Abstract
A drug challenge test in Parkinson’s disease, such as the levodopa challenge test (LCT), is an easy and generally safe procedure, which has been used by clinicians for various indications. The results of the test have significant implications in the management of patients, from preoperative evaluation for deep brain stimulation to providing the basis for medication adjustments to address motor or non-motor fluctuations and dyskinesias. This paper reviews the different indications and protocols commonly used in an acute LCT. Potential complications of the procedure and an overview of levodopa responsiveness and unresponsiveness are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A characteristic hallmark of Parkinson’s disease (PD) is its excellent response to dopaminergic medications, most notably levodopa [1]. The immediate motor benefit that lasts hours and which parallels the plasma levels of levodopa has been referred to as the short-duration response (SDR). Alternatively, the long-duration response (LDR) is the sustained motor improvement lasting days to weeks with repeated levodopa administration [2]. Both types of responses constitute the overall drug benefit; however, the extent to which the SDR or LDR contributes to the motor improvement may differ depending on the stage of the disease [2, 3]. Acute drug challenge tests such as the levodopa challenge test (LCT) have been used since the 1980s for various clinical and experimental purposes. By pharmacologically stimulating central dopamine receptors, the effects of dopaminergic transmission can be clinically observed, reflecting the SDR associated with levodopa intake.
Various medications have been used in acute dopaminergic drug challenge tests in PD, including levodopa, subcutaneous apomorphine, clozapine, biperiden, amantadine, piribedil, bromocriptine, intravenous lisuride, and ester prodrugs of levodopa [4]. The use of either levodopa or apomorphine has their own advantages and disadvantages but their effects are similar with regard to predicting chronic levodopa response [5]. Levodopa is more commonly used because it is cheaper and is more available and accessible compared to apomorphine. There are also more adverse effects observed in apomorphine challenge tests [5].
Published papers utilizing the LCT as part of their study have applied different methods of performing the drug challenge test. Several papers have cited the protocol outlined in the Core Assessment Program for Intracerebral Transplantations (CAPIT) and the Core Assessment Program for Surgical Interventional Therapies in Parkinson's Disease (CAPSIT-PD). These were based on the paper of Lindvall et al., which used the LCT as part of a preoperative assessment of patients undergoing fetal dopamine neuron grafting [6,7,8]. Over the years, there have been innovations in the LCT with the incorporation of different clinical and neurophysiologic parameters. Defining levodopa responsiveness has also been debated since the conception of the LCT, but recent studies have established some consensus in this regard. An updated review is, therefore, needed to succinctly discuss the essential aspects of this clinically useful procedure, such as its indications, the commonly used protocols, as well as its potential complications. The pertinent topics of levodopa responsiveness and unresponsiveness are also included in this review.
Indications for LCT
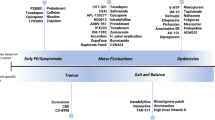
The LCT has different clinical uses aside from its experimental application in clinical trials to induce the effects of dopaminergic stimulation (Table 1). The most frequent indication of a LCT today is probably as an evaluation tool to ensure that a patient is a suitable candidate for invasive forms of treatment. Demonstration of levodopa responsiveness in an acute LCT has been considered an absolute requirement in the screening process for deep brain stimulation (DBS) [6]. This is also important to educate the patient and their caregivers of the possible outcome of surgery and the extent to which their symptoms will improve, i.e., to establish realistic expectations from the surgery [9]. In general, possibly apart from tremor, the symptoms that respond to levodopa are also expected to improve with surgery [10]. Demonstration of levodopa responsiveness, which may be best characterized through a drug challenge test, is also a requirement prior to continuous intestinal infusion therapy of levodopa/carbidopa [11, 12].
A LCT is also helpful in the re-evaluation of patients when they have issues regarding medication response, such as latency or onset of benefit, the magnitude of response, and the duration of benefit [4]. There are patients with a diagnosis of idiopathic PD who report having had a suboptimal response with levodopa despite the clinician noticing an improvement of their performance on rating scales [13]. The LCT is an excellent opportunity for both the clinician to confirm medication responsiveness and for the patient to get a better picture of the extent to which parkinsonian motor symptoms respond to levodopa. This can easily be done by evaluating the specific subcomponents of the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS UPDRS) Part III, i.e., bradykinesia sub-component score (sum of the scores for finger tapping, hand movements, pronation–supination movements, toe tapping, and leg agility scores), rigidity sub-component score (sum of the scores for rigidity of the neck and all four limbs), tremor sub-component score (sum of the scores for rest tremor, constancy of rest tremor, postural tremor, and kinetic tremor scores), axial symptom sub-component score (sum of the scores for arising from chair, posture, and postural instability), and gait. Gait assessment may also be done by recording the time it takes for the patient to walk a defined distance (e.g. 3 m or 12 m) [14]. Furthermore, a LCT may also be used to further characterize the other effects of levodopa, such as the nature and timing of dyskinesias, as well as non-motor symptoms [4, 15,16,17]. However, end-of-day dyskinesias (end-of-dose dyskinesias that are limited to the end of the day) and rebound off-state phenomenon may not be reflected in a routine LCT [4].
It has also long been debated whether a LCT can be used in the diagnosis of PD. While levodopa responsiveness demonstrated in a LCT can be used to support the diagnosis of PD [4], a single acute LCT is not recommended to diagnose PD [18]. Foremost, some PD patients predominantly have a long-duration response (LDR) to levodopa that is not reflected in an acute LCT [2, 3]. Different proposed mechanisms underly the LDR such as receptor sensitization, alteration of post-synaptic cellular elements, and central and peripheral pharmacokinetic properties of levodopa [2]. If the patient has been receiving chronic levodopa therapy, a LDR may mask the SDR in an acute challenge test [19]. Other factors might also affect the response seen in an acute LCT, such as an inappropriate LCT protocol and poor drug absorption. Acute side effects such as emesis and hypotension may limit assessment of efficacy despite efforts to avoid these (e.g., use of domperidone, additional carbidopa). In contrast, some recent studies claim that LCT, especially when combined with other objective parameters, can be used to increase the sensitivity of diagnosing early PD [14, 20, 21]. The accurate differentiation of PD from other forms of parkinsonism remains an unmet need to date. For example, this is important in conducting clinical trials, where exclusion of other parkinsonian disorders is crucial. In one study, LCT, combined with olfactory testing (Sniffin’ Sticks Test), had a 90% sensitivity in diagnosing PD among patients presenting with parkinsonism [20]. This study was conducted in a center where an acute LCT is routinely done in all patients with a recent complaint of tremor or parkinsonism. Another recent study, which utilized data from the DeNoPa cohort, showed that ≥ 33% levodopa responsiveness on an acute LCT, combined with other clinical data (urinary incontinence, fainting, asymmetric tremor, and amount of further drug-intake), can be considered a reliable tool to diagnose early de novo PD, with a sensitivity of 91% [14]. However, this was only based on a clinical diagnosis of PD after a 2-year follow-up period.
In relation to the use of the LCT in diagnosing PD patients in drug-naïve patients is its potential utility in differentiating PD from other forms of parkinsonism. The ability to differentiate various causes of parkinsonism remains an unmet need at present, given the lack of reliable biomarkers to correctly diagnose these disorders. This has implications not only for clinical trials of neuroprotective agents that need to recruit patients in the early stages of the disease but also in the selection of patients for DBS given the common experience that patients with atypical parkinsonism misdiagnosed as PD generally have a suboptimal response to the surgery [22]. Recommendations from clinical practice guidelines differ; the 2006 American Academy of Neurology (AAN) practice parameters stated that a LCT is probably useful in this aspect [23], but both the 2013 European Federation of Neurological Societies/Movement Disorder Society-European Section (EFNS/MDS-ES) and the 2017 National Institute for Health and Clinical Excellence (NICE) guidelines do not recommend using drug challenge tests in differentiating parkinsonian disorders [18, 24]. While an acute LCT may be able to predict the chronic response to levodopa therapy [5], a systematic review found no added benefit in performing an acute LCT to predict chronic levodopa responsiveness as a way of differentiating various forms of parkinsonism [25]. All patients with parkinsonism will still be tried on chronic levodopa therapy as a form of symptomatic treatment, although the initial positive response in atypical parkinsonism is usually not as marked and sustained as in PD [26]. Relevant to this issue and the use of a LCT as a method of diagnosing PD, chronic levodopa responsiveness is not infrequently evident in the absence of a significant levodopa response on an acute LCT in drug-naïve patients. Drug challenge tests in de novo PD patients generally have a false-negative rate of 40% [4]. Furthermore, acute problematic side effects (e.g., orthostatic hypotension) may occur on a LCT in patients with atypical parkinsonism more often than in those with PD [27]. Interestingly, this study found the difference in the frequency of side effects and tolerance to a LCT as a reliable way to differentiate PD from atypical parkinsonism, in contrast to an earlier study that did not find a significant difference in LCT intolerance between patients with PD and multiple system atrophy (MSA) [28].
Levodopa challenge protocol
Preparation and timing
Patients should withhold all dopaminergic medications for a minimum of 12 h to allow for an appropriate washout of levodopa and to induce a practically defined “off medication state” [6]. Thus, most of the time, the LCT is done early in the morning because it is easier to withdraw dopaminergic medications overnight when the patient is not active. A long washout period (15 days) is also necessary to minimize the effect of LDR in the motor response observed in a LCT, especially in patients on chronic levodopa therapy; this assessment is exclusively used in selected research protocols and is not part of the routine LCT [2, 19]. Occasionally, a LCT is done at a specific time of the day when the symptoms or the main concerns of the patient (e.g., dyskinesias) are typically more pronounced. There are also certain instances when the patient can be brought to the clinic for a LCT without having to be off their PD medications, as long as the objective of the test will not be compromised. For example, in some patients, withholding morning medications will result in a severe debilitating off state (including prolonged, painful dystonia), making transport to the clinic challenging. In this situation, it may be sufficient to perform the LCT after the benefit from the first medication dose has worn off, especially if the purpose of the test is just for the characterization of dopaminergic effects and dyskinesias. The evaluators can, therefore, decide on the duration of the drug withdrawal based on their clinical judgment while considering the patient's ability to tolerate being off medications [6]. The LCT is probably best conducted in the fasting state [5], but this is not necessary as long as there is at least a 45-min interval between the procedure and the last meal (to optimize gastric emptying and to avoid the interference of dietary protein on the absorption of levodopa). Furthermore, the levodopa tablets can be crushed and taken with 50–100 ml of a carbonated beverage to hasten its absorption [4, 7].
Domperidone, where available, may be given to patients who complain of levodopa-induced nausea and orthostatic hypotension, if the LCT is performed in drug naïve patients or if apomorphine is used as the challenge agent [4]. The recommended dose is between 10 and 20 mg, given 30 min prior to levodopa administration [29]. Sometimes, the patient has to be premedicated with domperidone for 2–3 days before the procedure, typically with 10–20 mg thrice daily and rarely up to a dose of 100–120 mg per day [4, 30]. More recent concerns about domperidone’s potential effects on the QT interval now make it difficult to justify the use of such high doses [31].
Dosing
The dose of levodopa used in an acute LCT depends on the purpose of the test (Table 1). In the CAPSIT protocol for DBS preoperative evaluation, the authors recommended keeping the dose before and after surgery the same and using the maintenance dose to define the dopaminergic responsiveness [6]. In many centers, including our own, a higher dose of 120% of the patient’s morning levodopa dose is used to compensate for other dopaminergic medications on hold and to overcome problems of drug absorption. Occasionally, the morning levodopa equivalent dose (LED) has to be computed. A peripheral dopa decarboxylase inhibitor such as carbidopa or benserazide, is always given together with levodopa. Furthermore, if the purpose of the test is for a re-evaluation of levodopa responsiveness, a higher dose of 120% may also be used for the same reasons. If the patient reports a suboptimal response with the current dose, then an additional + 20 to + 50% of the morning dose may be used to determine if there is a greater improvement of symptoms with higher doses. It is important to note, however, that not all motor symptoms are levodopa responsive, and some symptoms, such as tremor, may require considerably higher doses of levodopa before a significant improvement may be seen [4, 32]. One study proposed escalating doses of levodopa at 100, 150, 200, 300 mg on four consecutive days [33]. In this study, the authors stated that 150, 200, or 300 mg of levodopa could be used for the differential diagnosis of parkinsonism, and they also recommended 300 mg as the dose to be used for preoperative screening. Generally, a dose of 150% of the standard first morning levodopa dose is considered the suprathreshold dose in most studies on LCT, even in experimental studies where drug effects such as dyskinesias are being evaluated [16, 34,35,36]. Finally, if the objective of the LCT is the characterization of dyskinesias, the dose used in the LCT is typically the same as the maintenance dose of levodopa. As in the other indications, this is also an opportunity to educate the patients on how to differentiate dyskinesias from other motor symptoms such as tremor.
Periods of assessment
The duration of the LCT and the periods of assessment will depend again on the purpose of the test, but generally, an evaluation must be done in the “off state” (period A), during the onset of levodopa effect (period B), at the peak of levodopa effect (period C), and occasionally, as the effects wane (period D) and at the end of the entire dosing interval (period E) (Table 2). The latter two assessment periods are particularly crucial if wearing off, duration of benefit, and dyskinesias are an issue. The onset of levodopa effect is subjectively reported by the patient; objectively, it is defined as the time at which there is > 15% improvement in the motor scores compared to the off state [37]. The onset of levodopa effect is usually around 30 min after oral drug intake on an empty stomach [38]. The peak of levodopa effect is also subjectively reported by the patient as the best “on state” and is thus variable, but most studies on LCT set this assessment period at 60 min, knowing that the half-life of levodopa is around 1.5 h when taken with carbidopa [38]. It is crucial for both the patient and the evaluator to agree with the best “on state” as this will be used in the calculation of the levodopa responsiveness. In the postoperative evaluation of DBS, the assessment of the peak benefit (period C) can further be defined as two states: “medication on/stimulation off” and “medication on/stimulation on.”
Since the results of the LCT often have an impact on the management of the patient, it is highly recommended that the assessment be done by experienced physicians or nurses. One practical and standardized method of establishing the consistency of assessment is for the evaluator to become certified to conduct the MDS UPDRS [39] and Unified Dyskinesia Rating Scale (UDysRS) [40], especially since the rating scales used are subjective and rater dependent (see https://www.movementdisorders.org/MDS/MDS-Rating-Scales.htm for more information). If possible, all periods of assessment in a LCT should be videotaped for later re-evaluation. This is particularly important for patients undergoing surgical therapies since it can be very useful to be able to assess the preoperative state in light of postoperative issues. These include an apparent poor/suboptimal response to DBS as well as in patients who can be shown to have had a good response but who are dissatisfied in part because they do not adequately recall their preoperative status for comparison [22, 41].
Objective assessment parameters
Assessment of the motor symptoms is done using clinically validated scales, such as the MDS UPDRS III [42]. A detailed evaluation of the subcomponents of the test, i.e., bradykinesia, rigidity, tremor, and axial symptoms, is recommended to identify the specific symptoms that respond to levodopa. Other objective measures can be used, such as finger tapping speed, counting taps between two fixed points, 3 m or 12 m walk time, timed up and go test (TUG), 30-s step test as well as a variety of tests that can be used in selected circumstances (mainly for research) including heart rate variability through continuous ECG monitoring, olfactory testing, fiberoptic endoscopic evaluation of swallowing (FEES), ultrasound strain elastography, speech and voice analysis and orofacial strength [4, 16, 20, 36, 37, 43,44,45,46,47]. Levodopa-induced dyskinesias can be evaluated using the UDysRS, but independent of the purpose of the LCT, the presence and nature (body location, movement disorder phenomenology) of dyskinesias should always be noted in all assessment periods [40]. The blood pressure should also be obtained in the “off state” (period A) and at the peak of levodopa effect (period C), especially since levodopa-induced orthostatic hypotension can be a complication of the procedure but may also be an explanation of the patient’s complaints that are the reason for conducting the LCT (e.g., mental fogginess, falls, neck and shoulder pain, etc.). Moreover, non-motor symptoms can also be assessed in a LCT using the Non-motor Symptoms Scale (NMSS) [16, 17] or possibly an adaptation of the new Movement Disorder Society Nonmotor Rating Scale [48]. In addition, there are also scales for the specific non-motor symptoms of interest: visual analog scale (VAS) for pain and fatigue, Strait–Trait Anxiety (STAI) for anxiety, Neuropsychiatric Inventory test 12-items for various psychiatric symptoms in dementia patients, and the Geriatric Depression Scale (GDS) for depression [16].
Subjective assessment
At each period of assessment, the patient’s insight about his/her symptoms must also be asked. Not only is the LCT important to determine the onset and peak of levodopa benefit, it is also a venue to educate the patient about the symptoms which can improve with levodopa, dyskinesias, and signs of motor fluctuations. To date, there are no structured patient questionnaires for this subjective assessment. In one recent study that compared the association between patients’ reported subjective improvement (in percentage) and the objective measures used by clinicians in a LCT, the authors simply asked the percentage of improvement in their motor disability compared to the “off state” [13]. There was a good correlation between the reported improvement and the calculated levodopa responsiveness. In the same study, the presence of peak-dose dyskinesias and the axial symptom sub-component scores led to an underestimation of the perceived motor improvement [13]. Adequate patient education about dyskinesias and levodopa responsive motor symptoms can correct this underestimation.
Defining levodopa responsiveness
An acute LCT only reflects the SDR, which is the motoric improvement that comes with the rise of the plasma levels of levodopa [2]. The magnitude of improvement in the MDS UPDRS III score from the “off state” (period A) to the peak of levodopa effect (period C) is expressed in terms of % Levodopa Responsiveness (%LR), calculated as %\({\text{LR}} = \frac{{{\text{"OFF"}}\,{\text{MDS UPDRS}}\,{\text{III}}\,{\text{score }}{-}{\text{PEAK}}\,{\text{"ON"}}\,{\text{MDS UPDRS}}\,{\text{III}}\,{\text{score}}}}{{{\text{"OFF"}}\,{\text{MDS UPDRS}}\,{\text{III}}\,{\text{score}}}} \times 100\). In the preoperative evaluation of patients, %LR is a critical value that can mean the difference between proceeding with surgery or not. It is not surprising, therefore, that the cut-off value for %LR has been debated for many years. The cut-off value for %LR actually depends on its ability to predict chronic levodopa responsiveness. This was previously important when LCT was commonly used in de novo patients with parkinsonism to differentiate PD from atypical parkinsonian syndromes [5]. Previously, a significant %LR was arbitrarily set at 30% based on the supposition that placebo effects occur in one-third of patients [42], although it is unclear why a 30% placebo response rate was equated with a 30% improvement in UPDRS III. The CAPIT and CAPSIT-PD preoperative protocols have also arbitrarily set a minimum of 33% improvement in the UPDRS III score in the LCT [6, 7]. This cut-off value has been validated by two earlier studies based on its ability to predict chronic levodopa responsiveness [5, 42]. The CAPIT protocol used the UPDRS III, and the cut-off value was set at 30% to achieve an acceptable sensitivity, specificity, and positive predictive value of 70.9%, 81.4%, and 88.6%, respectively [5]. This was validated by a later study that used the MDS UPDRS III, and the cut-off value was set at 24% to attain the same degree of sensitivity and specificity [42]. A recent study in de novo PD patients showed that a %LR of 33% is an optimal cut-off that provides a modestly high sensitivity and specificity of 70%, with a positive predictive value of 92.3% and a negative predictive value of 32.1% for chronic levodopa response, confirming the value set in older validation studies [14]. Moreover, the onset (or latency) of levodopa response has been defined as the time when there is at least a 15% improvement in the motor scores compared to the “off state” [37]. Other objective clinical measures, such as a tapping test and a walk test, have lower cut-offs of 15% and 25%, respectively [37]. When the LCT is just performed to characterize dyskinesias, the %LR (using the total motor scores) is still calculated to help determine the phase at which the dyskinesias appear, i.e., whether the dyskinesias occur at the onset (i.e., “beginning-of-dose” dyskinesias), at the peak (i.e., “peak-dose” dyskinesias), or towards the end of the dosing interval (i.e., “end-of-dose” dyskinesias), or diphasic dyskinesias.
Dopaminergic responsiveness may differ depending on the stage of PD, with the LDR having a more significant role early in the disease and the SDR being the predominant response in late-stage PD [49]. Since the conventional levodopa responsiveness is expressed as %LR, a change in the UPDRS III score from 75 to 50 is seemingly similar to a change from 45 to 30, both representing a 33% benefit. Clearly, the first scenario reflects a worse motor state given the higher score in the “off state”, which is usually seen in late-stage PD.
As indicated above, in addition to the motor assessment, another very important aspect of drug challenge tests is the assessment of non-motor symptoms (NMS) in PD. This has been investigated in two recent studies, which included patients with late-stage PD [16, 17]. Both studies showed that NMS could also be objectively assessed in a LCT using scales such as the NMSS and STAI, among others, but the degree of levodopa responsiveness of NMS is less in late-stage PD, especially in patients demonstrating little improvement in their motor symptoms [16, 17]. Levodopa-induced orthostatic hypotension and sleepiness are also common in late-stage PD [16].
Levodopa unresponsiveness in an acute LCT
If the patient does not meet the cut-off value for a significant %LR, the LCT can be repeated twice according to the CAPIT protocol [7]. Here, repeat LCTs were spaced 1 week apart, with the same washout period and performed at the same time of the day, at an escalating dose of 150% and 200% of the normal levodopa dose before the patients were deemed ineligible for neural transplantation [7]. This was, however, not recommended as part of the CAPSIT-PD protocol for the preoperative evaluation of patients for DBS or pallidotomy [6]. If patients do not meet the significant cut-off for %LR defined in the CAPSIT protocol, DBS can still be considered in cases of severe dyskinesia, on/off motor fluctuations (which cannot be adequately assessed in an acute LCT) and in those with medication-refractory tremor [41].
There are several reasons why a PD patient does not show levodopa responsiveness in a LCT. Some of the motor features of PD are known to be relatively unresponsive (or less responsive than others) to levodopa, such as axial symptoms, postural instability, gait, and occasionally tremors [32]. Thus, PD patients with the postural instability–gait difficulty (PIGD) subtype and those with poorly levodopa responsive tremors may have a lower %LR. Poor drug absorption is also another reason for levodopa unresponsiveness [50]. Moreover, drug-naïve patients with early PD may not meet the cut-off of the %LR in an acute LCT [4]. The LCT, which best reflects the SDR to levodopa, may not acutely improve the motor symptoms in patients with a predominant LDR to the drug, such as in patients with early PD, especially in the first year of therapy [2]. In de novo PD patients, the LDR can be estimated through a subacute LCT as demonstrated by Quattrone et al. [51]. In the ELLDOPA study, the LDR was estimated as the difference between the early morning “off state” total UPDRS score after a 2 week washout period, although a longer washout period of 32 days was also suggested [52]. In addition, patients with a presumed LDR can also be tried on levodopa for at least 3–6 months at a dose of at least 800–1200 mg per day to show levodopa responsiveness [4].
Complications and side effects
The acute LCT is generally a safe procedure that can be performed in an outpatient or inpatient setting. Unless an adverse event occurred during the procedure, there is no need to monitor the patient after the test. There have been no serious adverse events reported related to withholding dopaminergic medications for 12 h, likely because of the long-duration response (LDR) with levodopa, which can last for a few days to weeks [2]. Patients must be apprised, however, regarding the “off state”, which may be severe and disabling for some. Off-period dystonia may be a consequence of withholding the morning dose of medications. Clinicians must also be aware that abrupt discontinuation of dopaminergic medications is a known trigger of neuroleptic-malignant-like (NMS-like) events in PD [53], but to our knowledge, this has not been reported to have occurred in the setting of a LCT. The risk of NMS-like events is probably low because of the brief duration of the drug withdrawal and the relatively stable condition of the patients when performing a drug challenge test (i.e., without intercurrent infection, etc.). Nausea, vomiting, hypotension, and profuse perspiration can be observed in a LCT, even among patients who were premedicated with domperidone [28]. In a study comparing the apomorphine challenge test with the LCT, levodopa was found to be better tolerated, with less frequent and milder occurrences of nausea and vomiting [54]. In one recent study concentrating on the side effects of LCT, a total of 63 patients with parkinsonism (34 with drug-naïve PD, 10 with MSA, 12 with Progressive Supranuclear Palsy and 7 with Corticobasal Degeneration) underwent LCT with 250 mg of levodopa, and the most frequent side effects noted were nausea (17.5%), sleepiness (11%), and dizziness (6%) [27]. There were no serious side effects, but there were more side effects noted among patients with atypical parkinsonism compared to those with PD. In two related studies where a supramaximal dose of levodopa was given at 150% of the morning levodopa equivalent dose, moderate drowsiness and orthostatic hypotension were observed [16, 35]. It is probably important to note that the patients included in these studies had late-stage PD, with a mean age of 78.8 years. Many of these side effects are preventable by pretreatment with domperidone, especially if the patient is known to have them beforehand. In cases of severe hypotension, it might be necessary to abort the routine LCT procedure and prioritize the management of the hypotension.
Conclusions
In summary, we have reviewed the different indications, protocols, and safety of the LCT. Levodopa responsiveness, the primary endpoint of this drug challenge test, is defined as the magnitude of improvement in the motor score at the peak of levodopa effect compared to the “off state.” Based on recent validation studies, a %LR of > 33%, using the MDS UPDRS III as the objective measure, can be considered a sensitive and specific cut-off value in predicting chronic levodopa responsiveness. Coincidentally, this is similar to the arbitrary cut-off value adapted by both the CAPIT and CAPSIT-PD protocol. Nevertheless, DBS candidates who do not meet the significant cut-off value for %LR can still undergo surgery after considering other aspects known to be responsive to surgery but that may not be adequately assessed in an acute LCT. Finally, dyskinesias, non-motor symptoms, and other dopaminergic effects can also be evaluated using the LCT. The LCT is an important, clinically valuable, simple, and safe procedure that can be used for various indications in the medical and surgical management of PD.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/S0140-6736(14)61393-3
Anderson E, Nutt J (2011) The long-duration response to levodopa: phenomenology, potential mechanisms and clinical implications. Parkinsonism Relat Disord 17:587–592. https://doi.org/10.1016/j.parkreldis.2011.03.014
Nutt J, Carter J, Woodward W (1995) Long-duration response to levodopa. Neurology 45:1613–1616
Albanese A, Bonuccelli U, Brefel C et al (2001) Consensus statement on the role of acute dopaminergic challenge in Parkinson’s disease. Mov Disord 16:197–201
Merello M, Nouzeilles MI, Arce GP, Leiguarda R (2002) Accuracy of acute levodopa challenge for clinical prediction of sustained long-term levodopa response as a major criterion for idiopathic Parkinson’s disease diagnosis. Mov Disord 17:795–798. https://doi.org/10.1002/mds.10123
Defer GL, Widner H, Marié RM et al (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 14:572–584. https://doi.org/10.1002/1531-8257(199907)14:4%3c572:AID-MDS1005%3e3.0.CO;2-C
Langston JW, Widner H, Goetz CG et al (1992) Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 7:2–13. https://doi.org/10.1002/mds.870070103
Lindvall O, Rehncrona S, Brundin P et al (1989) Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease: a detailed account of methodology and a 6-month follow-up. JAMA Neurol 46:615–631. https://doi.org/10.1001/archneur.1989.00520420033021
Lang AE, Widner H (2002) Deep brain stimulation for Parkinson’s disease: patient selection and evaluation. Mov Disord 17:S94–S101. https://doi.org/10.1002/mds.10149
Machado A, Fernandez HH, Deogaonkar M (2012) Deep brain stimulation: what can patients expect from it? Cleve Clin J Med 79:113–120. https://doi.org/10.3949/ccjm.78gr.11006
Abbruzzese G, Barone P, Bonuccelli U et al (2012) Continuous intestinal infusion of levodopa/carbidopa in advanced Parkinson’s disease: efficacy, safety and patient selection. Funct Neurol 27:147–154
Fernandez HH, Standaert DG, Hauser RA et al (2015) Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord 30:500–509. https://doi.org/10.1002/mds.26123
Rabel C, Le Goff F, Lefaucheur R et al (2016) Subjective perceived motor improvement after acute levodopa challenge in Parkinson’s disease. J Parkinsons Dis 6:779–785. https://doi.org/10.3233/JPD-160906
Schade S, Sixel-Döring F, Ebentheuer J et al (2017) Acute levodopa challenge test in patients with de novo Parkinson’s disease: data from the DeNoPa cohort. Mov Disord Clin Pract 4:755–762. https://doi.org/10.1002/mdc3.12511
Ko P-W, Kang K, Lee H-W (2018) Levodopa-induced respiratory dysfunction confirmed by levodopa challenge test: a case report. Medicine (Baltimore) 97:e12488–e12488. https://doi.org/10.1097/MD.0000000000012488
Fabbri M, Coelho M, Guedes LC et al (2017) Response of non-motor symptoms to levodopa in late-stage Parkinson’s disease: results of a levodopa challenge test. Parkinsonism Relat Disord 39:37–43. https://doi.org/10.1016/j.parkreldis.2017.02.007
Rosqvist K, Odin P, Hagell P et al (2018) Dopaminergic effect on non-motor symptoms in late stage Parkinson’s disease. J Parkinsons Dis 8:409–420. https://doi.org/10.3233/JPD-181380
Berardelli A, Wenning GK, Antonini A et al (2013) EFNS/MDS-ES recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol 20:16–34. https://doi.org/10.1111/ene.12022
Zappia M, Colao R, Montesanti R et al (1997) Long duration response to levodopa influences the pharmacodynamics of short-duration response in Parkinson’s disease. Ann Neurol 42:245–248. https://doi.org/10.1002/ana.410420217
Terroba Chambi C, Rossi M, Bril A et al (2017) Diagnostic value of combined acute levodopa challenge and olfactory testing to predict Parkinson’s disease. Mov Disord Clin Pract 4:824–828. https://doi.org/10.1002/mdc3.12517
Asayama S, Wate R, Kaneko S et al (2013) Levodopa challenge test and 123I-metaiodobenzylguanidine scintigraphy for diagnosing Parkinson’s disease. Acta Neurol Scand 128:160–165. https://doi.org/10.1111/ane.12104
Okun MS, Tagliati M, Pourfar M et al (2005) Management of referred deep brain stimulation failures. Arch Neurol 62:1250. https://doi.org/10.1001/archneur.62.8.noc40425
Suchowersky O, Reich S, Perlmutter J et al (2006) Practice parameter: diagnosis and prognosis of new onset Parkinson disease (an evidence-based review). Neurology 66:968–975. https://doi.org/10.1212/01.wnl.0000215437.80053.d0
National Collaborating Centre for Chronic Conditions (Great Britain) (2017) Parkinson’s disease: national clinical guideline for diagnosis and management in primary and secondary care. Royal College of Physicians, London
Clarke CE (2002) Systematic review of acute levodopa and apomorphine challenge tests in the diagnosis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 69:590–594. https://doi.org/10.1136/jnnp.69.5.590
Levin J, Kurz A, Arzberger T et al (2016) ÜBERSICHTSARBEIT: Differenzialdiagnose und Therapie der atypischen Parkinson-syndrome. Dtsch Arztebl Int 113:61–69. https://doi.org/10.3238/arztebl.2016.0061
Vasta R, Nicoletti A, Mostile G et al (2017) Side effects induced by the acute levodopa challenge in Parkinson’s disease and atypical parkinsonisms. PLoS ONE 12:1–6. https://doi.org/10.1371/journal.pone.0172145
Estévez S, Perez-Lloret S, Merello M (2009) Does clinical intolerance to a diagnostic acute levodopa challenge differentiate multiple system atrophy from pd? Int J Neurosci 119:2257–2261. https://doi.org/10.3109/00207450903139721
Müller T, Benz S, Przuntek H (2000) Choice reaction time after levodopa challenge in parkinsonian patients. J Neurol Sci 181:98–103. https://doi.org/10.1016/S0022-510X(00)00436-6
Müller T, Benz S (2002) Quantification of the dopaminergic response in Parkinson’s disease. Parkinsonism Relat Disord 8:181–186. https://doi.org/10.1016/S1353-8020(01)00010-4
Rhew K, Han N, Oh JM (2019) Impact of safety warning on domperidone prescribing for geriatric patients in South Korea: analysis of national insurance claim data. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16162985
Sethi K (2008) Levodopa unresponsive symptoms in Parkinson disease. Mov Disord 23:S521–S533. https://doi.org/10.1002/mds.22049
Feng T, Li W, Lu L et al (2009) Acute stepwise challenge test with levodopa in treated patients with parkinsonism. Parkinsonism Relat Disord 15:354–358. https://doi.org/10.1016/j.parkreldis.2008.08.010
Castrioto A, Kistner A, Klinger H et al (2013) Psychostimulant effect of levodopa: reversing sensitisation is possible. J Neurol Neurosurg Psychiatry 84:18–22. https://doi.org/10.1136/jnnp-2012-302444
Fabbri M, Coelho M, Abreu D et al (2016) Do patients with late-stage Parkinson’s disease still respond to levodopa? Parkinsonism Relat Disord 26:10–16. https://doi.org/10.1016/j.parkreldis.2016.02.021
Ruonala V, Tarvainen MP, Karjalainen PA et al (2015) Autonomic nervous system response to L-dopa in patients with advanced Parkinson’s disease. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS 2015:6162–6165. https://doi.org/10.1109/EMBC.2015.7319799
Lucetti C, Logi C, Del Dotto P et al (2010) Levodopa response in dementia with Lewy bodies: a 1-year follow-up study. Parkinsonism Relat Disord 16:522–526. https://doi.org/10.1016/j.parkreldis.2010.06.004
Khor S-P, Hsu A (2007) The pharmacokinetics and pharmacodynamics of levodopa in the treatment of Parkinson’s disease. Curr Clin Pharmacol 2:234–243. https://doi.org/10.2174/157488407781668802
Goetz CG, Tilley BC, Shaftman SR et al (2008) Movement disorder society-sponsored revision of the unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170. https://doi.org/10.1002/mds.22340
Goetz CG, Nutt JG, Stebbins GT (2008) The unified dyskinesia rating scale: presentation and clinimetric profile. Mov Disord 23:2398–2403. https://doi.org/10.1002/mds.22341
Morishita T, Rahman M, Foote KD et al (2011) DBS candidates that fall short on a levodopa challenge test: alternative and important indications. Neurologist 17:263–268. https://doi.org/10.1097/NRL.0b013e31822d1069
Merello M, Gerschcovich ER, Ballesteros D, Cerquetti D (2011) Correlation between the movement disorders society unified Parkinson’s Disease rating scale (MDS-UPDRS) and the Unified Parkinson’s Disease rating scale (UPDRS) during l-dopa acute challenge. Parkinsonism Relat Disord 17:705–707. https://doi.org/10.1016/j.parkreldis.2011.07.002
Warnecke T, Suttrup I, Schröder JB et al (2016) Levodopa responsiveness of dysphagia in advanced Parkinson’s disease and reliability testing of the FEES-Levodopa-test. Parkinsonism Relat Disord 28:100–106. https://doi.org/10.1016/j.parkreldis.2016.04.034
Fabbri M, Marini C, Bisulli F et al (2013) Clinical and polygraphic study of familial paroxysmal kinesigenic dyskinesia with PRRT2 mutation. Epileptic Disord 15:123–127. https://doi.org/10.1684/epd.2013.0569
Lechien JR, Blecic S, Ghosez Y et al (2018) Voice quality and orofacial strength as outcome of levodopa effectiveness in patients with early idiopathic parkinson disease: a preliminary report. J Voice. https://doi.org/10.1016/j.jvoice.2018.04.002
Seiffert P, Derejczyk J, Kawa J et al (2017) Frailty phenotype and the role of levodopa challenge test in geriatric inpatients with mild parkinsonian signs. Biogerontology 18:641–650. https://doi.org/10.1007/s10522-017-9716-6
Gao J, Du LJ, He W et al (2016) Ultrasound strain elastography in assessment of muscle stiffness in acute levodopa challenge test: a feasibility study. Ultrasound Med Biol 42:1084–1089. https://doi.org/10.1016/j.ultrasmedbio.2015.12.014
Chaudhuri KR, Schrag A, Weintraub D et al (2019) The movement disorder society nonmotor rating scale: initial validation study. Mov Disord. https://doi.org/10.1002/mds.27862
Wider C, Russmann H, Villemure J-G et al (2006) Long-duration response to levodopa in patients with advanced parkinson disease treated with subthalamic deep brain stimulation. JAMA Neurol 63:951–955. https://doi.org/10.1001/archneur.63.7.951
Ogawa N (2000) Factors affecting levodopa effects in Parkinson’s disease. Acta Med Okayama 54:95–101
Quattrone A, Zappia M, Aguglia U et al (1995) The subacute levodopa test for evaluating long-duration response in Parkinson’s disease. Ann Neurol 38:389–395. https://doi.org/10.1002/ana.410380308
Parkinson Study Group (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508. https://doi.org/10.1056/NEJMoa033447
Ikebe SI, Harada T, Hashimoto T et al (2003) Prevention and treatment of malignant syndrome in Parkinson’s disease: a consensus statement of the malignant syndrome research group. Parkinsonism Relat Disord 9:47–49. https://doi.org/10.1016/S1353-8020(02)00123-2
Rossi P, Colosimo C, Moro E, Tonali PAA (2001) Acute challenge with apomorphine and levodopa in parkinsonism. Focus Parkinson's Dis 13:60–63. https://doi.org/10.1159/000008142
Funding
This study was supported in part by the Parkinson’s Foundation through its support of the fellowship training of G.S.
Author information
Authors and Affiliations
Contributions
GS performed a detailed review of the literature and undertook the writing of the manuscript. AEL provided critical comments, recommendations, and editorial contributions.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval
The article is based on previously published studies and patient data and is in line with the journal's ethical guidelines.
Informed consent
Consent to participate: not applicable. Consent for publication: not applicable.
Rights and permissions
About this article
Cite this article
Saranza, G., Lang, A.E. Levodopa challenge test: indications, protocol, and guide. J Neurol 268, 3135–3143 (2021). https://doi.org/10.1007/s00415-020-09810-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09810-7