Abstract
The diagnostic criteria of menstrual migraine (MM), migraine related to menstruation and pure menstrual migraine, are placed in the appendix of the International Classification of Headache Disorders and are still primarily considered as research criteria that need validation. Although there is a great wealth of knowledge about the neurobiological processes underlying MM and its symptoms, the mechanisms by which an attack starts during the menstrual cycle remain baffling, and the disease is still undertreated. In this narrative review, we aim to summarize recent data on pathophysiology, epidemiology, burden of disease and treatment of MM. The vast majority of the literature focuses on the relationship between MM and hormonal factors. The role of falling in estrogen levels is believed to increase the susceptibility of blood vessels to prostaglandins, which have been implicated in neurogenic inflammation. Moreover, fluctuations of ovarian steroid hormone levels modulate calcitonin gene-related peptide in the trigeminovascular system. In addition, it has been observed that gonadal hormones modulate cortical spreading depression susceptibility in animal models. Sex hormone influences on MM affect not only the frequency and severity of headache attack but also its treatment. Understanding the mechanisms that contribute to neuroendocrine vulnerability in some women and some menstrual cycles may yield possible marker of the disease opening treatment options specifically targeting MM. An increased interest for future research on the subject will further elucidate how to manage this debilitating type of migraine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hormonal factors play a relevant impact on migraine. Menstruation is a common migraine trigger among female migraineurs. The International Classification of Headache Disorders ICHD-3 [1] recognizes two types of menstrual migraine (MM): migraine related to menstruation (MRM) and pure menstrual migraine (PMM). Women with a diagnosis of MM report that menstrual attacks are more painful, longer lasting, more disabling and less responsive to treatment [2,3,4,5,6,7]. During the last few years, the literature concerning this topic has added some relevant information widening definition and meaning of the relationship between hormonal events and migraine. According to new scientific evidence, ICHD-3 criteria for MM have been revised [1]. Considering the various lines of findings implicating the impact of hormonal and biochemical cyclic changes on trigeminovascular system in menstrual migraineurs, we aim to provide an updated overview of the literature to date on the subject. We hope also to inspire an increase in interest for future research aimed at further elucidating how to manage this type of debilitating migraine.
Methods
We searched the literature from MEDLINE, PubMed, Cochrane Library, and EMBASE databases for publications from 1972 to October 2019. Key search terms “Menstrual Migraine”, “Estrogen and migraine”, “Hormones and Migraine”, “CGRP and Hormones”, “CGRP and Menstrual Migraine”. were used. This narrative review included both quantitative and qualitative studies in addition to reviews and abstracts. Relevant literature was reviewed by the authors. Only English language studies were included. The final reference list was generated on the basis of relevance to the topics covered in this review.
Results
Diagnostic criteria
The definition of “menstrual migraine” appeared in the International Classification of Headache Disorders I in 1988 [8], although accepted criteria for this entity were not yet available. In ICHD II 2004, MM criteria were added in the Appendix, defined as migraine without aura occurring in the perimenstrual period, i.e. on day − 2 to + 3 of menstruation in at least two out of three menstrual cycles and subdivided into PMM without aura and MRM without aura [9]. PMM without aura was defined as migraine without aura attacks occurring exclusively in the perimenstrual period, while MRM without aura might have also migraine without aura attacks outside the perimenstrual period [9]. In ICHD III beta version published in 2013, MM diagnostic criteria appeared unchanged from ICHD II version [10]. At beginning, 2018 ICHD-3 criteria were published. MM criteria, although always added in the Appendix, were updated [1]. Which is the main novelty about MM in ICHD-3 criteria? The diagnostic criteria for PMM and MRM now include not only that one for PMM and MRM without aura, but also those for PMM and MRM with aura [1]. In both cases, as clearly pointed out in the guidelines, MM are defined attacks on day − 2 to + 3 of menstruation in at least two out of three menstrual cycles. The definition of the first day of menstruation which is day 1 and the preceding day is day − 1; there is no day 0. For research purposes, a prospective diary is recommended although not mandatory for clinical diagnosis. In the notes, some relevant information are again outlined including the criteria of menstruation considered to be endometrial bleeding resulting either from the normal menstrual cycle or from the withdrawal of exogenous progestogens, as in the use of combined oral contraceptives or cyclical hormone replacement therapy [1]. Since, as ICHD-3 guideline outlines, the mechanisms of migraine may be different with endometrial bleeding resulting from the normal menstrual cycle and bleeding due to the withdrawal of exogenous progestogens research should separate these distinct subpopulations even though the diagnostic criteria do not (Table 1).
Estrogen withdrawal hypothesis and pathophysiological implications
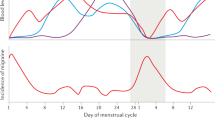
There is some evidence that MM attacks, at least in some women, result from oestrogen withdrawal, although other hormonal and biochemical changes at this time of the cycle may also be relevant (Fig. 1). Actually, the “estrogen withdrawal hypothesis” of migraine triggering described by Somerville in 1972 [11] has been confirmed and some further relevant information have been gained exploring menstrual hormonal patterns in women with migraine or comparing hormone levels and patterns between women with history of migraine and controls [4, 12,13,14,15]. More recently in the Study of Women’s Health Across the Nation (SWAN) Daily Hormone Study (DHS), authors evaluated whether hormone levels and rates of change differ for women with migraine compared with controls [16]. To explore the hypothesis that women with migraine have distinct hormone patterns, they compared peak and daily hormone levels (up to 5 days post-peak) as well as mean within-woman daily rates of decline in ovulatory menstrual cycles for women with history of migraine and controls. Interestingly, in this study, it has been observed that there is no significant difference in estrogen peak levels or mean daily levels between migraineurs and controls. Conversely, there is a significant difference between the two groups in the rate of estrogen decline, specifically in the late luteal phase [16]. Moreover, among migraineurs, the rate of estrogen decline does not distinguish cycles with and without an acute headache [16]. This finding suggests that a neuroendocrine vulnerability characterizes women with migraine and it may facilitate initiation of migraine attack [16]. Interestingly, these pathophysiological considerations concerning migraineurs in respect of controls might underlie also the more specific condition of menstrual migraineurs. On the other hand, no obvious relationship between progesterone fluctuations across the menstrual cycle and migraine attacks was found [11, 16].
Ovarian hormones’ cyclicity has been implicated in modulating also other chronic non-cancer pain conditions including endometriosis [17]. Indeed, there is good evidence of menstrual cyclicity to the pain in both these estrogen-dependent conditions [17].
A drop in estrogen may cause an increased susceptibility to prostaglandins (PGs). PGs might play a role in MM since a threefold increase in PG levels occurs from the follicular phase to the luteal phase, with a further increase occurring during menstruation [18]. PGs can cause neurogenic inflammation, leading to the release of neuropeptides such as substance P, neurokinins and calcitonin gene-related peptide (CGRP) [19,20,21,22,23,24].
Hormonal fluctuations of ovarian steroid hormone levels, estrogens and progesterone, influence CGRP in the trigeminovascular system during different reproductive milestones. In a clinical study, authors demonstrated that concentrations of immunoreactive plasma CGRP in healthy women were significantly increased throughout pregnancy and decreased after delivery [25]. Moreover, the sensitivity of various vascular beds to CGRP in rats appears to increase with advancing pregnancy. This increased sensitivity might be involved in regulating uteroplacental blood flow, in addition to other vascular adaptations that occur during normal pregnancy [26]. In addition, it was observed that in healthy subjects, immunoreactive plasma CGRP levels were significantly higher in females than in males and that women on contraceptive pills had significantly higher immunoreactive CGRP levels than women not taking contraceptives [27]. Moreover, circulating CGRP levels are influenced by menopausal status [28].
These data suggest that the CGRP system could be influenced directly by both endogenous and exogenous ovarian steroid hormones. In a rat model, 17β-estradiol enhanced neurogenic vasodilation, suggesting increased CGRP release from perivascular nerves [29, 30]. Authors suggested that this might be one of the mechanisms through which 17β-estradiol exacerbates migraine in women [29, 30]. In a clinical experimental model exploring gender differences in CGRP-dependent dermal blood flow in healthy subjects and migraineurs, dermal blood flow in males did not vary over time and was comparable between healthy subjects and migraineurs [31]. Conversely, in healthy women, fluctuations of ovarian steroid hormones influenced CGRP-dependent dermal blood flow. Interestingly, in female migraine patients, dermal blood flow responses were elevated, compared to healthy subjects, but these responses were independent of the menstrual cycle [31]. The authors postulate that their data confirm the preexisting theory that the premenstrual withdrawal of estradiol influences the trigeminovascular system. They conclude that this study supports the hypothesis of a disturbed systemic as well as trigeminovascular cyclicity in patients with menstrual-related migraine, which might augment their susceptibility to migraine around the time of menstruation [31].
An estrogen receptor-mediated mechanism contributes also to spreading depression (SD) events and pain in ovariectomized and intact rats [32]. This finding suggests that estrogens play multiple actions in migraine when intense hormonal fluctuations occur [32]. Gonadal hormone-mediated modulation of cortical SD susceptibility in female FHM1 mutant mice has also been observed [33]. The authors also found that ovariectomy and senescence influence cortical SD susceptibility in wild-type and homozygous R192Q mutant mice [33]. All these findings, even if obtained in different experimental and clinical models of migraine, may also have a relevance for the pathophysiology of a more specific condition such as MM.
The serotoninergic system and ovarian hormones play a complex and interconnected role in migraine pathogenesis. The cortical SD suppressive effect of 5-hydroxytryptophan has been shown to occur only in the presence of ovarian sex hormones and it is more pronounced during the estrous phase in cycling rats as well as after chronic estradiol administration in oophorectomized animals [34]. Estrogens and estrogen receptors are widely expressed in the brain and in the trigeminovascular system [35]. The density of estrogen receptors changes with changes in estrogen levels over the menstrual cycle [36, 37]. Estrogen receptors are mainly localized in periaqueductal grey, thalamus, amygdala, brain regions critically controlling pain perception [38] (Fig. 2).
The cyclic fluctuations of gonadal hormones may directly alter neuronal, glial and astrocyte function throughout the brain. There is also evidence of brain alterations across the menstrual cycle in females. Estrogen may influence brain areas, potentially involved to some migraine-related behaviors such as allodynia, mood changes, and food cravings [35] (Fig. 3).
Menstrual migraine and genetic aspects
Although estrogen levels are thought to be involved in MM, an association of functional polymorphisms in the estrogen metabolism genes COMT, CYP1A1 or CYP19A1 with MM has not been found [39]. Moreover, also variants in estrogen receptor 1 (ESR1) have not been found associated with MM, while SNPs in tumour necrosis factor alpha (TNFα) and Spectrin Repeat Containing Nuclear Envelope 1 (SYNE1), a gene neighbouring ESR1, were positively associated with MM [40]. The authors suggest that since TNFα is a pro-inflammatory cytokine, their finding might represent a link between the influence of estrogen and progesterone on inflammatory processes and MM. In addition, SYNE1, a spectrin repeat containing protein usually is directly adjacent to the estrogen receptor, and polymorphisms within SYNE1 have been linked strongly to other estrogen-mediated events [40].
On the other hand, the neuropilin 1 gene (NRP1), a protein coding gene, was found to be significantly associated with MM suggesting that NRP1 functions could be related to either the neuronal or vascular aspects of migraine pathophysiology [41]. NRP1 encodes a transmembrane protein that acts as a receptor and is considered involved in pathways of neurovascular tissue and menstruation, the authors thus suggest that this transmembrane protein could play a role in the pathophysiology and etiology of MM [41].
Epidemiology and burden
PMM is uncommon with respect to MRM. Few women reported migraine exclusively with menstruation and at no other time of the month in both headache clinic studies [42,43,44] and population studies [2, 45]. The prevalence of PMM varies widely in these studies, this large variation might be related to differences in study methodology and patient samples. Two large population-based studies published in the last few years have added some information to the epidemiology and burden of MM [46, 47]. In the Norwegian study, among 5000 women aged 30–34 years screened for MM women with self-reported MM in at least half of their menstrual cycles were invited to an interview and examination. A total of 237 women were included in the study. The prevalence for MM was 7.6%. In particular, prevalence for MM without aura was 6.1%, while MM with aura was 0.6%. PMM prevalence was around 1% among all women. In this population-based study, the authors confirmed the observation of some previous clinical studies that MM might also affect subjects with migraine with aura [46]. Moreover, an ancillary study of the same group has shown that, in women who were prospectively diagnosed with MM by diary, the MM without aura attacks were longer and more frequently accompanied by severe nausea than non-menstrual migraine (NMM) without aura attacks [7]. Conversely, no significant differences between menstrual and NMM without aura attacks were found among women with migraine without aura, but no MM [7]. Thus, the authors’ conclusion is that in women from the general population, MM without aura attacks differ from NMM attacks only in women fulfilling the ICHD criteria for MM [7].
In the American Migraine Prevalence and Prevention (AMPP) study [47], 1697 women aged 18–60 years old with at least 1 menstrual cycle in the previous 2 months have been investigated. MM was assessed by mean of a validated questionnaire asking: “Which statements best describe your headaches in relation to your period?” Respondents were divided into three groups: MM, attacks that only or predominantly occur at the time of menses; menstrual-associated migraine, attacks commonly associated with menses, but that also occur at other times of the month; menstrual-unassociated migraine self-reported menstrually unrelated migraine. Women with attacks that only or predominantly occur at the time of menses had a prevalence of 5.5% and an older age of migraine onset. Moreover, women with predominantly menstrually related attacks had fewer headache-days but appeared to be more impaired by attacks. Headache impact test (HIT-6) and the migraine disability assessment scale (MIDAS) scores, two well-known and validated clinical measures of headache-related disability, were found significantly higher for both the MM and menstrual-associated migraine groups compared with the menstrual-unassociated groups [47]. Although in this study, the subgroups of MM differed from the ICHD-recommended definitions and data relied on self-reported retrospective data; this descriptive study of women in the US population provides information on the self perception of these patients concerning the relationship between their migraine attacks and menstrual cycle and provide data on the burden of migraine associated with menstruation in a large demographic representative US population.
Moreover, in two different cohort of patients, it was observed that among patients with MRM attacks, allodynia was more frequent that in NMM-related attacks [48] and allodynia scores were higher in MRM attacks in comparison to NMM attacks [49]. These results suggest that hormonal factors might have a facilitating effect on the development of central sensitization and thus potentially play a role in the risk of chronicization.
In the two above-mentioned large population studies [46, 47], it has been confirmed and widened the knowledge concerning the significant impairment and impact of headache in menstrual migraineurs’ daily activities. These studies provided also methodological information relevant for the disease definition. However, the real prevalence of MM should be ascertained using three-step approach. First, in the general population, a validated questionnaire should screen women with possible MM; second, a clinical interview by a physician should confirm the suspect for MM; finally, the use of a prospective headache and menstruation diary will allow a defined diagnosis.
MM may change over a woman’s reproductive life and for this reason, this clinical condition might be underestimated either in the early reproductive life or in the proximity of the menopausal period.
Interestingly, a higher frequency of MM among female university students living together compared with a control group of university students living alone has also been observed [50]. The authors studied a group of migraineur female university students aged 18–30 years who lived together with two or more other students and a control group of age-matched students who lived alone. Subjects were interviewed with a specific questionnaire and assessed for 3 months by means of a paper pain diary. A higher occurrence of MM among women living together compared with women living alone was detected. This finding was not related to the main influencing factors detected such as use of a contraceptive, test stress, or sleep deprivation. These women also showed menstrual cycle synchrony with their roommates and the presence of headache crises during the menstruation of their colleagues [50]. The menstrual cycle synchrony is a controversial issue that might cause a further confounding element and might be one of the possible explanations concerning the high variability of prevalence data of both clinical and epidemiological studies in MM.
The natural history
The menstrual relation with migraine may change over a woman’s reproductive lifetime. Women with MM had a late age of migraine onset in the AMPP study (age at onset ≥ 30 in 34.4% of cases) [47]. Interestingly, women with MM reported higher headache intensity during early pregnancy and postpartum compared to migraineur women without MM [51]. However, both groups showed improvement during the second half of pregnancy, in line with the continuous extreme high estrogen concentrations of the second and third trimesters of pregnancy, and after delivery [51].
Major fluctuations in estrogen levels take place during perimenopause, ultimately leading to dropping levels. During the perimenopausal period, serum estradiol levels are low. Nevertheless, 8–13% of women with migraine report the onset of their migraine during this period [43, 44].
Moreover, fifty percent of subjects reported MRM during menopausal transition in a cross-sectional community-based survey [52]. However, since longitudinal studies have not yet been performed on menstrual migraineurs, the natural history of the disease remains incompletely defined.
Treatment options
Attacks of MM are usually more debilitating, of longer duration, more prone to recurrence, and less responsive to acute treatment than NMM attacks [2, 5,6,7, 47, 48]. Nevertheless at present, there is no specific treatment for MM approved by the Food and Drug Administration (FDA) or the European Medicine Agency (EMA). Treatments used in traditional migraine therapy are used for PMM and menstrual-related migraine as well. The greater severity and duration of MM requires pharmaceutical targeting with anti-migraine drugs. However, there are no treatment options licensed specifically for MM and ICHD-3 does not give any treatment recommendations. Thus, the validation of both symptomatic and preventive treatment options in MM represents an unmet need.
Currently available acute treatments include mainly triptans, nonsteroidal anti-inflammatory drugs (NSAIDs) and ergot derivatives for acute attacks [53]. The efficacy of a triptan in combination with a second agent has also been evaluated. Rizatriptan combined with dexamethasone in a randomized, double-blind, cross-over study was found more effective than rizatriptan alone, although was associated with higher rate of adverse events [54]. In an open-label trial, it was tested the efficacy of frovatriptan and dexketoprofen for treatment of acute attack of MM [55] and encouraging data concerning the benefit derived from combining dexketoprofen with frovatriptan rather than using frovatriptan alone was observed [55]. Since women suffering from MM often do not respond completely to abortive therapies, they may be candidates for preventive treatment. Women with a regular menstrual cycle and MM may benefit from short-term strategies with drugs administered some days before or during menses. On the other hand, in women with irregular menstrual cycles a continuous preventive treatment may be helpful. Various options are available for both short-term and continuous prophylaxis [53]. However, evidence supporting most categories of prophylactic MM and/or menstrual-related migraine treatments is weak [53]. Among short-term prophylactic therapies administered only during perimenstrual period there are triptans, oestrogen, and naproxen [53]. Continuous prophylactic treatment with hormonal contraceptives are a further option tested and shown to be effective in some open-label trial [53].
However, the increased risk of vascular disease in women with migraine [56] and the controversial issue on increased risk of stroke in women who use hormonal contraceptives [57] might concern about their use in women with migraine, at least in those with additional stroke risk factors such as smoking and hypertension. Moreover, oral contraceptives should be discouraged in women suffering from MM with aura since they may lead to a further increase in the vascular risk [58]. In a systematic review and meta-analysis of studies on the use of desogestrel progestin-only pill, it has been suggested a potential benefit [59]. However, current evidence is observational and based on small samples of women using one oral progestin-only formulation. Thus, further randomized trials on additional progestin-only contraceptives are required to confirm their role in migraine management [59]. Only two small studies have investigated the use of phytoestrogens, estrogen-like molecules derived from soy, for MM prophylaxis [60, 61]. Although both studies showed improvement in outcomes, the level of evidence is low. Moreover, non-invasive Vagus Nerve Stimulation mini-prophylaxis has been shown to be an effective treatment that reduces the number of MM and MRM days and acute analgesic use for subjects with MM and MRM without adding any treatment-related safety or tolerability concerns [62]. However, further randomised controlled studies are needed to validate these results.
Finally, concerning association of frovatriptan with oral contraceptive, it was observed a decrease in pain only in patients taking combined therapy [63]. In conclusion, so far, triptans have the strongest evidence for both preventive and acute MM treatment [53]. Available studies support the use of almotriptan, naratriptan, sumatriptan, and zolmitriptan as acute therapies and frovatriptan, naratriptan, and zolmitriptan as preventive therapies [53].
A therapeutic perspective with Onabotulinum A for the management of MM has also been hypothesized, although clinical trial on this topic are lacking [64].
The safety and efficacy of perimenstrual telcagepant, a CGRP receptor antagonist, for headache prophylaxis has been studied in a randomized, double-blind, placebo-controlled 6-month trial in women with migraine for ≥ 3 months who experienced perimenstrual headaches. Telcagepant 140 mg taken perimenstrually for 7 days was generally well tolerated, but was associated with transaminase elevations. Interestingly, telcagepant did not reduce monthly headache frequency, but reduced perimenstrual headaches [65].
Three novel antibodies directed against CGRP or its receptor are currently approved by both FDA and EMA and entered in clinical practice [66,67,68] and another is pending [69] for the prevention of migraine. Future studies should focus on how these drugs act in PMM and MRM attacks. Moreover, the development of ditans and gepants might represent a major progress also in MM acute treatment [70].
Discussion
The diagnostic criteria of MM are still placed in the appendix of the ICHD III and are primarily considered as research criteria that need validation, although have been updated at beginning of 2018 including those for MM with aura. Estrogen receptors are expressed in the trigeminovascular system. Fluctuations of estrogen levels modulate CGRP, serotonin and PGs. These factors might account for MM attacks. Further research investigating reasons behind the susceptibility to hormonal and biochemical changes in some MM attacks, migraine treatment target and preventative treatment options should be conducted. MM appears not rare in clinical practice. Currently, there is no specific preventive treatment for MM. It is important to treat women with PMM and MRM adequate, as the impact of MM attacks on daily life is high. The development of monoclonal antibodies, ditans and gepants might represent a major progress not only in migraine but also in MM treatment.
“Finally, although current ICHD-3 criteria for MM is more exhaustive in respect of the previous guidelines, we feel that this gender specific type of migraine might benefit from further extensive research efforts being considered as an entity in the next ICHD classification.”
References
Headache Classification Committee of the International Headache Society (IHS) (2018) The international classification of headache disorders, 3rd edition. Cephalalgia 38:1–211
Couturier EG, Bomhof MA, Neven AK, van Duijn NP (2003) Menstrual migraine in a representative Dutch population sample: prevalence, disability and treatment. Cephalalgia 23:302–308
Granella F, Sances G, Allais G, Nappi RE, Tirelli A, Benedetto C, Brundu B, Facchinetti F, Nappi G (2004) Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia 24:707–716
MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A (2006) Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology 67:2154–2158
MacGregor EA, Victor TW, Hu X, Xiang Q, Puenpatom RA, Chen W, Campbell JC (2010) Characteristics of menstrual vs nonmenstrual migraine: a post hoc, within-woman analysis of the usual-care phase of a nonrandomized menstrual migraine clinical trial. Headache 50:528–538
Pinkerman B, Holroyd K (2010) Menstrual and nonmenstrual migraines differ in women with menstrually-related migraine. Cephalalgia 30:1187–1194
Vetvik KG, Benth JŠ, MacGregor EA, Lundqvist C, Russell MB (2015) Menstrual versus non-menstrual attacks of migraine without aura in women with and without menstrual migraine. Cephalalgia 35:1261–1268
Classification and Diagnostic Criteria for Headache Disorders, Cranial Neuralgias and Facial Pain (1988) Headache classification committee of the International Headache Society. Cephalalgia 8(Suppl 7):1–96
Headache Classification Subcommittee of the International Headache Society (2004) The international classification of headache disorders: 2nd edition. Cephalalgia 24(Suppl 1):9–160
Headache Classification Committee of the International Headache Society (IHS) (2013) The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Somerville BW (1972) The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 22:355–365
Epstein MT, Hockaday JM, Hockaday TD (1975) Migraine and reproductive hormones throughout the menstrual cycle. Lancet 1:543–548
Horth CE, Wainscott G, Neylan C, Wilkinson MI (1975) Proceedings: progesterone, oestradiol and aldosterone levels in plasma during the menstrual cycle of women suffering from migraine. J Endocrinol 65:24P–25P
Martin VT, Wernke S, Mandell K, Ramadan N, Kao L, Bean J, Liu J, Zoma W, Rebar R (2005) Defining the relationship between ovarian hormones and migraine headache. Headache 45:1190–1201
MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A (2006) Prevention of menstrual attacks of migraine: a double-blind placebo-controlled crossover study. Neurology 67:2159–2163
Pavlović JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS, Lipton RB, Derby CA (2016) Sex hormones in women with and without migraine: evidence of migraine-specific hormone profiles. Neurology 5(87):49–56
Hassan S, Muere A, Einstein G (2014) Ovarian hormones and chronic pain: a comprehensive review. Pain 155:2448–2460
Downie J, Poyser NL, Wunderlich M (1974) Levels of prostaglandins in human endometrium during the normal menstrual cycle. J Physiol 236:465–472
Moskowitz MA (1984) The neurobiology of vascular head pain. Ann Neurol 16:157–168
Ho TW, Edvinsson L, Goadsby PJ (2010) CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol 6:573–582
Eftekhari S, Warfvinge K, Blixt FW, Edvinsson L (2013) Differentiation of nerve fibers storing CGRP and CGRP receptors in the peripheral trigeminovascular system. J Pain 14:1289–1303
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94:1099–1142
Antonova M, Wienecke T, Olesen J, Ashina M (2013) Prostaglandins in migraine: update. Curr Opin Neurol 26:269–275
Matsuda M, Huh Y, Ji RR (2019) Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 33:131–139
Stevenson JC, Macdonald DW, Warren RC, Booker MW, Whitehead MI (1986) Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed) 293:1329–1330
Yallampalli C, Chauhan M, Thota CS, Kondapaka S, Wimalawansa SJ (2002) Calcitonin gene-related peptide in pregnancy and its emerging receptor heterogeneity. Trends Endocrinol Metab 13:263–269
Valdemarsson S, Edvinsson L, Hedner P, Ekman R (1990) Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Investig 50:385–388
Gupta P, Harte A, Sturdee DW, Sharma A, Barnett AH, Kumar S, Mc Ternan PG (2008) Effects of menopausal status on circulating calcitonin gene-related peptide and adipokines: implications for insulin resistance and cardiovascular risks. Climacteric 11:364–372
Gupta S, Villalon CM, Mehrotra S, De Vries R, Garrelds IM, Pramod R, Saxena PR, MaassenVanDenBrink A (2007) Female sex hormones and rat dural vasodilatation to CGRP, periarterial electrical stimulation and capsaicin. Headache 47:225–235
Gupta S, Mehrotra S, Villalón C, De Vries R, Garrelds I, Saxena P, Vandenbrink AM (2007) Effects of female sex hormones on responses to CGRP, acetylcholine, and 5-HT in rat isolated arteries. Headache 47:564–575
Ibrahimi K, van Oosterhout WP, van Dorp W, Danser AH, Garrelds IM, Kushner SA, Lesaffre EM, Terwindt GM, Ferrari MD, van den Meiracker AH, MaassenVanDenBrink A (2015) Reduced trigeminovascular cyclicity in patients with menstrually related migraine. Neurology 84:125–131
Sandweiss AJ, Cottier KE, McIntosh MI, Dussor G, Davis TP, Vanderah TW, Largent-Milnes TM (2017) 17-β-Estradiol induces spreading depression and pain behavior in alert female rats. Oncotarget 8:114109–114122
Eikermann-Haerter K, Dileköz E, Kudo C, Savitz SI, Waeber C, Baum MJ, Ferrari MD, van den Maagdenberg Arn MJM, Moskowitz MA, Ayata C (2009) Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Investig 119:99–109
Chauvel V, Multon S, Schoenen J (2018) Estrogen-dependent effects of 5-hydroxytryptophan on cortical spreading depression in rat: modelling the serotonin-ovarian hormone interaction in migraine aura. Cephalalgia 38:427–436
Borsook D, Erpelding N, Lebel A, Linnman C, Veggeberg R, Grant PE, Buettner C, Becerra L, Burstein R (2014) Sex and the migraine brain. Neurobiol Dis 68:200–214
Amandusson A, Hermanson O, Blomqvist A (1995) Estrogen receptor-like immunoreactivity in the medullary and spinal dorsal horn of the female rat. Neurosci Lett 196:25–28
Shughrue PJ, Bushnell CD, Dorsa DM (1992) Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology 131:381–388
Amandusson A, Blomqvist A (2013) Estrogenic influences in pain processing. Front Neuroendocrinol 34:329–349
Sutherland HG, Champion M, Plays A, Stuart S, Haupt LM, Frith A, MacGregor EA, Griffiths LR (2017) Investigation of polymorphisms in genes involved in estrogen metabolism in menstrual migraine. Gene 5(607):36–40
Rodriguez-Acevedo AJ, Smith RA, Roy B, Sutherland H, Lea RA, Frith A, MacGregor EA, Griffiths LR (2014) Genetic association and gene expression studies suggest that genetic variants in the SYNE1 and TNF genes are related to menstrual migraine. J Headache Pain 14(15):62
Pollock CE, Sutherland HG, Maher BH, Lea RA, Haupt LM, Frith A, MacGregor EA, Griffiths LR (2018) The NRP1 migraine risk variant shows evidence of association with menstrual migraine. J Headache Pain 19:31
MacGregor EA, Chia H, Vohrah RC, Wilkinson M (1990) Migraine and menstruation: a pilot study. Cephalalgia 10:305–310
Granella F, Sances G, Zanferrari C, Costa A, Martignoni E, Manzoni GC (1993) Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache 33:385–389
Cupini LM, Matteis M, Troisi E, Calabresi P, Bernardi G, Silvestrini M (1995) Sex-hormone-related events in migrainous females. A clinical comparative study between migraine with aura and migraine without aura. Cephalalgia 15:140–144
Vetvik KG, MacGregor EA, Lundqvist C, Russell MB (2010) Self-reported menstrual migraine in the general population. J Headache Pain 11:87–92
Vetvik KG, Macgregor EA, Lundqvist C, Russell MB (2014) Prevalence of menstrual migraine: a population-based study. Cephalalgia 34:280–288
Pavlović JM, Stewart WF, Bruce CA, Gorman JA, Sun H, Buse DC, Lipton RB (2015) Burden of migraine related to menses: results from the AMPP study. J Headache Pain 16:24
Güven B, Güven H, Çomoğlu S (2017) Clinical characteristics of menstrually related and non-menstrual migraine. Acta Neurol Belg 117:671–676
Melhado EM, Thiers Rister HL, Belitardo GA, de Oliveira DR, Buttarello A, Belucio IS, Oliveira Marcos JM, Tonhá Xavier ML, Prieto Peres MF (2019) Allodynia in menstrually related migraine: Score Assessment by Allodynia Symptom Checklist (ASC-12). Headache. https://doi.org/10.1111/head.13677(Epub ahead of print)
Ferreira KS, Guilherme G, Faria VR, Borges LM, Uchiyama AA (2017) Women living together have a higher frequency of menstrual migraine. Headache 57:135–142
Petrovski BÉ, Vetvik KG, Lundqvist C, Eberhard-Gran M (2018) Characteristics of menstrual versus non-menstrual migraine during pregnancy: a longitudinal population-based study. J Headache Pain 19:27
Wang SJ, Fuh JL, Lu SR, Juang KD, Wang PH (2003) Migraine prevalence during menopausal transition. Headache 43:470–478
Nierenburg Hdel C, Ailani J, Malloy M, Siavoshi S, Hu NN, Yusuf N (2015) Systematic review of preventive and acute treatment of menstrual migraine. Headache 55:1052–1071
Bigal M, Sheftell F, Tepper S, Tepper D, Ho TW, Rapoport A (2008) A randomized double-blind study comparing rizatriptan, dexamethasone, and the combination of both in the acute treatment of menstrually related migraine. Headache 48:1286–1293
Allais G, Rolando S, Schiapparelli P, Airola G, Borgogno P, Mana O, Benedetto C (2013) Frovatriptan plus dexketoprofen in the treatment of menstrually related migraine: an open study. Neurol Sci 34:S179–S181
Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T (2009) Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 339:b3914
Carlton C, Banks M, Sundararajan S (2018) Oral contraceptives and ischemic stroke risk. Stroke 49:e157–e159
Sacco S, Merki-Feld GS, Ægidius KL, Bitzer J, Canonico M, Kurth T, Lampl C, Lidegaard Ø, MacGregor EA, MaassenVanDenBrink A, Mitsikostas DD, Nappi RE, Ntaios G, Sandset PM, Martelletti M, European Headache Federation (EHF), the European Society of Contraception, and Reproductive Health (ESC) (2017) Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain 18:108
Warhurst S, Rofe CJ, Brew BJ, Bateson D, McGeechan K, Merki-Field GS, Garrick R, Tomlinson SE (2018) Effectiveness of the progestin-only pill for migraine treatment in women: a systematic review and meta-analysis. Cephalalgia 38:754–764
Burke BE, Olson RD, Cusack BJ (2002) Randomized, controlled trial of phytoestrogen in the prophylactic treatment of menstrual migraine. Biomed Pharmacother 56:283–288
Ferrante F, Fusco E, Calabresi P, Cupini LM (2004) Phyto-oestrogens in the prophylaxis of menstrual migraine. Clin Neuropharmacol 27:137–140
Grazzi L, Egeo G, Calhoun AH, McClure CK, Liebler E, Barbanti P (2016) Non-invasive vagus nerve stimulation (nVNS) as mini-prophylaxis for menstrual/menstrually related migraine: an open-label study. J Headache Pain 17:91
Coffee AL, Sulak PJ, Hill AJ, Hansen DJ, Kuehl TJ, Clark JW (2014) Extended cycle combined oral contraceptives and prophylactic frovatriptan during the hormone-free interval in women with menstrual-related migraines. J Womens Health (Larchmt) 23:310–317
Dima L, Bălan A, Moga MA, Dinu CG, Dimienescu OG, Varga I, Neculau AE (2019) Botulinum toxin a valuable prophylactic agent for migraines and a possible future option for the prevention of hormonal variations-triggered migraines. Toxins (Basel) 11(8):E465. https://doi.org/10.3390/toxins11080465
Ho TW, Ho AP, Ge YJ, Assaid C, Gottwald R, MacGregor EA, Mannix LK, van Oosterhout WP, Koppenhaver J, Lines C, Ferrari MD, Michelson D (2016) Randomized controlled trial of the CGRP receptor antagonist telcagepant for prevention of headache in women with perimenstrual migraine. Cephalalgia 36:148–161
Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, Sapra S, Picard H, Mikol DD, Lenz RA (2017) A controlled trial of erenumab for episodic migraine. N Engl J Med 377:2123–2132
Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR (2018) Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol 75:1080–1088
Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, Grozinski-Wolff M, Yang R, Ma Y, Aycardi E (2018) Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA 319:1999–2008
Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J, for the ALD403 study investigators (2014) Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13:1100–1107
Do TP, Guo S, Ashina M (2019) Therapeutic novelties in migraine: new drugs, new hope? J Headache Pain 20:37. https://doi.org/10.1186/s10194-019-0974-3
Author information
Authors and Affiliations
Contributions
Category 1, (a) conception and design: LMC, IC, PS; (b) acquisition of data: LMC, IC, PS; (c) analysis and interpretation of data: LMC, IC, PS. Category 2, (a) drafting the manuscript: LMC, IC, PS; (b) revising it for intellectual content: LMC, IC, PS. Category 3, (a) final approval of the completed manuscript: LMC, IC, PS.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Cupini, L.M., Corbelli, I. & Sarchelli, P. Menstrual migraine: what it is and does it matter?. J Neurol 268, 2355–2363 (2021). https://doi.org/10.1007/s00415-020-09726-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09726-2