Abstract
Background
Symptomatic isolated carotid artery occlusions (ICAO) can lead to disability, recurrent stroke, and mortality, but natural history and best therapeutic management remain poorly known. The objective of this study was to describe our cohort of ICAO patients with an initial medical management.
Methods
We conducted a retrospective study including consecutive patients admitted to our Comprehensive Stroke Center for ICAO within 24 h after stroke onset between January 2016 and September 2018. Patients with immediate endovascular therapy (EVT) were excluded. Medical treatment was based on anticoagulation (delayed by 24 h if intravenous thrombolysis was performed). ‘Rescue’ EVT was considered if first-week neurological deterioration (FWND) occurred.
Results
Fifty-six patients were included, with a median National Institutes of Health Stroke Scale (NIHSS) of 3. Eleven patients (20%) had FWND during the first week, four benefited from rescue EVT. A mismatch volume > 40 cc on initial perfusion imaging and FLAIR vascular hyperintensities were associated with FWND (p = 0.007 and p = 0.009, respectively). Thirty-eight patients (69%) had a good outcome (modified Rankin Scale mRS 0–2) at 3 months, 36 (69%) had an excellent outcome (mRS 0–1). Seventeen patients (38%) had carotid patency on 3-month control imaging. Recurrences occurred in six (13%) of the survivors (mean follow-up: 13.6 months).
Conclusion
Our results suggest that the prognosis of patients with acute ICAO was favorable with a medical strategy, albeit a substantial rate of FWND and recurrence. FWND was well predicted by a core-perfusion mismatch volume > 40 cc. Randomized controlled trials are necessary to assess the benefit of EVT in ICAO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Internal carotid artery occlusions account for 6–15% of all acute ischemic stroke (AIS) and 15–25% of AIS in the carotid territory [1, 2], with atherosclerosis as the leading cause, followed by dissection and cardio-embolic sources [3]. AIS related to carotid occlusion are usually severe at presentation [4], with a high risk of recurrent in-hospital AIS, early deaths and poor functional outcome at 3 months [1,2,3]. In addition, the annual risk for recurrent stroke is about 6–10%, and 9.5% for death [1, 2, 5,6,7,8].
There are few data on isolated carotid artery occlusion (ICAO) (without associated occlusion of the circle of Willis, i.e. without T-shaped, L-shaped or tandem occlusions), as the diagnosis of carotid occlusion was mainly performed by cervical Doppler ultrasound, with missing data on the existence of associated intracranial occlusion. The clinical presentation of ICAO is highly variable (asymptomatic, transient ischemic attack (TIA), minor or severe stroke, chronic ocular ischemia) [9], and its prognosis is poorly known. Moreover, optimal treatment of acute ICAO is unknown.
Intravenous thrombolysis (IVT) has been described as poorly effective in patients with carotid occlusion [4, 10, 11] but the recanalization rate and clinical outcome could be higher in ICAO [12]. Since 2015, numerous randomized controlled trials (RCT) have demonstrated the superiority of mechanical thrombectomy (MT) compared to best medical treatment in patients with AIS and intracranial occlusion in the anterior circulation [13], but there was little data in ICAO, being an exclusion criteria of the majority of those RCT. Few observational studies have compared MT to IVT in patients with ICAO, with inconclusive results [14, 15]; a recent study suggested that immediate endovascular treatment (EVT) in patients with initial clinico-imaging mismatch (CIM) could lead to a high rate of recanalization and good outcome [16].
The best strategy during the subacute and chronic phases to prevent early neurologic deterioration and recurrences is also unknown. Some studies evaluated the interest of antithrombotic agents with inconclusive results. A RCT including 229 patients with severe cervical carotid stenosis or occlusion suggested the superiority of danaparoid versus placebo [17] on the outcome at 7 days and 3 months, except in the subgroup with carotid occlusion. In a recent observational study including 33 patients with symptomatic ICAO treated by antiplatelets or anticoagulation, early anticoagulation compared to antiplatelets was associated with less recurrent strokes within 7 days (6.7% versus 38.9%) and more carotid recanalization at 1 year (46.2% versus 9.1%) [18]. Intra–extra-cranial bypass has failed to show benefit for the prevention of recurrent strokes in two large RCTs [19, 20]. Similarly, no study to date has proven the efficacy of EVT in the prevention of recurrences in ICAO.
The objectives of this cohort study were to describe the clinical presentation, imaging features and outcome in patients with recent AIS due to ICAO admitted to a Comprehensive Stroke Center (CSC) and treated with an initial medical strategy, and to assess the factors associated with first-week neurological deterioration (FWND) and recurrences in the acute and subacute phases.
Methods
Study population
We carried out a retrospective study with data extraction from our prospective clinical registry of consecutive patients admitted for AIS to our CSC between January 2016 and September 2018. Patients with symptomatic ICAO were whether initially admitted to the CSC or transferred from five primary stroke centers of the Occitanie-Est area without neuro-interventional radiologists.
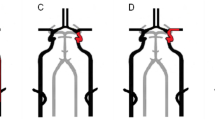
Consecutive patients were included if they fulfilled the following criteria: (i) AIS (hemispheric or retinal TIA, cerebral infarct) in the anterior circulation with symptom onset < 24 h before admission, (ii) ipsilateral ICAO without associated ipsilateral intracranial occlusion (T- and L- shaped occlusions, M1–M2 portions of the middle cerebral artery (MCA), A1–A2 portions of the anterior cerebral artery, P1–P2 portions in case of fetal posterior cerebral artery), confirmed on initial non-invasive vascular imaging [computed tomography angiography (CTA) or contrast-enhanced magnetic resonance angiography (MRA)]. Types of carotid occlusions are represented in Fig. 1.
Carotid occlusions. This figure shows the different types of carotid occlusions, with thrombus being represented in red. In this study, only patients with isolated carotid occlusions represented in a (short occlusive lesion), b (I occlusion) and c (long occlusive lesion, i.e. cervical–intracranial occlusion) were included [34]. Patients with L occlusion (d) and T occlusion (e) were excluded from this study, because of the existence of associated occlusion of the circle of Willis
Exclusion criteria were: (i) immediate EVT (MT and/or carotid stenting) at admission, due to CIM, defined as a severe neurologic deficit not explained by the size and/or the location of the recent cerebral infarct, (ii) ICAO previously documented, (iii) severe stenosis (≥ 50%) of M1-MCA ipsilateral to ICAO, (iv) associated intracranial large vessel occlusion in another territory (e.g. contralateral M1 or M2 segments, basilar artery).
Patient management
Immediate EVT (MT and/or carotid stenting) was systematically discussed on admission between the stroke neurologist and the neuro-interventional radiologist in case of CIM. Patients treated with immediate EVT were excluded.
Medical strategy during the acute phase was based on: (i) hospitalization in the CSC for at least 48 h for supine position, maintenance of hemodynamic function (objective of systolic blood pressure > 120 mmHg), and close monitoring of any neurological worsening, with repeated NIHSS; (ii) IVT in symptomatic patients before 4.5 h of stroke onset (or with a FLAIR-negative MRI in case of unknown time of symptoms onset). Among the patients transferred, IVT was given before or during the transfer to the CSC, (iii) anticoagulants in the absence of contraindication (large cerebral infarct, hemorrhagic transformation, other general bleeding risk) by low molecular weight or unfractionated heparin, associated with aspirin in atheromatous occlusion. This antithrombotic treatment was delayed by 24 h for patients treated with IVT. This medical strategy is resumed in Supplementary Figure.
Rescue EVT was discussed during the hospitalization in the CSC between the neurologist and the neuro-interventional radiologist in case of first-week neurologic deterioration (FWND), defined by an increase of NIHSS ≥ 4 points [21] or the apparition of relevant symptoms for asymptomatic patients at admission, despite best medical treatment during the first week.
Treatment during the subacute phase
In case of carotid revascularization under medical treatment with underlying severe atheromatous carotid stenosis, carotid stenting or endarterectomy was performed for secondary prevention. If ICAO persisted under anticoagulation, this treatment was maintained during the subacute phase for 4–6 weeks after hospital discharge (until follow-up imaging), by low molecular weight heparin or vitamin K antagonist. Patients were then treated for long-term secondary prevention depending on the cause of the AIS (anticoagulants or antiplatelets), following current guidelines [22].
Follow-up
Clinical Patients had repeated neurological examinations with NIHSS during the first 72 h and at discharge, and a follow-up visit at 3 months with a stroke neurologist to assess the mRS and recurrences.
ICAO CTA or MRA was systematically performed at 24 h after admission in patients treated by IVT, and within the five first days for others; most patients had additional follow-up arterial imaging (ultrasound, MRA, CTA) during hospitalization. Patients also had a follow-up carotid imaging at 1 to 3 months to evaluate carotid patency.
Data collection
Patient characteristics, imaging data, treatment, and follow-up were prospectively collected at the comprehensive stroke center (CSC) by the stroke neurologist:
-
1.
Clinical: age, sex, vascular risk factors (hypertension, diabetes, hypercholesterolemia, current smoking), history of ipsilateral acute ischemic stroke (TIA), cerebral infarct), history of atrial fibrillation, current medication, admission blood pressure, time delay from symptom onset or last seen well to admission to the CSC, initial clinical fluctuation before admission (defined as multiple TIAs or a National Institutes of Health Stroke Scale (NIHSS) increase ≥ 4 points between 2 evaluations); NIHSS at admission
-
2.
Imaging data: type of initial cerebral and arterial imaging, delay from symptoms onset to imaging, The Alberta Stroke Program Early CT Score (ASPECTS) on diffusion weighted-magnetic resonance imaging or computed tomography (CT), cerebral infarct (arterial territory, watershed infarct, contralateral stroke), localization of isolated carotid artery occlusion (ICAO), anatomy of the circle of Willis (existence of anterior and posterior communicating arteries), stenosis (≥ 50%) or occlusion of the contralateral internal carotid artery, FLAIR vascular hyperintensities (FVH) homolateral to ICAO;
-
3.
Perfusion data when available: MRI or CT imaging were analysed with RAPID® (iSchemaView Inc, Menlo Park, CA) or OLEA® (Olea Medical, Canon Medical Systems Corporation) softwares, which automatically generated hypoperfusion volumes (area in which Tmax > 6 s), necrosis volumes (area in which Apparent Diffusion Coefficient < 620 × 10−6 mm2 on MRI or CBF < 30% on CT scan) and mismatch volumes. A core-hypoperfusion mismatch was defined by a mismatch volume > 40 mL [23].
-
4.
Treatment: initial treatment (intravenous thrombolysis, anticoagulation, antiplatelet, abstention), rescue endovascular treatment and antithrombotic treatment during the follow-up;
-
5.
Follow-up during hospitalization: clinical (early neurological deterioration, NIHSS score at 24 h, complications of treatments) and imaging between 24 h and Day 5 (ASPECTS, carotid patency, hemorrhagic transformation according to European Cooperative Acute Stroke Study (ECASS) classification [24], and after hospitalization: occurrence of ischemic recurrences (TIA or AIS), carotid patency, and 3-month mRS.
-
6.
Etiology of ICAO was defined, according to the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) classification [25], as atheromatous, cardio-embolic, dissection, other cause (radiation-induced vascular disease, hemopathy, multiple definite causes), and as undetermined when no cause was found despite exhaustive exploration.
Outcome evaluation
Primary endpoint was functional outcome at 3 months, assessed by mRS. Outcome was defined as favorable for mRS ≤ 2, excellent for mRS ≤ 1. Secondary endpoints were treatment complications, FWND, ICA patency rate on follow-up imaging, and recurrences.
Statistical analysis
Continuous variables are presented as mean ± SD or median (IQR), and categorical variables are presented as frequency and percent. Univariate analysis was performed using the Wilcoxon rank-sum test for continuous variables, and the Fisher’s exact test for categorical variables.
The probabilities of favorable and excellent prognosis at 3 months were modeled by multivariate logistic regression. Variables were selected for multivariate analysis if their univariate p value was < 0.20, and the final model was built using a backward selection method (Supplementary Table). All tests were considered significant for a bilateral α < 0.05, and data were analyzed using SPSS Statistics Version 25 (IBM, Armonk, NY, USA) and SAS9.4.
Results
Baseline characteristics
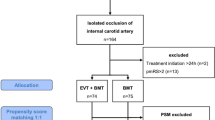
From January 2016 to September 2018, among 1378 patients admitted to our CSC for acute AIS and large vessel occlusion, 308 had extracranial carotid occlusion, of whom 76 patients presented with ICAO (Fig. 2). Nine patients (12%) were excluded because of a delay between symptom onset and admission > 24 h. Eleven (14%) patients were excluded because of immediate EVT due to a CIM [mean age 71 ± 11 years, 46% females, median initial NIHSS of 16 (13)]. Four other patients (7%) had a CIM at admission but did not benefit from immediate EVT due to a rapid spontaneous improvement of the NIHSS score (n = 2), a pre-stroke mRS at 4 (n = 1) and a medical decision (n = 1). These patients were included in this study.
Overall, 56 patients were included. Baseline characteristics are shown in Table 1. Mean age was 63 years, 13 patients (23%) were female. Median initial NIHSS score was 3 (IQR 0–6).
Forty-four (79%) patients had an acute cerebral infarct, of whom 31 (70%) had watershed infarcts. Among the 51 patients (91%) with an initial cerebral MRI, 32 (63%) had FLAIR vascular hyperintensities (FVH) in the carotid territory. Among the 21 patients (38%) who had perfusion imaging, 12 (57%) had a core-hypoperfusion mismatch > 40 cc. All these patients with mismatch > 40 cc also had FVH, while five out of nine patients without mismatch > 40 cc had FVH (with core-hypoperfusion mismatch volumes, respectively, of 3, 19, 30, 33, and 35 mL).
Initial management
Among the 17 patients admitted within 4 h 30 min after known stroke onset or unknown-onset strokes with absence of a lesion on FLAIR-imaging, six were treated with IVT followed by anticoagulation (n = 3) or antiplatelet (n = 3) after 24 h. The reasons for not giving IVT were a minor deficit (NIHSS ≤ 5, n = 8), or a contraindication to IVT (n = 3).
Of the remaining 50 patients, 42 (84%) were treated with anticoagulants (by low molecular weight or unfractionated heparin), of whom 15 had also antiplatelets. Among the patients with contraindication to anticoagulants, five were treated with antiplatelet agents alone; three patients did not have antithrombotic therapy. Overall, 45 (80%) received anticoagulation within 24 h after admission.
Eleven patients (20%) had FWND within the first week. Two of them had initial CIM but were not treated with immediate EVT because of a rapid neurological improvement in one case and a medical decision for the other one. The presumed mechanisms for these FWND were embolic for one patient (T-shaped occlusion on control imaging), hemodynamic for nine patients (no intracranial occlusions; presence of a core-hypoperfusion mismatch > 40 mL for the five patients with perfusion imaging), and status epilepticus for one patient. All but one patient had persistent carotid occlusion (the latter had subocclusive stenosis). Four of these 11 patients with FWND had rescue EVT. Four patients with FWND (36%) died during hospitalization, one after rescue EVT.
After univariate analysis, predictive factors of FWND were the male sex (p = 0.013), no current smoking (p = 0.019), the existence of FVH (p = 0.009), and the existence of a mismatch between ischemic core volume and Tmax > 6 s volume > 40 mL (p = 0.007). None of the 17 patients without FVH had FWND, while 10 out of 32 patients (31%) with FVH had. None of the 9 patients with a < 40 mL mismatch volume had FWND, while 5 out of 12 patients (42%) with a > 40 mL volume did.
Haemorrhages
Among the 56 patients of this study, there was no symptomatic cerebral hemorrhage; 6 patients (11%) had asymptomatic cerebral hemorrhages, after treatment by IVT (n = 1), anticoagulants ± antiplatelets (n = 2) and antiplatelets (n = 3). After univariate analysis, initial NIHSS (p = 0.008) and ASPECTS scores (p = 0.021) were associated with the occurrence of cerebral haemorrhages.
ICA recanalization
Endpoints are presented in Table 2.
On the first follow-up imaging (available for 53 patients), 7 (13%) patients had ICA recanalization, including the 2 patients who had rescue EVT on day 1. Thus, among the 51 patients with a medical strategy, 5 (10%) had carotid recanalization (1 of the 6 patients treated with IVT, 4 under anticoagulants).
On the follow-up imaging performed between 1 and 3 months in 45 patients, 17 (38%) had carotid patency, including 3 patients who had rescue EVT. Overall, among the 42 patients treated with a medical strategy, 14 (33%) had carotid recanalization after initial IVT (n = 2), anticoagulants ± antiplatelets (n = 11), antiplatelets then anticoagulants (n = 1). Six patients had complete ICA recanalization or residual stenosis < 50%, eight patients had residual carotid stenosis ≥ 50%, of whom two had carotid stenting and two had endarterectomy, without complications.
3-month mRS
One patient was lost for follow-up. Among the remaining 55 patients, excellent outcome (mRS ≤ 1) at 3 months was achieved in 36 (66%) and favorable outcome (mRS ≤ 2) in 38 (69%). Seven patients (13%) were dead at 3 months, from recurrent AIS (n = 4), mesenteric ischemia (n = 1), cardiogenic failure (n = 1), status epilepticus (n = 1).
In multivariate analysis (Supplementary Table), factors associated with a bad outcome at 3 months were: higher initial NIHSS score (p = 0.01), lower initial diastolic blood pressure (p = 0.04), and existence of FWND (OR 0.05; 95% CI 0.003–0.79; p = 0.03).
Recurrences
Among the 49 survivors, the median follow-up time was 409 (IQR 182–747) days. Six patients (13%) had a recurrence after hospitalization, of whom five during the first 3 months. Three patients had neurological deficit (transient, n = 1; persistent, n = 2) with new cerebral infarct; three had ocular symptoms without cerebral infarct. All patients with recurrence had been treated with initial anticoagulation (associated with aspirin in two), which was stopped for four patients within 15 days before recurrence; the other two patients had transient monocular blindness under anticoagulation treatment. All patients had persistent carotid occlusion at the time of the recurrences.
Discussion
The objective of this observational study was to focus on the short-term natural history of symptomatic ICAO in 56 consecutive patients treated with initial medical management in our regional CSC. There are few data about symptomatic ICAO. The initial presentation of ICAO patients seems to be notably different from patients with tandem, but also intracranial occlusions. Indeed, in our study, almost two-thirds of the patients presented with a persistent neurological deficit, 20% with a TIA, and 17% with a retinal symptomatology; many patients (43%) presented with multiple TIAs or clinical fluctuations. The highly heterogeneous presentation of ICAO could be explained by the variability in collateral flow supply by the circle of Willis. Interestingly, most patients had a minor stroke, with a median initial NIHSS of 3. This is partially explained by the exclusion of the 11 patients with a CIM on admission, who had a median NIHSS of 16 and underwent immediate EVT. Nevertheless, this low initial severity in our cohort is comparable with two other studies including respectively 33 (median NIHSS of 4) and 133 patients (median NIHSS of 5) with ICAO without EVT [18, 26]. In another study including 100 patients with ICAO treated with IVT, the initial median NIHSS was 19, but comparison with our cohort is unsuitable as probably most patients had CIM [12].
The optimal acute management of symptomatic ICAO has not yet been addressed. There are few data on MT in acute ICAO because it was an exclusion criterion of the majority of recent RCT focusing on intracranial occlusion in the anterior circulation [13], with only few patients included in the DEFUSE-3 trial [27]. In a recent retrospective cohort study with 107 ICAO patients, selected with an initial CIM or the existence of clinical instability, the recanalization rate after cervical carotid angioplasty stenting was high (92% at day 1) and 65% patients had a favorable prognosis at 3 months [16]. In our CSC, patients presenting with CIM usually had EVT, and were excluded for two reasons: first, the objective of this study was to focus on the natural history of ICAO with a medical strategy, and second, patients treated with medical treatment or EVT are very different in terms of initial severity.
In our study, the standardized medical strategy during the acute and subacute phases was mainly based on anticoagulants in the absence of contraindication (Supplementary Figure), and overall, 45 (80%) received anticoagulation within 24 h after admission. Intravenous thrombolysis was performed in symptomatic patients within 4 h 30 min after known stroke onset or with a FLAIR-negative MRI in case of unknown stroke onset. Antiplatelet treatment was given alone in case of contraindication to anticoagulants, or in association with anticoagulation for atheromatous occlusions without high risk of bleeding.
Only six patients had IVT, with a recanalization rate of 33% at Day 1. IVT has been described as poorly effective in patients with carotid occlusion, with a low recanalization rate of 4–26% [4, 10, 11]. However, the rate of recanalization could be higher than 50% in ICAO [12]. This has to be confirmed in further studies.
Few studies have evaluated the occurrence of early neurological deterioration in ICAO patients, whose main mechanism may be embolic or hemodynamic ischemic injury. In one study of 96 patients with ICAO treated by IVT, 17% had neurological deterioration during the first 24 h [28]. In our population treated mainly by anticoagulants, 20% had neurological deterioration during the first week, quite similar than the 24% rate reported in one study on 33 ICAO patients, where early anticoagulation was associated with fewer recurrent strokes and increased carotid recanalization than antiplatelets [18]. The mechanism by which patients with symptomatic ICAO can benefit from anticoagulation is unclear. Early anticoagulation may help by preventing ICA clot extension into circle of Willis end-arteries, which would deprive them of collateral supply from the anterior / posterior communicating arteries, and also by lowering risk of stump emboli from the occluded carotid [29]. In one study [26], among 133 patients with ICAO treated by antiplatelets, 7% of patients had recurrence, occurring within the 9 first days, presumed to be embolic in 10 cases as patients had normal cerebral perfusion, and hemodynamic in 5 cases. One major difference with our study was the low rate of cerebral hypoperfusion, which concerned only 16% of patients.
In our study, almost two-third of patients had FVH, known to reflect the existence of hypoperfusion [30]. Twenty-one patients were explored with perfusion imaging.
There is no definition of significative core-hypoperfusion mismatch for ICAO. Three RCT used perfusion-imaging–based selection for patients with proximal MCA or mostly intracranial carotid occlusions [27, 31, 32]. In DEFUSE-3 and SWIFT-PRIME, an absolute mismatch volume of at least 15 mL and a mismatch ratio (ratio of the volume of ischemic tissue on perfusion imaging to infarct volume) of 1.8 or more was required, and the ischemic core had to be less than 70 ml in DEFUSE-3 and less than 50 mL in SWIFT-PRIME trial,but the median mismatch volume was finally about 100 mL in DEFUSE-3 [13, 31]. In EXTEND-IA, any evidence of salvageable brain tissue was required, the median perfusion lesion volume at initial imaging was 116 mL (with a 20 mL ischemic core volume) [32]. In a recent case–control study focusing on large cerebral infarcts, EVT was effective for patients with a ≥ 40 mL mismatch [23]. For ICAO, further studies are necessary to evaluate the most accurate mismatch. In our study, we defined a significative mismatch as a mismatch volume > 40 mL on initial CT or MRI. To the best of our knowledge, this is the first study to show that a core-hypoperfusion mismatch on initial CT or MRI was associated with the occurrence of neurological deterioration in ICAO. Moreover, we similarly found that the presence of FVH was associated with higher risk of FWND. In line with this result, altered hemodynamic factors in patients with chronic carotid occlusions was linked to late recurrences in some studies using different perfusion tools (positron emission tomography [6, 8, 20], single-photon emission CT, transcranial Doppler, or stable xenon CT [6]). However, there are only two studies focusing on the importance of hemodynamic factors investigated at the acute phase to identify the risk of early neurological deterioration or late recurrences [12, 26]. Leptomeningeal collateral status on initial CT was a predictive factor of functional outcome and mortality in one study on 100 ICAO patients treated with IVT [12]. Exhausted cerebrovascular reserve ipsilateral to ICAO, explored by transcranial Doppler, was associated with recurrent ischemic events at 4.5 months in one other study [26]. The presence of a core-hypoperfusion mismatch or FVH at the acute phase could hence identify a subpopulation of ICAO with minor stroke at higher risk of deterioration, in which specific management (close monitoring and immediate EVT) should be discussed. This ‘a priori’ defined 40 cc cut-off will however have to be validated in further studies.
In our center, first-line treatment strategy for patients with ICAO and no CIM on admission relies on medical management, with the possibility of rescue EVT in case of FWND. Four patients had rescue MT in our study. There are currently no data on the interest of rescue EVT in ICAO with minor stroke. However, as up to 20% of ICAO patients experience FWND making them candidates to rescue EVT, this strategy has to be evaluated. This problematic is close to the one of minor strokes secondary to intracranial occlusion in which the best strategy between immediate and rescue MT is unknown [33], and currently under investigation in RCT.
In our cohort, one-third of our patients had complete or incomplete ICA recanalization after 1–3 months under medical treatment alone. The rate of ICA recanalization was 23% in patients treated by antiplatelets (with de novo dual antiplatelet therapy associated with higher rate of recanalization) in one study [26], and 46% at 1 year in another study in their subgroup of 15 patients under anticoagulants [18]. Whether anticoagulation therapy can increase recanalization rate of ICAO compared to antiplatelet therapy and its optimum duration remain to be addressed.
In our study, clinical outcome at 3 months was favorable with two-thirds of patients having an excellent outcome, and 69% being autonomous (mRS0 ≤ 2), a higher rate that one found by Haus (57% at 4 months). On the other hand, this 31% rate of dependence is quite high for one population presenting with a minor stroke, underlining the need to evaluate predictive factors of deterioration and the interest of EVT in this population.
Finally, the 13% rate of recurrences in our study was higher than two studies among 133 and 33 patients, respectively, [18, 26], which ranges from 0 to 4%, which could be secondary to the high proportion of patients with core-perfusion mismatch in our study.
Our study has several limitations: data analysis is retrospective (even if it was collected from a prospective record), our sample has small size, and the study is monocentric. Another limitation is the small number of patients with perfusion imaging (38%). Finally, the follow-up was relatively short (median: 13.6 months), with no information on the risk of long-term recurrence.
Conclusion
Our results suggest that in a cohort of consecutive patients with symptomatic ICAO without initial CIM and treated according to a standardized medical regional strategy including close monitoring, anticoagulation, IVT and ‘rescue’ EVT in case of neurological deterioration, most had good outcome at 3 months. On the other hand, one-third of patients had early neurological deterioration or recurrences during follow-up, and two-third of patients had persistent ICA occlusion at 3 months. We identified imaging hemodynamic parameters, defined by a mismatch volume > 40 cc on PWI or presence of FVH, as candidate risk markers for early neurological deterioration, which need to be further evaluated.
Our data justify the necessity of a close initial monitoring in Stroke Centers with Interventional Neuroradiology facilities for ICAO patients to discuss rescue therapy in case of deterioration and a close outpatient follow-up. Further rigorous prospective studies are needed to assess safety and efficacy of best medical and endovascular therapeutic strategies (immediate or rescue) during acute and subacute phase of ICAO.
References
Powers WJ (2003) Atherosclerotic carotid artery occlusion. Curr Treat Options Cardiovasc Med 5:501–509. https://doi.org/10.1007/s11936-003-0039-3
Flaherty ML, Flemming KD, McClelland R et al (2004) Population-based study of symptomatic internal carotid artery occlusion: incidence and long-term follow-up. Stroke 35:e349–e352. https://doi.org/10.1161/01.STR.0000135024.54608.3f
Paciaroni M, Caso V, Venti M et al (2005) Outcome in patients with stroke associated with internal carotid artery occlusion. Cerebrovasc Dis 20:108–113. https://doi.org/10.1159/000086800
Paciaroni M, Agnelli G, Caso V et al (2012) Intravenous thrombolysis for acute ischemic stroke associated to extracranial internal carotid artery occlusion: the ICARO-2 study. Cerebrovasc Dis 34:430–435. https://doi.org/10.1159/000345081
Hankey GJ, Warlow CP (1991) Prognosis of symptomatic carotid artery occlusion. Cerebrovasc Dis 1:245–256. https://doi.org/10.1159/000108851
Klijn CJ, Kappelle LJ, Tulleken CA, van Gijn J (1997) Symptomatic carotid artery occlusion. A reappraisal of hemodynamic factors. Stroke 28:2084–2093. https://doi.org/10.1161/01.str.28.10.2084
Klijn CJM, van Buren PA, Kappelle LJ et al (2000) Outcome in patients with symptomatic occlusion of the internal carotid artery. Eur J Vasc Endovasc Surg 19:579–586. https://doi.org/10.1053/ejvs.2000.1129
Grubb RL, Derdeyn CP, Fritsch SM et al (1998) Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 280:6. https://doi.org/10.1001/jama.280.12.1055
Lanari A, Silvestrelli G (2012) Acute and chronic carotid occlusion syndromes. In: Paciaroni M, Agnelli G, Caso V, Bogousslavsky J (eds) Frontiers of neurology and neuroscience. Karger, Basel, pp 185–190
Bhatia R, Hill MD, Shobha N et al (2010) Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke 41:2254–2258. https://doi.org/10.1161/STROKEAHA.110.592535
Paciaroni M, Balucani C, Agnelli G et al (2012) Systemic thrombolysis in patients with acute ischemic stroke and internal carotid artery occlusion: the ICARO study. Stroke 43:125–130. https://doi.org/10.1161/STROKEAHA.111.630624
Yeo LLL, Kong WY, Paliwal P et al (2016) Intravenous thrombolysis for acute ischemic stroke due to cervical internal carotid artery occlusion. J Stroke Cerebrovasc Dis 25:2423–2429. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.06.014
Goyal M, Menon BK, van Zwam WH et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet Lond Engl 387:1723–1731. https://doi.org/10.1016/S0140-6736(16)00163-X
Mokin M, Kass-Hout T, Kass-Hout O et al (2012) Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion: a systematic review of clinical outcomes. Stroke 43:2362–2368. https://doi.org/10.1161/STROKEAHA.112.655621
Paciaroni M, Inzitari D, Agnelli G et al (2015) Intravenous thrombolysis or endovascular therapy for acute ischemic stroke associated with cervical internal carotid artery occlusion: the ICARO-3 study. J Neurol 262:459–468. https://doi.org/10.1007/s00415-014-7550-1
Jadhav A, Panczykowski D, Jumaa M et al (2018) Angioplasty and stenting for symptomatic extracranial non-tandem internal carotid artery occlusion. J NeuroInterv Surg 10:1155–1160. https://doi.org/10.1136/neurintsurg-2018-013810
Adams HP, Bendixen BH, Leira E et al (1999) Antithrombotic treatment of ischemic stroke among patients with occlusion or severe stenosis of the internal carotid artery: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53:122–125. https://doi.org/10.1212/wnl.53.1.122
Damania D, Kung NT-M, Jain M et al (2016) Factors associated with recurrent stroke and recanalization in patients presenting with isolated symptomatic carotid occlusion. Eur J Neurol 23:127–132. https://doi.org/10.1111/ene.12819
EC/IC Bypass Study Group (1985) Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. N Engl J Med 313:1191–1200. https://doi.org/10.1056/NEJM198511073131904
Powers WJ, Clarke WR, Grubb RL et al (2011) Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: the carotid occlusion surgery study randomized trial. JAMA 306:1983–1992. https://doi.org/10.1001/jama.2011.1610
Seners P, Turc G, Tisserand M et al (2014) Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke 45:2004–2009. https://doi.org/10.1161/STROKEAHA.114.005426
Kernan WN, Ovbiagele B, Black HR et al (2014) Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45:2160–2236. https://doi.org/10.1161/STR.0000000000000024
Rebello LC, Bouslama M, Haussen DC et al (2017) Endovascular treatment for patients with acute stroke who have a large ischemic core and large mismatch imaging profile. JAMA Neurol 74:34. https://doi.org/10.1001/jamaneurol.2016.3954
Hacke W (1995) Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA J Am Med Assoc 274:1017–1025. https://doi.org/10.1001/jama.274.13.1017
Adams HP, Bendixen BH, Kappelle LJ et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41. https://doi.org/10.1161/01.STR.24.1.35
Hause S, Oldag A, Breja A et al (2019) Acute symptomatic extracranial internal carotid occlusion—natural course and clinical impact. Vasa. https://doi.org/10.1024/0301-1526/a000826
Albers GW, Marks MP, Kemp S et al (2018) Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 378:708–718. https://doi.org/10.1056/NEJMoa1713973
Mazya MV, Cooray C, Lees KR et al (2018) Minor stroke due to large artery occlusion. When is intravenous thrombolysis not enough? Results from the SITS International Stroke Thrombolysis Register. Eur Stroke J 3:29–38. https://doi.org/10.1177/2396987317746003
Xu B, Li C, Guo Y et al (2017) Current understanding of chronic total occlusion of the internal carotid artery (review). Biomed Rep. https://doi.org/10.3892/br.2017.1033
Legrand L, Tisserand M, Turc G et al (2015) Do FLAIR vascular hyperintensities beyond the DWI lesion represent the ischemic penumbra? Am J Neuroradiol 36:269–274. https://doi.org/10.3174/ajnr.A4088
Saver JL, Goyal M, Bonafe A et al (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372:2285–2295. https://doi.org/10.1056/NEJMoa1415061
Campbell BCV, Mitchell PJ, Kleinig TJ et al (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372:1009–1018. https://doi.org/10.1056/NEJMoa1414792
Dargazanli C, Arquizan C, Gory B et al (2017) Mechanical thrombectomy for minor and mild stroke patients harboring large vessel occlusion in the anterior circulation: a multicenter cohort study. Stroke 48:3274–3281. https://doi.org/10.1161/STROKEAHA.117.018113
Cagnazzo F, Dargazanli C, Lefevre P-H et al (2019) Chronic occlusion of the internal carotid artery: endovascular revascularization technique of long occlusive lesions. J Neuroradiol. https://doi.org/10.1016/j.neurad.2019.05.005
Acknowledgements
Authors would like to thank Mrs Hattinguais Jessica for assistance in data collection.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
CA and AS planned the study data collection, identified the patient cohort, gathered the data, and drafted the manuscript; CD and AS did the statistical analysis. All authors contributed to data collection, made suggestions on analysis, and approved the final manuscript. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study has been approved by local institutional review board (IRB 202000337) of Montpellier University Hospital, waiving need for informed consent due to the observational design.
Consent for publication
Informed consent has been waving by local IRB due to the study observational design.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ter Schiphorst, A., Gaillard, N., Dargazanli, C. et al. Symptomatic isolated internal carotid artery occlusion with initial medical management: a monocentric cohort. J Neurol 268, 346–355 (2021). https://doi.org/10.1007/s00415-020-10118-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10118-9