and had a lower relative loss than the two other groups. This is consistent with the hypothesis of a protective effect of strength training as the program in which the subjects participated focused on lower limb muscle strength, namely on the knee extensors [16]. These participants still lost MIMS in their lower limbs, probably due to the limited time they were training (12-week training program over a 3-year period) [16]. However, as they were only men, more studies are needed to be able to generalize these conclusions to the whole DM1 population. Interestingly, there was a significant increase of MIMS for the knee flexors for the physical activity subgroup. Although strength gains are surprising, this may be due to a coping mechanism to compensate for strength loss in other muscle groups.
Subgroup analysis for within and between interactions brings further insight into the progression of deficiencies in this cohort of DM1 subjects. In the sex subgroup analysis, the majority of between interactions were significant, indicating that women performed significantly differently than men, which hinders our capacity to differentiate if there was a true difference in progression. This was confirmed in the baseline comparisons, where men performed significantly better than women in almost every evaluation. It is therefore difficult to distinguish if greater capacity allows for more loss or if there is a true difference in progression due to sex. LEFS and lateral pinch were the only dependent variables where the within-participant was significant without a significant between-participant interaction for sex subgroups and therefore, we can conclude of a true influence of sex in the progression of these variables. Subgroup analysis for phenotype showed that none of the between interactions are statistically significant, showing that adult and juvenile phenotypes were not different in every test and evaluation performed. This was confirmed in baseline comparisons were the adult and juvenile phenotypes had no significant differences at P3. However, many within interactions were significant, showing a difference in progression for the mini-BESTest, the LEFS and MIMS in prehension, lateral pinch, hip abduction, knee extension and ankle dorsiflexion. This reinforces that juvenile and adult phenotypes should not be pooled together to assess the progression of the disease, bringing further weight to the 5-category classification presented by De Antonio et al. [7]. In the physical activity type subgroup analysis, only the mini-BESTest had a significant within interaction in the functional tests. The significant within interaction was probably driven by the sedentary subgroup, which was the only one with a significant difference between P3 and P4, showing the protective effect of physical activity and strength training on balance. The LEFS score showed the only significant within interaction among the questionnaires. Interestingly, only the strength training and sedentary subgroups had a significant between interaction, showing that the physical activity subgroup progressed differently than other two subgroups, where physical activity had no significant change in their LEFS score between P3 and P4. An interesting trend shows that none of the between interactions for physical activity and sedentary subgroups were significant for every MIMS test. Their muscle strength scores were also statistically similar at P3 for every muscle group except the knee extensors. Every other between interaction, except for strength training and physical activity for lateral pinch, were significant. This may initially seem surprising, since the physical activity subgroup had significant changes between P3 and P4 in three different muscle groups while there were no significant changes in the sedentary subgroup. Some caution needs to be taken when interpreting this data as there are a very low number of subjects in each subgroup. Although it was not significant, the sedentary subgroup lost between 3.8 and 23.2% MIMS (except for the shoulder flexors). More subjects would be needed in this subgroup to better understand the effect of a sedentary lifestyle in DM1. Further caution should be taken as general physical activity was self-reported and not a controlled intervention as with the strength training subgroup. For lateral pinch, when compared to the strength training subgroup, where the between interaction with the physical activity subgroup was not significant, it was the physical activity subgroup that progressed the most in three years. It is to be noted that physical activity and strength training did not specifically train the muscles involved in lateral pinch and therefore the subgroup categories may have had no influence on this MIMS progression.
The present study has some limitations: the small sample size may not represent the whole DM1 population, more participants would decrease the risk of type II error. Furthermore, the decrease of statistical power of this study limits further interaction analysis, such as phenotype X sex. This could have provided better insights to factors influencing the progression of the disease. Another limitation is that some participants were unable to perform some of the tests at T4, due to loss in functional capacities and/or muscle strength. This data was therefore not available for analysis, probably eliminating the greatest decline rates for these tests. Also, for a more comprehensive clinical evaluation, the use of a quality of life questionnaire specific for neuromuscular diseases that captures physical limitations specifically relevant to the muscle condition would have been interesting. Lastly, even if all evaluations were done in 2 separate visits, the effect of fatigue per se on motor performance has not been assessed [38]. However, the order of assessment was standardized and designed to minimize fatigue.
Conclusion
In conclusion, the aim of the study was to document the progression of muscular strength and functional capacities on a 3-year period in the adult and juvenile DM1 population. For the whole group, the Mini-BESTest is the only functional test that showed a significant decline, despite the significant loss of strength observed in many muscle groups. Overall, quantified MIMS evaluations are a better disease progression indicator in DM1 than functional tests. A better understanding of the disease progression is essential to measure the impact of any intervention that aims to reduce functional and strength decline. It also enables professionals to provide evidence-based prognostics to patients and their families. Thereby, with a better understanding of the progression, clinicians would be able to use evidence-based evaluation and interventions for a better management of signs and symptoms in DM1.
Availability of data and materials
Data can be available upon reasonable requests to the corresponding author.
References
Harper P (2009) Myotonic dystrophy. Oxford University Press, New York
Bird TD (1999) Myotonic dystrophy type 1. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH et al (eds) GeneReviews(R). University of Washington, Seattle
Bouchard G, Roy R, Declos M, Mathieu J, Kouladjian K (1989) Origin and diffusion of the myotonic dystrophy gene in the Saguenay Region Quebec. Can J Neurol Sci 16:119–122. https://doi.org/10.1017/s0317167100028651
Fu YH, Pizzuti A, Fenwick RG Jr, King J, Rajnarayan S, Dunne PW et al (1992) An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 255:1256–1258
Mathieu J, Boivin H, Richards CL (2003) Quantitative motor assessment in myotonic dystrophy. Can J Neurol Sci 30:129–136
Petitclerc E, Hebert LJ, Mathieu J, Desrosiers J, Gagnon C (2018) Relationships between lower limb muscle strength impairments and physical limitations in DM1. J Neuromuscul Dis 5:215–224. https://doi.org/10.3233/jnd-170291
De Antonio M, Dogan C, Hamroun D, Mati M, Zerrouki S, Eymard B et al (2016) Unravelling the myotonic dystrophy type 1 clinical spectrum: a systematic registry-based study with implications for disease classification. Rev Neurol (Paris) 172:572–580. https://doi.org/10.1016/j.neurol.2016.08.003
Turner C, Hilton-Jones D (2010) The myotonic dystrophies: diagnosis and management. J Neurol Neurosurg Psychiatry 81:358–367. https://doi.org/10.1136/jnnp.2008.158261
Petitclerc E, Hebert LJ, Mathieu J, Desrosiers J, Gagnon C (2017) Lower limb muscle strength impairment in late-onset and adult myotonic dystrophy type 1 phenotypes. Muscle Nerve 56:57–63. https://doi.org/10.1002/mus.25451
Dogan C, De Antonio M, Hamroun D, Varet H, Fabbro M, Rougier F et al (2016) Gender as a modifying factor influencing myotonic dystrophy type 1 phenotype severity and mortality: a nationwide multiple databases cross-sectional observational study. PLoS ONE 11:e0148264. https://doi.org/10.1371/journal.pone.0148264
Gagnon C, Petitclerc E, Kierkegaard M, Mathieu J, Duchesne E, Hebert LJ (2018) A 9-year follow-up study of quantitative muscle strength changes in myotonic dystrophy type 1. J Neurol. https://doi.org/10.1007/s00415-018-8898-4
Raymond K, Levasseur M, Mathieu J, Desrosiers J, Gagnon C (2017) A 9-year follow-up study of the natural progression of upper limb performance in myotonic dystrophy type 1: a similar decline for phenotypes but not for gender. Neuromuscul Disord 27:673–682. https://doi.org/10.1016/j.nmd.2017.04.007
Voet NB, van der Kooi EL, Riphagen II, Lindeman E, van Engelen BG, Geurts AC (2013) Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003907.pub2
Gagnon C, Chouinard MC, Laberge L, Brisson D, Gaudet D, Lavoie M et al (2013) Prevalence of lifestyle risk factors in myotonic dystrophy type 1. Can J Neurol Sci 40:42–47
Brady LI, MacNeil LG, Tarnopolsky MA (2014) Impact of habitual exercise on the strength of individuals with myotonic dystrophy type 1. Am J Phys Med Rehabil 93:739–750. https://doi.org/10.1097/PHM.0000000000000088
Roussel M-P, Hebert LJ, Duchesne E (2020) Strength-training effectively alleviates skeletal muscle impairments in myotonic dystrophy type 1. Neuromuscul Disord 30:283–293. https://doi.org/10.1016/j.nmd.2020.02.015
Lindeman E, Leffers P, Reulen J, Spaans F, Drukker J (1998) Quadriceps strength and timed motor performances in myotonic dystrophy, Charcot–Marie–Tooth disease, and healthy subjects. Clin Rehabil 12:127–135
Raymond K, Levasseur M, Mathieu J, Gagnon C (2019) Progressive decline in daily and social activities: a 9-year longitudinal study of participation in myotonic dystrophy type 1. Arch Phys Med Rehabil 100:1629–1639. https://doi.org/10.1016/j.apmr.2019.01.022
Hammaren E, Kjellby-Wendt G, Lindberg C (2015) Muscle force, balance and falls in muscular impaired individuals with myotonic dystrophy type 1: a five-year prospective cohort study. Neuromuscul Disord 25:141–148. https://doi.org/10.1016/j.nmd.2014.11.004
Bohannon RW (1999) Intertester reliability of hand-held dynamometry: a concise summary of published research. Percept Mot Skills 88:899–902
Seagraves FE, Horvat M (1995) Comparison of isometric test procedures to assess muscular strength in elementary school girls. Pediatr Exerc Sci 7:61–68
Gagnon C, Meola G, Hebert LJ, Laberge L, Leone M, Heatwole C (2015) Report of the second outcome measures in myotonic dystrophy type 1 (OMMYD-2) international workshop San Sebastian, Spain, October 16, 2013. Neuromuscul Disord 25:603–616. https://doi.org/10.1016/j.nmd.2015.01.008
Hebert LJ, Maltais DB, Lepage C, Saulnier J, Crete M, Perron M (2011) Isometric muscle strength in youth assessed by hand-held dynamometry: a feasibility, reliability, and validity study. Pediatr Phys Ther 23:289–299. https://doi.org/10.1097/PEP.0b013e318227ccff
Gagnon C, Heatwole C, Hebert LJ, Hogrel JY, Laberge L, Leone M et al (2018) Report of the third outcome measures in myotonic dystrophy type 1 (OMMYD-3) international workshop Paris, France, June 8, 2015. J Neuromuscul Dis 5:523–537. https://doi.org/10.3233/JND-180329
Franchignoni F, Horak F, Godi M, Nardone A, Giordano A (2010) Using psychometric techniques to improve the balance evaluation systems test: the mini-BESTest. J Rehabil Med 42:323–331. https://doi.org/10.2340/16501977-0537
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148. https://doi.org/10.1111/j.1532-5415.1991.tb01616.x
Hermans MC, Faber CG, De Baets MH, de Die-Smulders CE, Merkies IS (2010) Rasch-built myotonic dystrophy type 1 activity and participation scale (DM1-Activ). Neuromuscul Disord 20:310–318. https://doi.org/10.1016/j.nmd.2010.03.010
Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A (2013) Comparison of reliability, validity, and responsiveness of the mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther 93:158–167. https://doi.org/10.2522/ptj.20120171
Duchesne E, Hebert LJ, Mathieu J, Cote I, Roussel MP, Gagnon C (2020) Validity of the Mini-BESTest in adults with myotonic dystrophy type 1. Muscle Nerve. https://doi.org/10.1002/mus.26893
Wiles CM, Busse ME, Sampson CM, Rogers MT, Fenton-May J, van Deursen R (2006) Falls and stumbles in myotonic dystrophy. J Neurol Neurosurg Psychiatry 77:393–396. https://doi.org/10.1136/jnnp.2005.066258
Berends J, Tieleman AA, Horlings CGC, Smulders FHP, Voermans NC, van Engelen BGM et al (2019) High incidence of falls in patients with myotonic dystrophy type 1 and 2: a prospective study. Neuromuscul Disord 29:758–765. https://doi.org/10.1016/j.nmd.2019.08.012
Kierkegaard M, Petitclerc E, Hebert LJ, Mathieu J, Gagnon C (2018) Responsiveness of performance-based outcome measures for mobility, balance, muscle strength and manual dexterity in adults with myotonic dystrophy type 1. J Rehabil Med 50:269–277. https://doi.org/10.2340/16501977-2304
Gallais B, Montreuil M, Gargiulo M, Eymard B, Gagnon C, Laberge L (2015) Prevalence and correlates of apathy in myotonic dystrophy type 1. BMC Neurol 15:148. https://doi.org/10.1186/s12883-015-0401-6
Hammaren E, Ohlsson JA, Lindberg C, Kjellby-Wendt G (2012) Reliability of static and dynamic balance tests in subjects with myotonic dystrophy type 1. Adv Physiother 14:48–54
Perera S, Mody SH, Woodman RC, Studenski SA (2006) Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 54:743–749
Mateos-Aierdi AJ, Goicoechea M, Aiastui A, Fernandez-Torron R, Garcia-Puga M, Matheu A et al (2015) Muscle wasting in myotonic dystrophies: a model of premature aging. Front Aging Neurosci 7:125. https://doi.org/10.3389/fnagi.2015.00125
Hebert LJ, Vial C, Hogrel JY, Puymirat J (2018) Ankle strength impairments in myotonic dystrophy type 1: a five-year follow-up. J Neuromuscul Dis. https://doi.org/10.3233/JND-180311
Baldanzi S, Ricci G, Bottari M, Chico L, Simoncini C, Siciliano G (2017) The proposal of a clinical protocol to assess central and peripheral fatigue in myotonic dystrophy type 1. Arch Ital Biol 155:43–53. https://doi.org/10.12871/000398292017125
Acknowledgements
Firstly, we would like to thank all the participants of this study. We would also like to thank the nurses from the neuromuscular disease clinic of Jonquière, Saguenay-Lac-St-Jean with Dr Jean Mathieu for their work in precisely phenotyping all the participants. We also want to thank Isabelle Côté, M.Sc. and Hélène Simard for their respective work in project coordination and recruitment of participants. Finally, we want to thank Djamal Berbiche, Ph.D. (Senior Statistician) for his support in the statistical analyses.
Funding
This study was funded by the Programme de soutien au développement de la mission universitaire: Enseignement et recherche du CIUSSS-SLSJ and the Marigold Foundation. CG holds a career—grant funding from Fonds de recherche du Québec—santé (no 31011). M-PR holds a Ph.D. study grant from Fonds de recherche du Québec-santé.
Author information
Authors and Affiliations
Contributions
MPR contributed to the design conception of the study, collected and analyzed the data and has written the manuscript. MMF, LG, CL, EMN and LP contributed to data analysis and the writing of the manuscript. CG and ED contributed to the design conception of the study and data analysis as well as revised the manuscript.
Corresponding author
Ethics declarations
Ethical standards
This study was approved by the committee of ethics of research of the CIUSSS of Saguenay—Lac-St-Jean.
Informed consent
All participants give their written informed consent before the beginning of the study.
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Roussel, MP., Fiset, MM., Gauthier, L. et al. Assessment of muscular strength and functional capacity in the juvenile and adult myotonic dystrophy type 1 population: a 3-year follow-up study. J Neurol 268, 4221–4237 (2021). https://doi.org/10.1007/s00415-021-10533-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10533-6