Abstract
Background
Cognitive impairment is an essential feature of Huntington’s disease (HD) and dementia is a predictable outcome in all patients. However, validated instruments to assess global cognitive performance in the field of HD are lacking.
Objectives
We aimed to explore the utility of the Parkinson’s disease-Cognitive Rating Scale (PD-CRS) for the screening of global cognition in HD.
Methods
A multicenter cohort of 132 HD patients at different disease stages and 33 matched healthy controls were classified as having preserved cognition, mild cognitive impairment (HD-MCI) or dementia (HD-Dem) according to the Clinical Dementia Rating and Functional Independence Score. The PD-CRS and the Mini-Mental State Examination were administered. Receiver operating characteristic curve analysis was used to determine optimal cutoffs to differentiate patients according to their cognitive status.
Results
A PD-CRS cutoff score ≤ 81/82 was optimal to detect HD-MCI (sensitivity = 93%; specificity = 80%; area under the curve (AUC) = 0.940), and ≤ 63/64 was optimal to detect HD-Dem (sensitivity = 90%; specificity = 87%; AUC = 0.933). MMSE scores failed to show robust psychometric properties in this context.
Discussion
The PD-CRS is a valid and reliable instrument to assess global cognition in HD in routine clinical care and clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Huntington’s disease (HD) is a monogenetic, autosomal dominant neurodegenerative disorder caused by a CAG polyglutamine expansion in the HTT gene [1]. Clinical diagnosis is based on the presence of unequivocal motor abnormalities such as chorea, dystonia, parkinsonism and gait abnormalities. However, almost all patients with HD will also exhibit behavioral disturbances and cognitive decline [2]. Cognitive deterioration is progressive and can be tracked as early as 15 years before the emergence of the first motor symptoms [3, 4]. Although a clear pattern of transition from normal cognition to mild cognitive impairment has not been strictly defined in HD, dementia appears to be a predictable outcome [2, 5].
Cognitive impairment and dementia in HD have mostly been ascribed to progressive basal ganglia atrophy [4, 6]. Accordingly, it has been proposed that disruption of the frontal–subcortical circuitry is the main contributor to the prototypical frontal–executive neuropsychological profile of cognitive impairment and dementia in HD [5, 6]. More recently, widespread brain changes involving white matter alterations and posterior–cortical thinning have also been shown to contribute to the clinical picture of this disorder [4, 7,8,9,10].
Cross-sectional and longitudinal observational studies of HD have illustrated that performance in many cognitive tasks, especially in those addressing executive functions and processing speed, worsens linearly as disease progresses [11, 12]. These studies have identified important cognitive biomarkers to monitor the progression of HD. From a clinical and practical perspective, these measures allow clinicians to track disease progression or capture differences with healthy individuals, but they do not classify patients according to their global cognitive status in terms of cognitive normality, mild cognitive impairment, or dementia.
Formally, mild cognitive impairment (MCI) can be suspected when performance in standardized neuropsychological measures is below age- and education-adjusted means but no significant impact on activities of daily living (ADL) is evident. Dementia is assumed when objective cognitive impairment is sufficiently severe to significantly interfere with ADL. In neurodegenerative diseases, both MCI and dementia are formally diagnosed on the basis of Level II assessment, a comprehensive neuropsychological examination covering several cognitive domains. However, Level I assessments, that is, screening approaches using brief instruments that have been tested in specific diseases is also accepted. Testing general screening instruments in different diseases is of major importance, because cutoff scores for MCI and dementia may differ significantly between diseases. Such differences indicate that the same diagnostic criteria and assessment approaches do not apply equally for all neurodegenerative diseases. Although cognitive deterioration is a predictable outcome in HD, specific diagnostic criteria for MCI or dementia and validated instruments in HD are lacking [13]. Furthermore, few studies have addressed the prevalence and profile of MCI and dementia in HD [5, 14, 15].
The validation of instruments to evaluate global cognition in HD is of major importance [13]. Besides the value of characterizing global cognitive status for the work-up of HD patients in clinical practice, global cognitive assessments may be a requirement for the inclusion of patients in clinical trials. Currently, however, validated HD-specific scales for measuring global cognition are lacking and the global cognitive instruments usually used in clinical practice, such as the Mini-Mental State Examination (MMSE) or the Montreal Cognitive Assessment Battery (MoCA), or the HD Cognitive Assessment Battery (HD-CAB) or the Unified Huntington’s Disease Rating Scale (UHDRS) cognitive score, offer no specific cutoff scores for the screening of cognitive status in the HD population [13, 16, 17].
The Parkinson’s Disease-Cognitive Rating Scale (PD-CRS) is a screening instrument that addresses global cognition. It is freely accessible for non-profit scientific research (www.movementscales.com). It was specifically developed to capture the whole spectrum of cognitive changes in Parkinson’s disease (PD) [18, 19]. In PD, frontal–executive deficits characterize the profile of early cognitive changes and MCI, but the addition of posterior–cortical alterations define the transition from MCI to dementia [18, 20]. Accordingly, the PD-CRS tests performance in frontal–executive and posterior–cortical-dependent tasks, and could be a powerful approach to characterize both frontal–striatal and posterior–cortical alterations in HD. It has demonstrated excellent psychometric attributes to differentiate patients with normal cognition from those with MCI and dementia in PD [18, 19], and it has been recommended by the NINDS and MDS task force on PD-MCI. It has an alternative form for use in re-testing as it has been translated into 19 languages. Besides, it has shown reliability and discriminative capacity equivalent to a comprehensive neuropsychological assessment using two tests for each cognitive domain [21]. In the present work, using a large multicenter HD sample, we validated and tested the psychometric properties of the PD-CRS as a screening instrument to assess global cognition in this population.
Methods
Participants
One hundred and seventy-two participants were recruited in a multicentric study from ten hospitals in five European countries (78 participants from Spain, 59 from Italy, 11 from Portugal, 21 from Germany, and 3 from Poland). One hundred and thirty-nine participants were symptomatic gene mutation carriers (CAG > 38) and 33 were gene-negative healthy controls. Participants were classified as symptomatic based on a UHDRS-TMS > 4 and a diagnostic confidence level = 4, indicating that motor abnormalities were unequivocal signs of HD with ≥ 99% of confidence. All participants were free of any neurological disorder other than HD. We excluded individuals with a history of traumatic brain injury, epilepsy, drug abuse, or non-compensated systemic disease (i.e., diabetes).
Assessments
Clinical and sociodemographic variables recorded were age, gender, education, and CAG repeat length. The severity of motor symptoms was rated by trained neurologists using the UHDRS-TMS [22]. The UHDRS Functional Independence Scale (FIS) and total functional capacity (TFC) test were administered to obtain measures of independence in instrumental and basic activities of daily living (ADL) [22, 23]. The FIS scale is based on 25 questions (with yes/no responses) that qualify abilities to independently perform ADLs. A score of “independence” is computed from the answers provided, ranging from 100% (no special care needed) to 10% (tube fed, total bed care). Patients at a more advanced disease stage were excluded due to their incapacity to perform the assessments.
The TFC rates occupation, finances, domestic chores, ADLs and care, giving a total score from 0 to 13. Disease stage was determined according to the Shoulson and Fahn criteria for HD staging (TFC > 10 for stage I, TFC 6–10 for stage II and TFC < 6 for stage III). The disease burden score (DBS)—a measure of livelong exposure to mutant Huntington—was calculated using the formula based on age and CAG repeat length: [age × (CAG − 35.5)] [24].
In the absence of a comprehensive neuropsychological assessment and a validated gold standard to classify patients according to their cognitive status in HD, we followed the approach previously used during the development of the PD-CRS [18] and the study of its psychometric properties for MCI in PD [19]. Accordingly, we used the information provided by the Clinical Dementia Rating Scale (CDR) [25]. The CDR assesses cognitive and functional performance in six areas: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. A CDR of 0 indicates no cognitive deficits, 0.5 indicates very mild cognitive impairment, and 1–3 indicate mild to severe cognitive impairment. The CDR was used as the gold standard in the first validation study of the PD-CRS. This scale has been used as the main cognitive outcome in many studies [18]. Moreover, to follow the same approach as that used in previous studies addressing the usefulness of a screening instrument to assess cognition in HD, we added the FIS [5]. As in these previous works, we used a FIS score > 80%, which indicates a decline from pre-disease level of employment, impaired performance in household chores, and difficulties managing finances. Accordingly, patients with a CDR score of 0 and an FIS score > 80% were classified as cognitively preserved (HD-NC), patients with a CDR = 0.5 and a FIS > 80% were classified as MCI (HD-MCI), and patients with a CDR > 0.5 and a FIS < 80% were classified as demented (HD-Dem).
The PD-CRS was administered to all study participants. It is composed of nine subtests that assess immediate verbal memory, naming, sustained attention, working memory, unprompted drawing of a clock, copy of a clock, delayed free recall, alternating verbal fluency, and action verbal fluency. The PD-CRS provides a total score ranging from 0 to 134 and two independent frontal–subcortical and posterior–cortical subscores. The frontal–subcortical score is obtained by adding the immediate verbal memory, sustained attention, working memory, unprompted drawing of a clock, delayed free recall, alternating verbal fluency, and action verbal fluency. The posterior–cortical score is obtained by adding naming and the copy of a clock. Administration and scoring procedures for the different tasks composing the PD-CRS are stated in the source document of the scale which is available at www.movementscales.com. Time of administration varies as a function of the patient’s cognitive status but is around 15–25 min in patients with PD. We also administered the commonly used MMSE screening test for comparative analyses. The idea behind adding this test was to explore the comparative accuracy in terms of discriminative properties of the MMSE vs the PD-CRS. Presence and severity of behavioral symptoms were addressed using the short form of the Problem Behavior Assessment Scale for HD (PBA-s) [26].
All procedures performed in the present study were approved by the ethics committee at Hospital de la Santa Creu i Sant Pau in Barcelona and conducted in accordance with the 1964 Helsinki Declaration and its later amendments.
Statistical analysis
Data are expressed as means ± standard deviations (SDs) for continuous variables and as percentages for the categorical variables. Group comparisons were performed using independent t tests and analyses of variance (ANOVAs) for continuous variables, Mann–Whitney for ordinal data, and the χ2 test for categorical variables. To calculate the effect size of the differences observed between cognitive groups we used Cohen’s d coefficient (d value: 0–0.3, small effect size; 0.3–0.6, moderate effect size; > 0.6, large effect size). Binary logistic regression analysis was performed to test the classification capacity of the obtained cognitive measures and the influence of other collected variables. Receiver operator characteristic (ROC) curves were generated to explore the discriminative capacity of both the PD-CRS and the MMSE. Total scores in the PD-CRS and MMSE were used as predictor variables and cognitive groups as state variables. We calculated sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the obtained cutoffs. Scores reflecting the maximum accuracy cutoff (maximum combined sensitivity and specificity) were chosen. To explore the PD-CRS performance in each group, we conducted comparisons between each subtest of the PD-CRS. All the statistical procedures were performed using the SPSS v16.0 statistical software package.
Results
Clinical and sociodemographic data
The sample consisted of 139 symptomatic gene mutation carriers (mean age = 51.6 ± 10; mean CAG repeat length = 43 ± 2; mean education = 12.2 ± 4.5) and 33 healthy controls (mean age = 52.1 ± 8.7; mean education = 12.7 ± 4) matched for age, gender and education. As expected, significant differences were found in all the clinical (motor, cognitive, functional and behavioral) variables when comparing healthy controls and the whole HD group.
According to the CDR and the FIS, all healthy controls were classified as cognitively preserved. In the HD sample, 36 cases were classified as HD-NC, n = 63 were classified as HD-MCI, and n = 41 were classified as HD-Dem. Regarding disease stage, 75% of the HD sample were stage I, 43% were stage II, and 21% were stage III. Regarding cognitive groups, in the HD-NC group 35 patients were stage I, 1 was stage II and no patients were stage III. In the HD-MCI group, 31 patients were stage I, 29 were stage II, and 2 were III. In the HD-Dem group, 9 patients were stage I, 13 were stage II and 19 were stage III (see Table 1).
Correlation analysis
Partial bivariate correlation analysis was performed between the PD-CRS, the MMSE, age, education, CAG repeat length, PBA scores, and TFC. In this analysis, the influence of motor symptoms in terms of UHDRS-TMS was controlled. The PD-CRS and the MMSE showed moderate correlation coefficients with educational level (PD-CRS: r = 0.522; P < 0.001; MMSE: r = 0.403; P < 0.001) and with TFC (PD-CRS: r = 0.294; P < 0.005; MMSE: r = 0.346; P < 0.001). Moreover, the MMSE showed a moderate correlation with the severity of psychotic symptoms (r = − 0.322; P < 0.001) and apathy (r = − 0.201; P < 0.05). A mild association was found between total PD-CRS score and severity of depressive symptoms (r = − 0.195; P < 0.05).
Discriminative validity between cognitive groups
One-way ANOVA showed significant differences between the HD cognitive groups in education (P < 0.001), CAG repeat length (P < 0.002), PD-CRS total score (P < 0.001), MMSE (P < 0.001), UHDRS-TMS (P < 0.001), TFC (P < 0.001), FIS (P < 0.001), PBA apathy score (P < 0.005), and PBA executive dysfunction score (P < 0.005).
We used stepwise logistic regression analysis (forward; conditional) to determine the variables that independently differentiated HD-MCI and HD-Dem from HD-NC. The variables found to be significantly different between cognitive groups in the one-way ANOVA were included in the analysis to assess their contribution to group discrimination. The PD-CRS total score (P < 0.001; odds ratio, 0.90; 95% confidence interval, 0.81–0.91), the PBA executive dysfunction score (P < 0.01; odds ratio, 0.84; 95% confidence interval, 0.78–0.91) and the TFC (P < 0.01; odds ratio, 0.47; 95% confidence interval, 0.78–0.92) were identified as the best variables differentiating HD-NC from HD-MCI. The PD-CRS total score (P < 0.001; odds ratio, 0.90; 95% confidence interval, 0.87–0.94) was the best variable to differentiate HD-Dem from non-demented HD patients.
The PD-CRS total score was excluded from the stepwise logistic regression analysis to focus on the capacity of the MMSE to differentiate between cognitive groups. The performance of the MMSE at independently differentiating HD-NC from HD-MCI was poor (P > 0.05; odds ratio, 0.74; 95% confidence interval, 0.714–0.970) but it discriminated HD-Dem from non-demented HD (P < 0.001; odds ratio, 0.60; 95% confidence interval, 0.498–0.721). Moreover, education and UHDRS-TMS contributed significantly to differentiating HD-MCI (P < 0.005; odds ratio, 0.80; 95% confidence interval, 0.693–0.913) and UHDRS-TMS contributed significantly to differentiating HD-Dem (P < 0.001; odds ratio, 0.64; 95% confidence interval, 0.529–0.786).
ROC curve analysis conducted within the HD sample indicated that a PD-CRS total score ≤ 81/82 of 134 was the maximum accuracy cutoff to detect MCI (sensitivity, 93%; specificity, 80%; PPV, 65%. NPV, 89%; area under the ROC curve, 0.940; 95% confidence interval, 0.908–0.971) and a PD-CRS total score ≤ 63/64 of 134 was the maximum accuracy cutoff to classify patients as HD-Dem (sensitivity, 90%; specificity, 87%; PPV, 80%. NPV, 88%; area under the ROC curve, 0.933; 95% confidence interval, 0.896–0.970). Table 2 summarizes the different accuracy using different PD-CRS cutoff scores.
For the MMSE, the maximum accuracy cutoff to detect MCI was a total score ≤ 26/27 of 30 (sensitivity, 80%; specificity, 39%; PPV, 58%; NPV, 80%; area under the ROC curve, 0.773; 95% confidence interval, 0.674–0.871) and at a total score ≤ 24/25 of 30 to detect dementia (sensitivity, 85%; specificity, 65%; PPV, 53%; NPV, 91%; area under the ROC curve, 0.884; 95% confidence interval, 0.825–0.942). Table 2 shows the cutoff values and their sensitivity, specificity, PPV, and NPV (see Fig. 1).
PD-CRS performance between cognitive groups
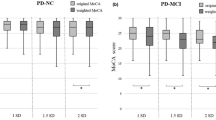
We compared the PD-CRS total score and all the scores obtained in each subtest of the PD-CRS between each cognitive group (see Fig. 2 in supplementary data). As seen in Table 3, the only subtest that differed between healthy controls and HD-NC was the unprompted drawing of a clock (P < 0.01). Significant differences were also found in all the subtests between healthy controls and HD-MCI and HD-Dem. Focusing on the HD sample, the only subtests that did not differ significantly between HD-NC and HD-MCI groups were the unprompted drawing of a clock (P = 0.846) and the copy of a clock (P < 0.210). As reflected by Cohen’s d, large effect sizes were found for all the significantly different comparisons.
Discussion
The present study demonstrates the utility of the PD-CRS as a practical and valid method to capture global cognitive deficits in HD. To our knowledge, this is one of the first studies to address the psychometric properties of a screening instrument for global cognition in HD [5, 24, 27,28,29]. Importantly, specific cutoff scores were determined to detect not only patients with dementia but also patients with mild cognitive deficits that were not sufficiently severe to significantly interfere with their functional independence.
In absence of a formally validated cognitive gold standard for use as a comparator, we used the combination of the CDR and the FIS [5, 18]. Using this classification, we found no significant differences between HD patients classified as cognitively preserved and age and education-matched healthy controls, thus supporting the reliability of our gold standard.
The PD-CRS total score showed excellent discriminative capacity to differentiate between cognitive groups. Notably, although the prevalence of HD-Dem was higher in Stage III, a non-depreciable prevalence of cases with HD-Dem was already observed in stages I and II. A PD-CRS total score ≤ 81/82 was found to be the optimum cutoff score to detect mild cognitive changes that associate mild to no interference with functional independence in HD. Conversely, a PD-CRS total score ≤ 63/64 was found to indicate that cognitive impairment is severe and compatible with the general definition of dementia based on the degree of interference on ADL. Interestingly, the observed PD-CRS cutoff scores for MCI and dementia in this large HD sample are equivalent to those previously found in the PD population [18, 19]. Equivalent properties were not found for a commonly used instrument like the MMSE. The MMSE cutoff score ≤ 26/27 showed very low specificity (36%) at detecting MCI and the cutoff score ≤ 24/25 showed poor specificity (65%) to detect dementia.
In HD, progressive cognitive deterioration is inseparable from the progression of other coexisting motor and behavioral symptoms [5, 11, 12]. When addressing the possible influence of these additional symptoms on the discrimination among cognitive groups, the PD-CRS total score was the best variable independently differentiating each cognitive group. In contrast, the MMSE on its own failed to serve this purpose, and when the PD-CRS was excluded from the model, both the UHDRS-TMS and education level were better predictors of cognitive status than the MMSE.
In all groups, both the PD-CRS and the MMSE showed strong correlations with education and mild correlations with TFC. However, in the logistic regression analysis, neither education nor TFC appeared to influence the capacity of the PD-CRS to predict the cognitive status. Conversely, performance on the MMSE was associated with the severity of apathy and psychotic symptoms. Accordingly, as seen with other cognitive measures, educational level seemed to influence the PD-CRS performance but did not alter its discriminative capacity.
The PD-CRS was significantly lower in the main HD sample than in healthy controls. According to the PD-CRS subtests, it was lower in the HD sample than in healthy controls. Looking at each group separately, PD-CRS was lower in HD-NC but not significantly different from that in healthy controls. The only task that was significantly different between these two groups was the unprompted clock drawing. Beyond constructional abilities, this task assesses planning, sequencing and conceptualization, a set of processes that are intimately associated with frontal–striatal functioning and known to be disrupted early in the course of HD. The comparison between HD-NC and HD-MCI revealed that performance was significantly lower in the HD-MCI group for all the frontal–subcortical tasks but comparable for the posterior–cortical tasks (confrontation naming and copy of a clock). Conversely, compared to the HD-NC and HD-MCI groups, performance of the HD-Dem group was significantly lower in all the tasks, including those associated with posterior–cortical areas. These findings suggest that although early cognitive changes in HD are prominently circumscribed to frontal–subcortical dysfunction, the transition to more severe cognitive impairment is accompanied by additional cognitive alterations in more cortical-dependent tasks (i.e., confrontation naming and visuoconstructional/visuospatial abilities) [30,31,32]. Interestingly, these results are comparable with those reported in previous studies addressing other neurodegenerative diseases, such as PD [18, 20].
Although progressive cognitive impairment leading to dementia will affect mostly all individuals with HD, standardized methods to address cognitive assessment and specific diagnostic criteria for MCI and dementia in HD are lacking [13]. Several cognitive measures were found to be extremely sensitive for tracking the progression of the disease from the prodromal stage or to distinguish patients from healthy controls. All these measures, including the SDMT, the Stroop word-reading and color-naming tests, the indirect circle drawing test, the facial emotion recognition test and the UHDRS cognitive composite score, provide valuable indirect information regarding cognitive and disease progression. However, they do not allow patients to be classified according to global cognitive status in terms of cognitive normality, MCI or dementia. To achieve this, specific standards must be followed. However, although specific diagnostic criteria for MCI and dementia and assessment recommendations are available for almost all neurodegenerative processes involving cognitive deterioration (i.e., Alzheimer’s disease, Parkinson’s disease, ALS, fronto-temporal lobar degeneration, multiple sclerosis), such criteria are lacking for HD [13]. Studies from other disorders have highlighted that diagnostic criteria for one disease do not necessarily capture the specificity of cognitive changes characterizing other diseases. Accordingly, when general and unspecific approaches are used the rate of false negatives/positives increases significantly. To solve this issue, cutoff scores in specific diseases have been established by addressing the discriminative properties of screening instruments against a gold standard. This approach illustrates that some screening instruments have good discriminative properties in one disease but poor properties in others, or that a specific cutoff score in a given test is valid in one disease but not in another. Although such testing has been performed systematically in some fields, little has been done in HD. As a result, currently used cutoff scores for the MMSE or the MoCA in HD are those originally developed to assess diseases with cognitive characteristics that differ from those in HD. In this sense, although the absence of a validated gold standard and a formal definition of MCI and dementia in HD implies a limitation, our results provide evidence on the utility of the PD-CRS as a screening procedure in HD. Instruments currently being used in care settings, clinical trials and observational studies in HD (i.e., MoCA or the MMSE) also have this limitation, highlighting the need to develop a formal definition of MCI and dementia in HD [13]. At the same time, further research is needed to determine the psychometric properties of the PD-CRS in HD.
In conclusion, our results support the usefulness of the PD-CRS as a screening instrument to assess global cognition in HD in clinical routine and in clinical trials. As our results show that the trajectory of global cognitive deterioration in HD is heterogeneous between patients, the characterization of different cognitive phenotypes in HD should be further addressed.
Change history
05 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00415-020-10305-8
References
Walker FO (2007) Huntington's disease. Lancet 369(9557):218–228. https://doi.org/10.1016/S0140-6736(07)60111-1
Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ (2014) Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 10(4):204–216. https://doi.org/10.1038/nrneurol.2014.24
Paulsen JS, Miller AC, Hayes T, Shaw E (2017) Cognitive and behavioral changes in Huntington disease before diagnosis. Handb Clin Neurol 144:69–91. https://doi.org/10.1016/B978-0-12-801893-4.00006-7
Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC (2009) Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol 8(9):791–801. https://doi.org/10.1016/S1474-4422(09)70170-X
Peavy GM, Jacobson MW, Goldstein JL, Hamilton JM, Kane A, Gamst AC, Lessig SL, Lee JC, Corey-Bloom J (2010) Cognitive and functional decline in Huntington's disease: dementia criteria revisited. Mov Disord 25(9):1163–1169. https://doi.org/10.1002/mds.22953
Dogan I, Eickhoff SB, Schulz JB, Shah NJ, Laird AR, Fox PT, Reetz K (2013) Consistent neurodegeneration and its association with clinical progression in Huntington's disease: a coordinate-based meta-analysis. Neuro-degener Dis 12(1):23–35. https://doi.org/10.1159/000339528
Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B (2005) Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology 65(5):745–747. https://doi.org/10.1212/01.wnl.0000174432.87383.87
Nopoulos PC, Aylward EH, Ross CA, Johnson HJ, Magnotta VA, Juhl AR, Pierson RK, Mills J, Langbehn DR, Paulsen JS (2010) Cerebral cortex structure in prodromal Huntington disease. Neurobiol Dis 40(3):544–554. https://doi.org/10.1016/j.nbd.2010.07.014
Sampedro F, Martinez-Horta S, Perez-Perez J, Horta-Barba A, Lopez-Mora DA, Camacho V, Fernandez-Leon A, Gomez-Anson B, Carrio I, Kulisevsky J (2019) Cortical atrophic-hypometabolic dissociation in the transition from premanifest to early-stage Huntington's disease. Eur J Nucl Med Mol Imaging 46(5):1111–1116. https://doi.org/10.1007/s00259-018-4257-z
Gregory S, Crawford H, Seunarine K, Leavitt B, Durr A, Roos RAC, Scahill RI, Tabrizi SJ, Rees G, Langbehn D, Orth M (2018) Natural biological variation of white matter microstructure is accentuated in Huntington's disease. Hum Brain Mapp. https://doi.org/10.1002/hbm.24191
Paulsen JS, Long JD, Johnson HJ, Aylward EH, Ross CA, Williams JK, Nance MA, Erwin CJ, Westervelt HJ, Harrington DL, Bockholt HJ, Zhang Y, McCusker EA, Chiu EM, Panegyres PK (2014) Clinical and biomarker changes in premanifest Huntington disease show trial feasibility: a decade of the PREDICT-HD study. Front Aging Neurosci 6:78. https://doi.org/10.3389/fnagi.2014.00078
Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, Jones R, Johnson H, Craufurd D, Hicks SL, Kennard C, Landwehrmeyer B, Stout JC, Borowsky B, Scahill RI, Frost C, Langbehn DR (2012) Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol 11(1):42–53. https://doi.org/10.1016/S1474-4422(11)70263-0
Mestre TA, Bachoud-Levi AC, Marinus J, Stout JC, Paulsen JS, Como P, Duff K, Sampaio C, Goetz CG, Cubo E, Stebbins GT, Martinez-Martin P (2018) Rating scales for cognition in Huntington's disease: critique and recommendations. Mov Disord 33(2):187–195. https://doi.org/10.1002/mds.27227
Julayanont P, McFarland NR, Heilman KM (2020) Mild cognitive impairment and dementia in motor manifest Huntington's disease: classification and prevalence. J Neurol Sci 408:116523. https://doi.org/10.1016/j.jns.2019.116523
Duff K, Paulsen J, Mills J, Beglinger LJ, Moser DJ, Smith MM, Langbehn D, Stout J, Queller S, Harrington DL (2010) Mild cognitive impairment in prediagnosed Huntington disease. Neurology 75(6):500–507. https://doi.org/10.1212/WNL.0b013e3181eccfa2
Videnovic A, Bernard B, Fan W, Jaglin J, Leurgans S, Shannon KM (2010) The Montreal Cognitive Assessment as a screening tool for cognitive dysfunction in Huntington's disease. Mov Disord 25(3):401–404. https://doi.org/10.1002/mds.22748
Ringkobing SP, Larsen IU, Jorgensen K, Vinther-Jensen T, Vogel A (2019) Cognitive screening tests in huntington gene mutation carriers: examining the validity of the mini-mental state examination and the montreal cognitive assessment. J Huntington's Dis. https://doi.org/10.3233/JHD-190350
Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A (2008) Parkinson's disease-cognitive rating scale: a new cognitive scale specific for Parkinson's disease. Mov Disord 23(7):998–1005. https://doi.org/10.1002/mds.22007
Fernandez de Bobadilla R, Pagonabarraga J, Martinez-Horta S, Pascual-Sedano B, Campolongo A, Kulisevsky J (2013) Parkinson's disease-cognitive rating scale: psychometrics for mild cognitive impairment. Mov Disord 28(10):1376–1383. https://doi.org/10.1002/mds.25568
Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA (2009) The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 132(Pt 11):2958–2969. https://doi.org/10.1093/brain/awp245
Skorvanek M, Goldman JG, Jahanshahi M, Marras C, Rektorova I, Schmand B, van Duijn E, Goetz CG, Weintraub D, Stebbins GT, Martinez-Martin P (2018) Global scales for cognitive screening in Parkinson's disease: critique and recommendations. Mov Disord 33(2):208–218. https://doi.org/10.1002/mds.27233
Group HS (1996) Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord 11(2):136–142. https://doi.org/10.1002/mds.870110204
Shoulson I, Fahn S (1979) Huntington disease: clinical care and evaluation. Neurology 29(1):1–3. https://doi.org/10.1212/wnl.29.1.1
Penney JB Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH (1997) CAG repeat number governs the development rate of pathology in Huntington's disease. Ann Neurol 41(5):689–692. https://doi.org/10.1002/ana.410410521
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Callaghan J, Stopford C, Arran N, Boisse MF, Coleman A, Santos RD, Dumas EM, Hart EP, Justo D, Owen G, Read J, Say MJ, Durr A, Leavitt BR, Roos RA, Tabrizi SJ, Bachoud-Levi AC, Bourdet C, van Duijn E, Craufurd D (2015) Reliability and factor structure of the Short Problem Behaviors Assessment for Huntington's disease (PBA-s) in the TRACK-HD and REGISTRY studies. J Neuropsychiatry Clin Neurosci 27(1):59–64. https://doi.org/10.1176/appi.neuropsych.13070169
Begeti F, Tan AY, Cummins GA, Collins LM, Guzman NV, Mason SL, Barker RA (2013) The Addenbrooke's Cognitive Examination-Revised accurately detects cognitive decline in Huntington's disease. J Neurol 260(11):2777–2785. https://doi.org/10.1007/s00415-013-7061-5
Stout JC, Queller S, Baker KN, Cowlishaw S, Sampaio C, Fitzer-Attas C, Borowsky B (2014) HD-CAB: a cognitive assessment battery for clinical trials in Huntington's disease 1,2,3. Mov Disord 29(10):1281–1288. https://doi.org/10.1002/mds.25964
Mickes L, Jacobson M, Peavy G, Wixted JT, Lessig S, Goldstein JL, Corey-Bloom J (2010) A comparison of two brief screening measures of cognitive impairment in Huntington's disease. Mov Disord 25(13):2229–2233. https://doi.org/10.1002/mds.23181
Coppen EM, Grond JV, Hafkemeijer A, Barkey Wolf JJH, Roos RAC (2018) Structural and functional changes of the visual cortex in early Huntington's disease. Hum Brain Mapp 39(12):4776–4786. https://doi.org/10.1002/hbm.24322
Johnson EB, Rees EM, Labuschagne I, Durr A, Leavitt BR, Roos RA, Reilmann R, Johnson H, Hobbs NZ, Langbehn DR, Stout JC, Tabrizi SJ, Scahill RI (2015) The impact of occipital lobe cortical thickness on cognitive task performance: an investigation in Huntington's Disease. Neuropsychologia 79(Pt A):138–146. https://doi.org/10.1016/j.neuropsychologia.2015.10.033
Labuschagne I, Cassidy AM, Scahill RI, Johnson EB, Rees E, O'Regan A, Queller S, Frost C, Leavitt BR, Durr A, Roos R, Owen G, Borowsky B, Tabrizi SJ, Stout JC (2016) Visuospatial processing deficits linked to posterior brain regions in premanifest and early stage Huntington's disease. J Int Neuropsychol Soc 22(6):595–608. https://doi.org/10.1017/S1355617716000321
Acknowledgements
The present study was partially funded by a Spanish Government Grants (PI17/001885). The authors want to thank Renata Wallner, PhD and Adrianna Senczyszyn, MA (Department of Psychiatry, Wroclaw Medical University, Wroclaw, Poland) for their collaboration in the polish translation of the PD-CRS.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
All procedures were performed in accordance with the standards of each regional local Ethic Review Board after protocol approval.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martinez-Horta, S., Horta-Barba, A., Perez-Perez, J. et al. Utility of the Parkinson’s disease-Cognitive Rating Scale for the screening of global cognitive status in Huntington’s disease. J Neurol 267, 1527–1535 (2020). https://doi.org/10.1007/s00415-020-09730-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09730-6