Abstract
Background
Chronic levodopa treatment in Parkinson’s disease (PD) may promote undesirable motor and non-motor fluctuations. Compared to chronic oral levodopa treatment, continuous infusion of levodopa/carbidopa intestinal gel (LCIG) in advanced PD reduces motor fluctuations. However, differences in their effect on acute non-motor changes were not formally demonstrated.
Objective
We performed a randomized, double-blind, double-dummy, crossover study to compare acute non-motor changes between intermittent oral immediate-release carbidopa/levodopa (LC-IR) and LCIG.
Methods
After > 12-h OFF, thirteen PD patients chronically treated with LCIG and without history of non-motor swings, were allocated to receive first, LCIG infusion plus three oral doses of placebo, or placebo infusion plus three oral doses of LC-IR. Over-encapsulated oral medication (LC-IR or placebo) was administered every 2 h. We monitored plasmatic levels of levodopa, motor status (UPDRS-III), mood, anxiety, and frontal functions at baseline (0-h) and hourly after each oral challenge.
Results
Repeated-measures ANOVAs showed significant group by treatment interaction indicating more fluctuations of levodopa plasma levels with LC-IR. No significant interactions were seen in the temporal profile of motor status, anxiety, mood and cognition. However, point-to-point parametric and nonparametric tests showed a significant more marked and more sustained improvement in anxiety scores under LCIG. A significant improvement of mood and verbal fluency was seen a + 3-h only under LCIG.
Discussion
Our sample of advanced PD patients exhibited moderate but significant non-motor fluctuations. LCIG was associated with a more favorable profile of acute affective and cognitive fluctuations that was particularly expressed at the first part of the infusion curve.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Levodopa is the most common, effective and well-tolerated oral drug for the symptomatic treatment of Parkinson’s disease (PD) [1, 2]. Yet, more as a function of disease duration than cumulative levodopa exposure [3], patients chronically treated with oral levodopa often develop disabling motor fluctuations and troublesome involuntary movements, coinciding with the rise and fall of levodopa plasma concentrations [4, 5]. Pulsatile availability of dopamine due to the short half-life and erratic gastric emptying of levodopa is considered responsible for the “ON/OFF” response, while progressive receptor and neurotransmitter changes associated with phasic dopaminergic stimulation are thought to be responsible for dyskinesia [3, 6].
Non-motor symptoms are highly common and disabling in PD [7]. They may vary in frequency and severity in the period between levodopa doses and can be more distressing than the accompanying motor fluctuations [8,9,10,11,12,13,14]. Affective and cognitive fluctuations are the most common clinically observed non-motor fluctuations [15, 16]. Not rarely, some patients shift from being depressed to euphoric [17], from relaxed to anxious [18], from clear minded to cognitive confused [19], or to a combination of these symptoms. Nevertheless, the most frequent pattern is a relatively lower mood and higher anxiety when in “OFF” and a higher mood and lower anxiety when in “ON”. In a study specifically addressing the emergence of non-motor fluctuations with reference to motor fluctuations, anxiety (22.2%), dullness of thinking (18.9%), difficulty in concentrating (19.8%), and sadness (18.9%) were among the most fluctuating non-motor symptoms both at onset of fluctuations and throughout the 36-month follow-up [16].
Remarkably, these fluctuations are often under-recognized by patients and under-treated by physicians [15, 20,21,22]. Accordingly, double-blind randomized controlled studies of the acute response to levodopa in wearing-off PD subjects without clinically evident affective fluctuations have shown significant positive and negative changes in mood and anxiety ratings that parallel the “ON/OFF” motor status and the rise and fall of plasmatic levels of levodopa [23, 24].
While the response of mood and anxiety to an acute levodopa challenge appears relatively straightforward and predictable [23,24,25], the relationship between cognitive performance and pulsatile elevations of brain dopamine from levodopa seems manifold [26, 27]. Studies in patients without clinically evident cognitive fluctuations have shown that acute changes in the level of dopamine stimulation with levodopa may improve, not affect, or alter performance in certain frontal tasks in relation to various factors. These factors include the basal level of dopamine function in the cortico-striatal circuitry [28,29,30], the stable or wearing-off response [31], the nature of the task and its ventral or dorsal striatum dependency [32, 33], and the more acute or slower rise of levodopa plasma levels [34].
The phenomenology of affective and cognitive fluctuations in PD patients under different pharmacokinetic profiles of levodopa has been little studied using a randomized, double-blind, placebo-controlled design and controlling for levodopa plasma levels. One pilot study showed a tendency to fewer mood fluctuations with continuous intravenous infusion than with immediate-release oral levodopa [25]. Other studies have observed significant mood elevation in wearing-off patients [23] and a worse profile of acute cognitive changes [34], with a more rapid rise in levodopa plasma levels from immediate-release formulations than from controlled-release formulations [34].
Continuous delivery of levodopa-carbidopa intestinal gel (LCIG) through a percutaneous intrajejunal infusion has proven to be an efficacious, safe and tolerable approach for the treatment of uncontrollable motor fluctuations in PD [35,36,37,38,39,40,41,42]. Compared to immediate release oral levodopa/carbidopa (LC-IR), LCIG results in lower variability and fluctuations in levodopa plasma concentrations [43]. Based on these premises, we sought to compare for the first time the acute affective and cognitive response of a continuous delivery of LCIG versus repeated doses of LC-IR. We hypothesized that at a comparable level of motor improvement, patients under LCIG infusion show a more stable or favorable profile on measures of affect and cognition than when repeatedly challenged with oral doses of LC-IR.
Patients and methods
Design
We used an intra-subject, randomized, double-blind, double-dummy, placebo-controlled, crossover design to compare two therapeutic interventions, LCIG and LC-IR, acutely administered in two sessions separated by a 2-week interval. Sample size planning was based on information from two of our previous works where 20 and 14 patients were studied [31, 34]. A minimum sample size of 12 patients was calculated according to the probability of a type error I (alpha) = 0.05 and a power of 0.8.
The protocol was approved by our institutional review board. Written informed consent was obtained before any study-related procedures were performed. Trial is registered with Eudra CT number 2015-002631-17.
Eligibility
Enrolled were thirteen patients with advanced PD according to the UK Brain Bank criteria and criteria for infusion therapy [44, 45]. All patients were under stable treatment with LCIG for more than 4 weeks before enrollment. Among all potential participants, we excluded all of these accomplishing any of the items listed as exclusion criteria (see below). Applying these criteria, we excluded approximate 60% of the candidates. Two weeks before the experimental sessions, a clinical interview was conducted with all participants and their caregivers. Baseline demographic data assessed included the levodopa equivalent dose (LED) [46], Hoehn and Yahr staging, motor status [Movement Disorders Society Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS-III)] [47] in ‘ON’ and ‘OFF’, global cognitive status (Parkinson’s Disease—Cognitive Rating Scale) [48], anxiety and depression (Hospital Anxiety and Depression Scale; HADS) [49], and apathy [50].
Main exclusion criteria were: Severe dementia; cognitive deterioration severe enough to interfere with test performance; deep brain stimulation; major depression; a “yes” answer on item 4 or 5 on the Columbia Suicide Severity Rating Scale; severe dyskinesia; hallucinations; and typical neuroleptics or any other drug which, in the opinion of the investigator, would interfere with the cognitive tests. Additionally, as we aimed to investigate possible affective and cognitive swings in a PD sample with no obvious non-motor fluctuations, we used a previously described semi-structured questionnaire to exclude subjects under LCIG who described acute affective or cognitive fluctuations in response to dopaminergic medication [34].
Medication
Patients were randomly assigned to receive either treatment with oral over-encapsulated 100/25 LC-IR plus placebo LCIG infusion, or an LCIG infusion plus oral over-encapsulated placebo 100/25 LC-IR. Randomization of treatment sequences followed Grecolatin square designs. To blind the study treatments, we used a double-dummy technique with matching placebos. LCIG was delivered either as an aqueous intestinal gel (containing 20 mg/ml levodopa and 5 mg/ml carbidopa monohydrate solution) in 100 ml cassettes or as matching placebo gel (sodium carboxymethylcellulose solution alone) administered as a bolus of 5–10 ml at time 0 h of the experimental session followed by continuous infusion at a constant rate for the ensuing six hours. The total LCIG dose administered was based on each patient’s individually optimized dose prior to randomization. LC-IR capsules containing 25/100 mg of carbidopa/levodopa were administered in three separate, equal doses every 2 h throughout the experimental sessions. To obtain a comparable motor effect in each subject across sessions using formulations of different bioavailability, we calculated the total LC-IR dose for each subject based on the usual and stable dose of LCIG they were receiving at the time of inclusion in the study. Since each phase of the study lasted six hours, and as each LCIG ml is equivalent to 20 mg of levodopa, the total dose of LCIG for that period was calculated including the first bolus and the continuous infusion dose. The dose of LC-IR that patients received every 2 h corresponded to the LCIG that the patient was receiving in this 2 h period (calculated on the basis of 1 ml of LCIG equivalent to 20 mg of LC-IR) rounding to the nearest 50 mg. Safety measures were recorded at + 6 h.
Procedure
Patients were evaluated in both sessions at our clinical drug research facility (CIM-Sant Pau) by neurologists, nurses and neuropsychologists with expertise in movement disorders. On the day before the first experimental session, we conducted a practice session using tests similar to those to be employed in the trials. The trial consisted of two experimental 6-h sessions conducted after 12-h overnight withdrawal of all antiparkinsonian medication (12-h off medication). Usual non-parkinsonian medication was not changed between sessions. Treatment started at 8.30 a.m. with intestinal infusion and first oral dose of LC-IR or placebo, immediately after obtaining basal assessments in “OFF” condition (time-point 0-h). At this time-point, patients were systematically asked whether they considered being in clinical ON or OFF state according to their own previous experience. To obtain a comparable time-to-peak levodopa level and motor improvement, treatment started at time-point 0-h with the patient’s usual bolus of LCIG or placebo followed by regular infusion rate. Subsequent oral doses followed a fixed order of administration with LC-IR or placebo added to the running infusion at time-points + 2-h and + 4-h after first treatment. Six consecutive hourly assessments were performed at time-point 0-h, and at + 1-h, + 2-h, + 3-h, + 4-h and + 5-h for pharmacokinetic sampling of levodopa plasma levels, and to evaluate motor status, anxiety, and mood ratings. Four consecutive cognitive assessments were obtained by the same neuropsychologist at time-point 0 h and 1 h after each oral treatment, at time-points + 1-h, + 3-h and + 5-h.
Blood samples to determine the pharmacokinetics of levodopa were obtained and analyzed as previously described [31]. All samples were analyzed at the same time at the end of the trial using standard high-pressure chromatography with electrochemical detection. Motor status was assessed with MDS-UPDRS-III scored by an independent investigator. Affective ratings were obtained with validated visual analogue scales for mood (VAS-M) and anxiety (VAS-A) with a range of 0–10 (not at all-very much, respectively). Two separate sheets were used to ensure blind answers, as previously described [23].
Two cognitive frontal-executive tasks, administered in a counterbalanced manner at each of the four cognitive testing times, were selected for their brevity and sensitivity to detect changes in PD [51]. Phonetic verbal fluency tasks were used to assess frontal-executive functions [52]. In the phonetic verbal fluency task, participants were requested to generate as many words as possible beginning with a given letter. This letter differed at each assessment (P, M and R). A computerized version of the Sternberg memory task with 2, 4 and 6-letter conditions was used to assess short-term retention and manipulation of information within working memory. The percentage of correct responses in each condition was used as outcome [53].
Data analysis
Differences between treatments were examined by repeated-measures analysis of variance (ANOVA). Factors considered were type of medication (LC-IR and LCIG) and time. Post hoc comparisons were performed using Student’s paired t test or Wilcoxon test when appropriate. P values for the ANOVAs were calculated after Greenhouse–Geisser corrections. The data that support the findings of this study are available from the corresponding author upon reasonable request. Data are presented as mean ± SD unless otherwise stated. Data were analyzed using SPSS15 software.
Results
Fourteen patients met eligibility criteria and signed informed consent forms. Thirteen patients completed the study. One patient was unable to complete the second experimental session due to family problems and was excluded from the analysis.
Clinical and sociodemographic baseline data are provided in Table 1. No specific tolerability issues with oral medication and no serious or clinically significant adverse events were observed.
LD levels in plasma (ng/ml)
Doses of the study drug, levodopa, ranged from 340 to 1120 mg during the 6-h study period. T test comparison showed no significant differences in basal levodopa doses between study conditions [t(12) = 1.31, p > 0.05).
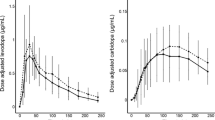
Figure 1 shows the participants’ pharmacokinetic profiles after both types of levodopa administration. Repeated measures ANOVA showed a significant group x treatment interaction [F(1,22) = 3.53, p < 0.05]. Consecutive paired t test showed equivalent levodopa levels in the “12-h off medication” period at baseline [t(24) = − 0.505, p > 0.05] and + 1-h after first treatment [t(24) = 0.001, p > 0.05] between the two groups.
Time course of levodopa plasma levels. Levodopa plasma concentrations for subjects with advanced Parkinson’s disease receiving LCIG or LC-IR. Paired t tests showed a comparable significant increase in levodopa plasma levels between baseline (12-h off medication; time-point 0-h) and one hour (time-point + 1-h) after first treatment (p < 0.005 for both treatments). A sustained maintenance of levodopa levels through subsequent time-points is observed for LCIG while significant increases and decreases of levodopa levels are seen for the LC-IR condition. LCIG levodopa-carbidopa intestinal gel, LC-IR oral levodopa-carbidopa immediate release
(LCIG: [t(12) = − 4.64, p < 0.005]; LC-IR: [t(12) = − 3.83, p < 0.005]. As depicted in Fig. 1, compared to LC-IR, the LCIG condition showed a sustained maintenance of levodopa levels after the first dose. Conversely, the LC-IR condition showed significant fluctuations at each time-point in the form of significant increases and decreases of the LD levels ranging from − 2132 ± 1644 to 1330 ± 2480 ng/ml. In the LCIG condition, oscillations ranged from − 182 ± 380 to 318 ± 970 ng/ml.
MDS-UPDRS III
MDS-UPDRS-III scores were similar at baseline between procedures [LCIG 68.25 ± 10; LC-IR 68.17 ± 10; t(24) = − 0.20, p > 0.05] with comparable ‘ON’ improvement between LCIG [t(12) = 2.98, p < 0.05] and LC-IR condition [t(12) = 4.02, p < 0.05]. ANOVA analysis showed no group-by-treatment interaction along time [F(1,22) = 0.37, p > 0.05]. Figure 2 shows the temporal profiles of the subjects after both types of levodopa administration.
Time course of motor assessments. Temporal profile of the Movement Disorders Society Unified Parkinson’s Disease Rating Scale part-III (MDS UPDRS-III) scores. ANOVA analysis showed no group x treatment interaction in motor status according to the MDS-UPDRS III (p = 0.818). Paired sample analysis showed that both treatment conditions (LCIG and LC-IR) were associated with a comparable significant motor benefit between ‘12-h off medication’ (time-point 0-h) and ‘on’ situation at one hour after first treatment administration (time-point + 1-h). No significant motor differences between treatments were observed at subsequent time-points. Direction of improvement: top to bottom
Anxiety
Baseline VAS-anxiety scores were high and comparable in both conditions (LCIG 6.2 ± 2; LC-IR 5.5 ± 2.3) [t(24) = 0.824, p > 0.05]. ANOVA revealed no group by treatment interaction [F(1,22) = 1.69, p > 0.05]. Point-by-point Wilcoxon analysis of the curve (Fig. 3) showed that while patients improved significantly under both conditions at + 1-h, there was a more pronounced and sustained reduction of anxiety scores with respect to baseline (0-h) in the LCIG condition with significant differences at all time-points (p < 0.005). Contrarily, in the LC-IR condition significant differences were only found at + 1-h (p < 0.05) and + 2-h (p < 0.05) losing significance at the rest of the curve. Between-group comparison showed a significant difference at time-point + 3-h with more pronounced lower anxiety score in the LCIG condition (LCIG = 1.6 vs LC-IR = 2.8; p < 0.05) .
Mood
Figure 3 shows the temporal profile of subjects’ VAS-mood scores after both types of LD administration. At baseline, both groups showed equivalent mood scores (LCIG 59.17 ± 20; LC-IR 60 ± 24; [t(24) = − 0.90, p > 0.05]). ANOVAs revealed no significant group x treatment interaction between time-points. Post hoc comparisons (Fig. 3) showed that while patients improved significantly under both conditions at + 1-h with respect to baseline (0-h), this significance was maintained only on LCIG at the rest of the curve. Between-group comparison showed a significant difference at time-point + 3-h with better mood score in the LCIG condition (LCIG = 71.6 ± 17 vs LC-IR = 56.6 ± 15; p < 0.05).
Sternberg test
In the 2-letter, 4-letter and 6-letter conditions, the percentage of correct responses at baseline was equivalent between treatments.
In the 2-letter and 4-letter conditions, repeated measures ANOVA showed no significant group by treatment interaction. Paired t test showed an equivalent percentage of change between treatment groups in all time-points (Fig. 4).
Phonetic verbal fluency test and Sternberg test. a Temporal profile of phonetic verbal fluency. A significant improvement was seen at time-point + 3-h compared to that at 0-h. b Temporal profile of short-term retention memory in conditions of increasing complexity. An opposite pattern of improvement (LCIG) vs worsening (LC-IR) was found between + 3-h and + 5-h
In the 6-letter condition, repeated measures ANOVA showed a significant group by treatment interaction [F(1,22) = 11.2; p < 0.05]. Paired t test revealed that this effect was mediated by a sustained improvement on performance in the LCIG condition along different time-points but a significant worsening in the LC-IR condition at time-point + 5-h [t(24) = 2.23; p < 0.05]. This pattern of worsening was exclusively seen in the LC-IR condition between time-point + 3-h and + 5-h [t(12) = − 3.83; p < 0.05].
Discussion
In this study we examined and compared acute non-motor changes associated with oral and intrajejunal administration of levodopa in advanced PD patients without clinically evident non-motor fluctuations. While the amount of oral levodopa administered was enough to obtain a similar motor improvement along the experiments under both treatment conditions, plasmatic levels of levodopa were more stable and patients exhibited an overall more favorable profile of affective and cognitive fluctuations with LCIG treatment. Although the differences observed under LCIG, are subtle and a trend to equitation between groups is seen at + 5-h mostly in all the measures, we consider that our exploratory findings may have notable implications in terms of understanding the importance of daily subclinical non-motor fluctuations and the long-term improvement of non-motor symptoms with LCIG.
A double dissociation was observed between motor status and affective and cognitive status along the experimental sessions. While the equivalent doses of LCIG and LC-IR resulted in equivalent improvement and maintenance of the UPDRS-III scores between sessions, non-motor ratings exhibited different curves.
Compared to 12-h off medication, anxiety ratings improved significantly and achieved its maximum significance at + 3-h in both conditions. Contrarily to what was observed in motor status, patients under LCIG condition showed a more significant change after + 1-h that reached it maximum at + 3-h. In spite of the worsening observed in both conditions after the + 3-h point, significant differences indicating improvement with respect to baseline were only maintained in the LCIG condition.
A slightly different pattern was observed in the mood ratings where there was again an improvement with respect to baseline in both conditions, but there was a significant divergence at + 3-h. At this latter point, the LCIG condition achieved its maximum improvement while a worsening was observed in patients in the LC-IR condition. Whether this LCIG improvement at + 3-h depended on a better stability of levodopa levels or it is just a carryover of the significant improvement of anxiety at + 3-h is difficult to establish.
A slightly different pattern was observed in the mood ratings where there was again an improvement with respect to baseline in both conditions, but there was a significant divergence at + 3-h. At this latter point, the LCIG condition achieved its maximum improvement while a worsening was observed in patients in the LC-IR condition. Whether this LCIG improvement at + 3-h depended on a better stability of levodopa levels or it is just a carryover of the significant improvement of anxiety at + 3-h is difficult to establish.
No changes on verbal fluency task were observed after acute administration of both types of treatment (levodopa bolus in the LCIG group and LC-IR) [28, 31]. However, LC-IR showed a plain curve, a significant improvement was observed at + 3-h with LCIG. Regarding the Sternberg test, a significant dissociation was seen for the more cognitively demanding 6-letter part at + 5-h, with improvement under LCIG and worsening with LC-IR. The expected worsening associated with “overdosing” effects after acute administration of levodopa was not observed at + 1-h, but it occurred after the second oral administration of LC-IR. This effect of worsening on frontal-related after acute administration of l-dopa has been assumed to reflect the inverted-U relationship between dopamine levels and prefrontal functioning [31]. Interestingly, this well-known “overdosing” effect here is seen in the LC-IR condition but not in the LCIG. Accordingly, it may result from the fluctuating pattern observed for the plasmatic levels in the LC-IR, which in contrast to the profile exhibited under LCIG is characterized by a fluctuating response with significant increases and decreases.
Stabilization of plasmatic levodopa levels under LCIG may explain that in LCIG condition an improvement was observed. It is possible that although the first administration was not enough to cause a significant worsening on cognitive performance, repeated raisings and augmentations in levodopa levels exert a negative effect.
We consider that our study may offer some lessons for clinical practice. The frequent schedule of administration of oral levodopa in our study demonstrates that it is possible to maintain similar motor status using this 2-h schedule during the short period of the experimental sessions. However, it will probably be not the case for longer periods. However, as seen in the results, repeated oral dosing may increase levodopa levels to a threshold that any new administration of LC-IR exposes the patients to a worsening of their affective and cognitive status.
Moreover, our results suggest that non-motor fluctuations—even subclinical—should be taken into account to eventually advance the indication of device aided methods to stabilize levodopa levels in advanced PD patients still not accomplishing condition of having severe motor fluctuations. Patients’ PD diaries should include items that enable uncovering clinical and subclinical non-motor fluctuations [44, 54].
This study has strengths and limitations. First, the cross-over design allowed statistical efficiency in a relatively small sample size and minimized bias in assessing subjective outcomes such as mood and anxiety. Second, the practice session helped moderate anxiety related to the exploratory paradigm, and the 2-week interval between sessions helped reduce potential learning effects on the cognitive task and dismiss any potential carry-over effect. Third, the individual titration of the total amount of oral levodopa (proportional to the first 6-h of the usual regime of LCIG) and the relatively frequent administration helped to achieve an optimal and comparable sustained motor benefit throughout the two sessions. Fourth, monitoring the pharmacokinetics of levodopa revealed significant oscillations in the LC-IR arm in spite of similar motor status, further strengthening the concept that the pattern of less favorable non-motor changes in this arm was not simply a reaction to motor function. Rather, these affective and cognitive changes were consistently associated with the fluctuating pharmacokinetics of LC-IR and probably also with the subsequent pharmacodynamic variation in dopamine stimulation resulting from LC-IR. And fifth, we selected a representative sample of patients with active LCIG. Excluding patients with clinically evident non-motor swings served to confirm that even patients without these complaints may present subclinical but significant affective and cognitive fluctuations associated with levodopa dosing. This latter can also be considered a limitation. Our findings should be reproduced in patients with clinically meaningful non-motor fluctuations.
Another limitation was the fact that our sample of advanced PD patients was not cognitively homogeneous and in fact, the influence of cognitive status over the observed results merits further exploration. In any case, the lack of cognitive homogeneity could be considered to reflect clinical practice. According to the PD-CRS scoring, we included cognitively preserved patients (n = 8), PD-MCI (n = 1) and even a few patients with mild dementia (n = 4), a diagnosis that is not presently considered an exclusion criterion for LCIG [44, 45]. While cognitively deteriorated patients could be at more risk of cognitive fluctuations, no outliers were observed among these patients. A third limitation is the possible influence of other drugs. Three PD participants were on a mixture of dopamine agonists and LCIG, two were taking low doses of clozapine or quetiapine, four were on antidepressants, and one was taking rivastigmine. However, each patient was their own control and maintained their usual medication between the experimental sessions. This minimizes possible bias and supports the notion that the observed changes likely represent specific pharmacodynamics effects of levodopa [55]. Fourth, a number of central factors can influence the different responses to LCIG and LC-IR, such as transport across the blood–brain barrier, enzymatic conversion of levodopa to dopamine, storage capacity for dopamine in the dopaminergic nerve terminals, dopamine release at the effect site, and changes in pre- and post-synaptic dopamine receptor sensitivity [43]. Dopaminergic PET imaging could have helped to determine whether different patterns of striatal, cortical or limbic dopamine changes can emerge from the two treatments [56].
Our study confirms and extends previous findings showing that advanced PD patients exhibit subclinical affective and cognitive fluctuations in response to significant variations in levodopa plasma levels. Our findings provide novel evidence supporting that these fluctuations can be relatively weakened by inducing more stable plasma levodopa levels with methods like LCIG. Long-term stabilization of plasmatic levodopa levels in advanced PD patients presenting or not clinical non-motor fluctuations might contribute to a long-term reduction in non‐motor symptom burden.
References
Olanow CW (2019) Levodopa is the best symptomatic therapy for PD: nothing more, nothing less. Mov Disord 34(6):812–815. https://doi.org/10.1002/mds.27690
Verschuur CVM, Suwijn SR, Boel JA, Post B, Bloem BR, van Hilten JJ, van Laar T, Tissingh G, Munts AG, Deuschl G, Lang AE, Dijkgraaf MGW, de Haan RJ, de Bie RMA (2019) Randomized delayed-start trial of levodopa in Parkinson's disease. N Eng J Medi 380(4):315–324. https://doi.org/10.1056/NEJMoa1809983
Espay AJ, Morgante F, Merola A, Fasano A, Marsili L, Fox SH, Bezard E, Picconi B, Calabresi P, Lang AE (2018) Levodopa-induced dyskinesia in Parkinson disease: current and evolving concepts. Ann Neurol 84(6):797–811. https://doi.org/10.1002/ana.25364
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson's disease. A community-based study. Brain 123(Pt 11):2297–2305. https://doi.org/10.1093/brain/123.11.2297
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16(3):448–458. https://doi.org/10.1002/mds.1090
Nutt JG, Chung KA, Holford NH (2010) Dyskinesia and the antiparkinsonian response always temporally coincide: a retrospective study. Neurology 74(15):1191–1197. https://doi.org/10.1212/WNL.0b013e3181d90050
Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR (2011) The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord 26(3):399–406. https://doi.org/10.1002/mds.23462
Hillen ME, Sage JI (1996) Nonmotor fluctuations in patients with Parkinson's disease. Neurology 47(5):1180–1183. https://doi.org/10.1212/wnl.47.5.1180
Nissenbaum H, Quinn NP, Brown RG, Toone B, Gotham AM, Marsden CD (1987) Mood swings associated with the 'on-off' phenomenon in Parkinson's disease. Psychol Med 17(4):899–904. https://doi.org/10.1017/s0033291700000702
Racette BA, Hartlein JM, Hershey T, Mink JW, Perlmutter JS, Black KJ (2002) Clinical features and comorbidity of mood fluctuations in Parkinson's disease. J Neuropsychiatry Clin Neurosci 14(4):438–442. https://doi.org/10.1176/jnp.14.4.438
Riley DE, Lang AE (1993) The spectrum of levodopa-related fluctuations in Parkinson's disease. Neurology 43(8):1459–1464. https://doi.org/10.1212/wnl.43.8.1459
Storch A, Schneider CB, Wolz M, Sturwald Y, Nebe A, Odin P, Mahler A, Fuchs G, Jost WH, Chaudhuri KR, Koch R, Reichmann H, Ebersbach G (2013) Nonmotor fluctuations in Parkinson disease: severity and correlation with motor complications. Neurology 80(9):800–809. https://doi.org/10.1212/WNL.0b013e318285c0ed
van der Velden RMJ, Broen MPG, Kuijf ML, Leentjens AFG (2018) Frequency of mood and anxiety fluctuations in Parkinson's disease patients with motor fluctuations: a systematic review. Mov Disord 33(10):1521–1527. https://doi.org/10.1002/mds.27465
Witjas T, Kaphan E, Azulay JP, Blin O, Ceccaldi M, Pouget J, Poncet M, Cherif AA (2002) Nonmotor fluctuations in Parkinson's disease: frequent and disabling. Neurology 59(3):408–413. https://doi.org/10.1212/wnl.59.3.408
Gallagher DA, Lees AJ, Schrag A (2010) What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord 25(15):2493–2500. https://doi.org/10.1002/mds.23394
Kim A, Kim HJ, Shin CW, Kim Y, Jang M, Jung YJ, Lee WW, Park H, Jeon B (2018) Emergence of non-motor fluctuations with reference to motor fluctuations in Parkinson's disease. Parkinsonism Relat Disord 54:79–83. https://doi.org/10.1016/j.parkreldis.2018.04.020
Delpont B, Lhommee E, Klinger H, Schmitt E, Bichon A, Fraix V, Castrioto A, Quesada JL, Pelissier P, Kistner A, Carnicella S, Luscher C, Broussolle E, Pollak P, Thobois S, Krack P (2017) Psychostimulant effect of dopaminergic treatment and addictions in Parkinson's disease. Mov Disord 32(11):1566–1573. https://doi.org/10.1002/mds.27101
Vazquez A, Jimenez-Jimenez FJ, Garcia-Ruiz P, Garcia-Urra D (1993) “Panic attacks” in Parkinson’s disease. A long-term complication of levodopa therapy. Acta Neurol Scand 87(1):14–18
Kulisevsky J, Garcia-Sanchez C, Berthier ML, Barbanoj M, Pascual-Sedano B, Gironell A, Estevez-Gonzalez A (2000) Chronic effects of dopaminergic replacement on cognitive function in Parkinson's disease: a two-year follow-up study of previously untreated patients. Mov Disord 15(4):613–626. https://doi.org/10.1002/1531-8257(200007)15:4<613:aid-mds1005>3.0.co;2-f
Martinez-Fernandez R, Schmitt E, Martinez-Martin P, Krack P (2016) The hidden sister of motor fluctuations in Parkinson's disease: a review on nonmotor fluctuations. Mov Disord 31(8):1080–1094. https://doi.org/10.1002/mds.26731
Schmitt E, Krack P, Castrioto A, Klinger H, Bichon A, Lhommee E, Pelissier P, Fraix V, Thobois S, Moro E, Martinez-Martin P (2018) The neuropsychiatric fluctuations scale for Parkinson’s disease: a pilot study. Mov Disord Clin Practice 5(3):265–272. https://doi.org/10.1002/mdc3.12607
Stacy M, Bowron A, Guttman M, Hauser R, Hughes K, Larsen JP, LeWitt P, Oertel W, Quinn N, Sethi K, Stocchi F (2005) Identification of motor and nonmotor wearing-off in Parkinson’s disease: comparison of a patient questionnaire versus a clinician assessment. Mov Disord 20(6):726–733. https://doi.org/10.1002/mds.20383
Kulisevsky J, Pascual-Sedano B, Barbanoj M, Gironell A, Pagonabarraga J, Garcia-Sanchez C (2007) Acute effects of immediate and controlled-release levodopa on mood in Parkinson's disease: a double-blind study. Mov Disord 22(1):62–67. https://doi.org/10.1002/mds.21205
Maricle RA, Nutt JG, Valentine RJ, Carter JH (1995) Dose-response relationship of levodopa with mood and anxiety in fluctuating Parkinson's disease: a double-blind, placebo-controlled study. Neurology 45(9):1757–1760. https://doi.org/10.1212/wnl.45.9.1757
Richard IH, Frank S, LaDonna KA, Wang H, McDermott MP, Kurlan R (2005) Mood fluctuations in Parkinson's disease: a pilot study comparing the effects of intravenous and oral levodopa administration. Neuropsychiatric Dis Treat 1(3):261–268
Cools R (2006) Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev 30(1):1–23. https://doi.org/10.1016/j.neubiorev.2005.03.024
Kulisevsky J (2000) Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson's disease. Drugs Aging 16(5):365–379. https://doi.org/10.2165/00002512-200016050-00006
Gotham AM, Brown RG, Marsden CD (1988) 'Frontal' cognitive function in patients with Parkinson's disease 'on' and 'off' levodopa. Brain 111(Pt 2):299–321. https://doi.org/10.1093/brain/111.2.299
Poewe W, Berger W, Benke T, Schelosky L (1991) High-speed memory scanning in Parkinson's disease: adverse effects of levodopa. Ann Neurol 29(6):670–673. https://doi.org/10.1002/ana.410290616
Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW (2000) Probabilistic learning and reversal deficits in patients with Parkinson's disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia 38(5):596–612. https://doi.org/10.1016/s0028-3932(99)00103-7
Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A (1996) Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson's disease patients at different levodopa plasma levels. Brain 119(Pt 6):2121–2132. https://doi.org/10.1093/brain/119.6.2121
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 11(12):1136–1143. https://doi.org/10.1093/cercor/11.12.1136
MacDonald PA, MacDonald AA, Seergobin KN, Tamjeedi R, Ganjavi H, Provost JS, Monchi O (2011) The effect of dopamine therapy on ventral and dorsal striatum-mediated cognition in Parkinson's disease: support from functional MRI. Brain 134(Pt 5):1447–1463. https://doi.org/10.1093/brain/awr075
Pascual-Sedano B, Kulisevsky J, Barbanoj M, Garcia-Sanchez C, Campolongo A, Gironell A, Pagonabarraga J, Gich I (2008) Levodopa and executive performance in Parkinson's disease: a randomized study. J Int Neuropsychol Soc 14(5):832–841. https://doi.org/10.1017/S1355617708081010
Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtosek Z, Szasz J, Valldeoriola F, Winkler C, Bergmann L, Yegin A, Onuk K, Barch D, Odin P (2017) Levodopa-carbidopa intestinal gel in advanced Parkinson's: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20. https://doi.org/10.1016/j.parkreldis.2017.09.018
Buongiorno M, Antonelli F, Camara A, Puente V, de Fabregues-Nebot O, Hernandez-Vara J, Calopa M, Pascual-Sedano B, Campolongo A, Valldeoriola F, Tolosa E, Kulisevsky J, Marti MJ (2015) Long-term response to continuous duodenal infusion of levodopa/carbidopa gel in patients with advanced Parkinson disease: the barcelona registry. Parkinsonism Relat Disord 21(8):871–876. https://doi.org/10.1016/j.parkreldis.2015.05.014
Lang AE, Rodriguez RL, Boyd JT, Chouinard S, Zadikoff C, Espay AJ, Slevin JT, Fernandez HH, Lew MF, Stein DA, Odin P, Fung VS, Klostermann F, Fasano A, Draganov PV, Schmulewitz N, Robieson WZ, Eaton S, Chatamra K, Benesh JA, Dubow J (2016) Integrated safety of levodopa-carbidopa intestinal gel from prospective clinical trials. Mov Disord 31(4):538–546. https://doi.org/10.1002/mds.26485
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson's disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13(2):141–149. https://doi.org/10.1016/S1474-4422(13)70293-X
Lopiano L, Modugno N, Marano P, Sensi M, Meco G, Solla P, Gusmaroli G, Tamma F, Mancini F, Quatrale R, Zangaglia R, Bentivoglio A, Eleopra R, Gualberti G, Melzi G, Antonini A (2019) Motor and non-motor outcomes in patients with advanced Parkinson's disease treated with levodopa/carbidopa intestinal gel: final results of the GREENFIELD observational study. J Neurol 266(9):2164–2176. https://doi.org/10.1007/s00415-019-09337-6
Isacson D, Bingefors K, Kristiansen IS, Nyholm D (2008) Fluctuating functions related to quality of life in advanced Parkinson disease: effects of duodenal levodopa infusion. Acta Neurol Scand 118(6):379–386. https://doi.org/10.1111/j.1600-0404.2008.01049.x
Dafsari HS, Martinez-Martin P, Rizos A, Trost M, Dos Santos Ghilardi MG, Reddy P, Sauerbier A, Petry-Schmelzer JN, Kramberger M, Borgemeester RWK, Barbe MT, Ashkan K, Silverdale M, Evans J, Odin P, Fonoff ET, Fink GR, Henriksen T, Ebersbach G, Pirtosek Z, Visser-Vandewalle V, Antonini A, Timmermann L, Ray Chaudhuri K, Europar, the International P, Movement Disorders Society Non-Motor Parkinson's Disease Study G ( 2019) EuroInf 2: Subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson's disease. Mov Disord 34(3):353–365. https://doi.org/10.1002/mds.27626
Nyholm D, Askmark H, Gomes-Trolin C, Knutson T, Lennernas H, Nystrom C, Aquilonius SM (2003) Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol 26(3):156–163. https://doi.org/10.1097/00002826-200305000-00010
Othman AA, Rosebraugh M, Chatamra K, Locke C, Dutta S (2017) Levodopa-carbidopa intestinal gel pharmacokinetics: lower variability than oral levodopa-carbidopa. J Parkinson's Dis 7(2):275–278. https://doi.org/10.3233/JPD-161042
Catalan MJ, Antonini A, Calopa M, Bajenaru O, de Fabregues O, Minguez-Castellanos A, Odin P, Garcia-Moreno JM, Pedersen SW, Pirtosek Z, Kulisevsky J (2017) Can suitable candidates for levodopa/carbidopa intestinal gel therapy be identified using current evidence? eNeurologicalSci 8:44–53. https://doi.org/10.1016/j.ensci.2017.06.004
Volkmann J, Albanese A, Antonini A, Chaudhuri KR, Clarke CE, de Bie RM, Deuschl G, Eggert K, Houeto JL, Kulisevsky J, Nyholm D, Odin P, Ostergaard K, Poewe W, Pollak P, Rabey JM, Rascol O, Ruzicka E, Samuel M, Speelman H, Sydow O, Valldeoriola F, van der Linden C, Oertel W (2013) Selecting deep brain stimulation or infusion therapies in advanced Parkinson's disease: an evidence-based review. J Neurol 260(11):2701–2714. https://doi.org/10.1007/s00415-012-6798-6
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25(15):2649–2653. https://doi.org/10.1002/mds.23429
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. https://doi.org/10.1002/mds.22340
Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A (2008) Parkinson's disease-cognitive rating scale: a new cognitive scale specific for Parkinson's disease. Mov Disord 23(7):998–1005. https://doi.org/10.1002/mds.22007
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6):361–370. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x
Starkstein SE, Merello M, Jorge R, Brockman S, Bruce D, Power B (2009) The syndromal validity and nosological position of apathy in Parkinson's disease. Mov Disord 24(8):1211–1216. https://doi.org/10.1002/mds.22577
Marras C, Troster AI, Kulisevsky J, Stebbins GT (2014) The tools of the trade: a state of the art "How to Assess Cognition" in the patient with Parkinson's disease. Mov Disord 29(5):584–596. https://doi.org/10.1002/mds.25874
Lezak MHD, Bigler E, Tranel D (2012) Neuropsychological assessment. Oxford University Press, Oxford
Sternberg S (1966) High-speed scanning in human memory. Science 153(3736):652–654. https://doi.org/10.1126/science.153.3736.652
Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VS, Klostermann F, Lew MF, Odin P, Steiger M, Yakupov EZ, Chouinard S, Suchowersky O, Dubow J, Hall CM, Chatamra K, Robieson WZ, Benesh JA, Espay AJ (2015) Levodopa-carbidopa intestinal gel in advanced Parkinson's disease: final 12-month, open-label results. Mov Disord 30(4):500–509. https://doi.org/10.1002/mds.26123
Black KJ, Hershey T, Hartlein JM, Carl JL, Perlmutter JS (2005) Levodopa challenge neuroimaging of levodopa-related mood fluctuations in Parkinson's disease. Neuropsychopharmacology 30(3):590–601. https://doi.org/10.1038/sj.npp.1300632
Christopher L, Marras C, Duff-Canning S, Koshimori Y, Chen R, Boileau I, Segura B, Monchi O, Lang AE, Rusjan P, Houle S, Strafella AP (2014) Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson's disease with mild cognitive impairment. Brain 137(Pt 2):565–575. https://doi.org/10.1093/brain/awt337
Acknowledgements
We are grateful to all patients and caregivers for their generous participation in the study. This study was partially supported by FIS Grant PI15/00962; Rio Hortega CM17/00209 and CIBERNED (Instituto de Salud Carlos III, Spain); La Marató de TV3, Expedient 20142910, 2014/U/477, and an unrestrictive research grant from Abbvie.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
J Kulisevsky received compensation for consultancy and speaker activities from UCB, AbbVie, Neuroderm, Teva, Roche and Zambon. He received research support from Teva, Zambon, Abbvie, Ciberned and Carlos III Institute Research Grant FIS PI18/0717; S Martinez-Horta received compensation for speaker activities from UCB, AbbVie and Roche. He received research support from the Huntington's Disease Society of America; H Bejr-kasem received compensation for speaker activities in scientific meetings supported by Zambon, and non-financial support for congress attendance from Abbvie, Zambon and Allergan.I Aracil-Bolaños has received research support from Carlos III Institute Research Grant CM19/00156 G; B Pascual-Sedano received compensation for consultancy from Ferrer and speaker activities from AbbVie, UCB and Teva; C Izquierdo received compensation for speaker activities from UCB and Abbvie; A Campolongo received compensation for consultancy and speaker activities from UCB, AbbVie and Teva; J Pagonabarraga has received honoraria as Speaker or as member of Advisory Board from Zambon, UCB, Abbvie, Allergan and Ipsen; J Marín received compensation for consultancy and speaker activities from UCB; J Perez-Perez, O de Fàbregues, V Puente, A Crespo-Cuevas and M Calopa have no conflict of interest to declare.
Ethical standard
The study has been approved by the ethical committee of sant Pau Hospital (Barcelona, Spain). Written informed consent was obtained from all participants and the study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Rights and permissions
About this article
Cite this article
Kulisevsky, J., Bejr-Kasem, H., Martinez-Horta, S. et al. Subclinical affective and cognitive fluctuations in Parkinson's disease: a randomized double-blind double-dummy study of Oral vs. Intrajejunal Levodopa. J Neurol 267, 3400–3410 (2020). https://doi.org/10.1007/s00415-020-10018-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10018-y