Abstract
Aim
The second consensus statement for the diagnosis of multiple system atrophy type cerebellar (MSA-C) includes pons and middle cerebellar peduncle (MCP) atrophy as MRI features. However, other MRI abnormalities such as MCP hyperintensity, hot cross bun sign (HCB), putaminal hypointensity and hyperintense putaminal rim have been described.
Objectives
To evaluate, in patients with sporadic late-onset cerebellar ataxia (SLOCA), the discriminative value of several MRI features for the diagnosis of MSA-C, to follow their evolution during the course of MSA-C, and to search for correlations between these MRI features and clinical signs.
Methods
Consecutive patients referred for SLOCA underwent comprehensive clinical evaluation and laboratory investigations, brain MRI, DaTscan and a 1-year follow-up.
Results
Among 80 patients, 26 had MSA-C, 22 another diagnosis, and 32 no diagnosis at the end of the follow-up. At baseline, MCP hyperintensity and HCB were more frequent in patients finally diagnosed with MSA-C than in other patients with SLOCA (p < 0.0001), and had the highest specificity (98.5%) and positive predictive value (91.7%) for the diagnosis of MSA-C, compared to all other MRI signs. The most relevant MRI sequence regarding HCB sign was the T2-proton density (DP) weighted. All MRI features were more frequent with disease duration. No correlation was found between any MRI feature and neither clinical data, nor dopaminergic neuronal loss (p = 0.5008), except between vermis atrophy and UPDRSIII score.
Conclusion
MCP hyperintensity and HCB sign should be added into the list of additional features of possible MSA-C. MRI signal abnormalities suggestive of MSA-C should be searched for in suitable sequence.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple system atrophy (MSA) is a sporadic, degenerative, progressive, adult onset disorder of unknown cause, which involves striatonigral, olivopontocerebellar and spinal cord structures and whose pathological hallmark is glial cytoplasmic inclusions. Two clinical forms have been described: MSA-P with predominant parkinsonism, and MSA-C, with predominant cerebellar ataxia in early stages. Although a recent prospective study demonstrated that MSA-C was the most frequent cause of sporadic late-onset cerebellar ataxia (SLOCA) [1], and since there are numerous causes of SLOCA [2], the diagnosis of MSA-C can be challenging. According to the second consensus statement on the diagnosis of MSA [3], the diagnosis criteria for MSA-C include three categories: definite MSA-C, requiring pathologic confirmation, probable MSA-C, requiring autonomic failure in addition to cerebellar ataxia; and possible MSA-C, requiring cerebellar ataxia associated to autonomic dysfunction and at least one additional features including parkinsonism, Babinski sign with hyperreflexia, stridor, putamen, pons or middle cerebellar peduncle (MCP) atrophy, hypometabolism on [18F]fluorodeoxyglucose(FDG)-PET in putamen, or presynaptic nigrostriatal dopaminergic denervation on SPECT or PET. Other magnetic resonance imaging (MRI) features, such as “hot cross bun” (HCB) sign, MCP hyperintensity, putaminal hypointensity, hyperintense putaminal rim sign have been described in MSA patients, but are not included in the second consensus statement on the diagnosis of MSA. The discriminative value of these MRI features for the diagnosis of MSA has been studied rather in patients with parkinsonism [4,5,6,7,8,9] than in patients with cerebellar ataxia which are rarer. Only one study suggests that pons atrophy, MCP atrophy, MCP hyperintensity and HCB sign can help to differentiate MSA-C from other SLOCA [10].
The objectives of this prospective study were (1) to compare the discriminative value of each of the MRI features (atrophy or signal abnormalities) for the diagnosis of MSA-C, (2) to follow their evolution during the course of the disease, (3) to search for correlations between each of these MRI features and some clinical (presence of autonomic dysfunction or failure, parkinsonism, cerebellar ataxia severity) and paraclinical (nigrostriatal dopaminergic denervation) signs.
Methods
From January 2013 to April 2017, all patients consecutively sent to our referral centre for SLOCA, were included [1]. Inclusion criteria were: (i) cerebellar ataxia (CA) either isolated or not, (ii) lack of family history of CA; (iii) onset of CA after 40 years, (iv) CA duration longer than 6 months. The only exclusion criterion was MRI contraindication. Patients had a prospective follow-up with collection of clinical and imaging data as well as laboratory investigations at baseline, and 1 year later.
Collected clinical data included: Scale for the Assessment and Rating of Ataxia (SARA) score, Spinocerebellar Degeneration Functional Score (SDFS), Unified Parkinson’s Disease Rating Scale III (UPDRS III) score, assessment of dysautonomia (urinary signs, erectile dysfunction, orthostatic hypotension). To evaluate the severity of disease progression, scales scores were corrected by disease duration (DD). Probable or possible MSA were diagnosed according to the second consensus statement of 2008, which included MRI features (pons and MCP atrophy) [3]. Autonomic failure was established by urinary incontinence, with erectile dysfunction in males or orthostatic hypotension (defined by a decrease of blood pressure by a least 30 mmHg systolic or 15 mmHg diastolic after 3 min of standing) and autonomic dysfunction by urinary emergency, frequency or incomplete bladder emptying, erectile dysfunction in males or significant orthostatic blood pressure that does not meet the level required in probable MSA.
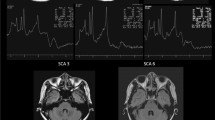
Brain MRI were performed at baseline (MRI 1) and one year later (MRI 2) with 1.5 or 3 T imaging system. MRI protocol included sagittal T1-weighted, axial T2-weighted, axial T2-proton density (PD)-weighted, axial T2 FLAIR-weighted, axial DWI-weighted, and axial T2*-weighted sequences. MRI data, which were collected by both a neuroradiologist and a neurologist of the referral centre, blinded from the suspected diagnosis, were the following: HCB sign, posterior putaminal hypointensity (on axial T2*), hyperintense putaminal rim sign (on T2 FLAIR [11], MCP hyperintensity (on T2 and T2 FLAIR), cerebellar vermis and cerebellar hemispheres atrophy (on sagittal T1, with the following visual scale, 0: absent, 1: mild, 2: moderate, 3: severe), and pons, midbrain and MCP sizes (Fig. 1). Pons and midbrain size were measured on sagittal T1-weighted sections as previously described [12], and MCP on axial T2 FLAIR-weighted sequences.
MRI features observed in MSA: Putaminal hypointensity (a), atrophy of middle cerebellar peduncle (MCP) (b), hyperintense putaminal rim sign (c), atrophy of pons and cerebellar vermis (d), hot cross bun sign in T2-PD-weighted (e), HCB sign in T2*-weighted (f), HCB sign and hyperintensity of MCP in T2-weighted (g), HCB sign in T2 FLAIR-weighted (h)
Brain MRI data from 30 age-matched controls, randomly extracted from MRI database at the author’s centre, were collected to establish reference sizes. For each healthy control, the MRI protocol included sagittal T1-weighted sequences (for pons and midbrain measurements), and axial T2 FLAIR-weighted sequences (for MCP measurement). Pons, midbrain and MCP atrophies were defined by a size which was inferior to the lowest value observed in controls.
123I-Ioflupane-SPECT (DaTscan) were performed and evaluated as previously described [13] by an expert nuclear radiologist blindly to the diagnosis suspected.
A comprehensive laboratory investigation was performed in all patients including alpha-fetoprotein serum level, albumin, vitamin E, copper, ceruloplasmin, acanthocytosis, very long-chain fatty acids, aryl sulfatase, hexosaminidase A + B, cholestanol, phytanic acid, homocystein, anti-neuronal antibodies, anti-thyropéroxydase antibodies, cerebrospinal fluid analysis (including 14-3-3 protein, Whipple PCR, Lyme serology, anti-neuronal antibodies), thoraco-abdomino-pelvic tomodensitometry, electromyography, electroencephalography, genetics study including SCA 1, 2, 3, 6, 7, 17 and DRPLA, Friedreich’s ataxia, FMR1 premutation for fragile X associated tremor/ataxia syndrome and, according to the phenotype, MELAS, MERFF, POLG1, episodic ataxia type 1 and 2, SLC20A2, PDGFRB, PDGFB and XPR1 genes sequencing.
Statistical analyses used the statistical software package Statistical Analysis System (SAS) for Windows, release 9.3 (SAS Institute Inc., Cary, NC, USA). The Shapiro–Wilk test and Q–Q plots were used to assess the normality of distribution of quantitative variables. Non-parametric statistical tests were used for the analysis of data, as the variables were not normally distributed. We applied Wilcoxon and Kruskall–Wallis tests for comparisons of quantitative variables across two or more independent groups. In case of statistically significant results, groups were pairwise compared and the p values were adjusted using the Bonferroni–Holm method. We used the Bonferroni–Holm correction for multiple comparisons. The linear relationship between quantitative variables was assessed using the Spearman correlation procedure. The Chi-square and the Fisher’s exact test were applied to test for differences in proportions between groups or association between categorical variables. Survival Estimates were calculated by the Kaplan–Meier method using the SAS Lifetest procedure. For all statistical tests, we considered p values < 0.05 as statistically significant.
Local ethics committee approved the study. Patients gave written consent to participate to the study.
Results
Demographic and clinical profiles of the 80 SLOCA patients and of 30 controls are presented in Table 1. At the end of the study, a diagnosis of possible or probable MSA-C (group 1) has been established for 26 patients (32.5%), another diagnosis for 22 (27.5%) (group 2), and no diagnosis was found for 32 (40.0%) (group 3).
Among MSA-C patients, 8 patients (10%, 4 males, 4 females) met the diagnosis criteria for possible MSA-C, and 18 (22.5%, 11 males, 7 females) for probable MSA-C. Diagnosis in group 2 were the following: Very Late Onset Friedreich Ataxia (vLOFA) (N = 2), Mitochondrial disease (N = 2), primary progressive multiple sclerosis (N = 4), paraneoplastic syndrome (N = 2), Creutzfeldt-Jakob disease (N = 1), superficial siderosis (N = 1), Arnold-Chiari malformation (N = 1), alcoholic cerebellar degeneration (N = 1), Hashimoto’s encephalopathy (N = 1), primary brain calcification due to SLC20A2 mutation (N = 3), cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS) (N = 1), coeliac disease (N = 1), SCA 3 (N = 1), severe essential tremor with cerebellar ataxia (N = 1).
Atrophy was defined by a size under 21 mm (mm) for pons, under 15 mm for the midbrain, and under 14 mm for the MCP (Table 2).
Discriminative value of brain MRI signs at baseline for the diagnosis of MSA-C following a 1-year follow-up
Hyperintensity of MCP, and HCB sign had the higher specificities and positive predictive values (PPV) for the diagnosis of MSA-C at baseline (Table 3, Fig. 1).
HCB sign was present in 44% of patients from group 1 and hyperintensity of MCP in 42% (Table 3), while in group 2 only 1 patient who had SCA 3, showed the HCB sign and another one, who had multiple sclerosis, showed a MCP hyperintensity.
The HCB sign was significantly more frequent on T2-PD sequences (p = 0.0074 on MRI 1 and p = 0.012 on MRI 2) and T2* sequences (p = 0.0416 on MRI1) than on T2 FLAIR sequences (Table 4).
Hypointensity of the putamen was significantly more frequent in group 1 (p < 0.0001), but observed in 10 patients (18.52%) from groups 2 and 3 [2 with SLC20A2 mutation, 1 with CANVAS, 1 with severe Essential tremor, 1 with paraneoplastic syndrome, and 5 with idiopathic late onset cerebellar ataxia (ILOCA)]. The hyperintense putaminal lateral rim was found in only one patient from group 1 and in none from groups 2 or 3 (Table 3).
Vermis and cerebellum hemispheres atrophies were present in all groups, and were not discriminant for MSA-C diagnosis (Table 3). Sizes of pons and MCP (Table 1) were lower in group 1 than in groups 2 and 3, while size of midbrain was not significantly different in the three groups. As expected, pons and MCP atrophies were significantly more frequent in group 1 than in groups 2 and 3 (Table 3).
In the MSA-C group, at baseline, MRI features were not necessary for the diagnosis of possible or probable MSA-C in 15/26 patients according to the second consensus statement. However, in 3/26 patients (11.5%) with ataxia and autonomic dysfunction (but neither autonomic failure, nor parkinsonism), presence of pons and MCP atrophy allowed the diagnosis of possible MSA, and in 3/26 patients (19.2%) with isolated cerebellar ataxia, other MRI features, not present in the second consensus statement [HCB sign and MCP hyperintensity (two patients), putaminal hypointensity (one patient)] were already present.
Natural history of MRI features in MSA-C (group1)
Kaplan–Meier curves (Fig. 2), demonstrated that the various MRI features were more frequent with disease progression. Furthermore, the probability to find each of all the MRI features increased with DD.
Kaplan–Meier curves: probability of presence of various MRI features during the course of MSA-C. a Pons, midbrain, cerebellum, middle cerebellum peduncle (MCP) atrophy. a: comparison between midbrain atrophy and MCP atrophy; b: comparison between midbrain atrophy and atrophy of cerebellar hemisphere; c: comparison between midbrain atrophy and atrophy of vermis. b Hot cross bun sign, MCP hyperintensity, putamen hypointensity, putamen hyperintense lateral rim
Comparing MRI 1 and MRI 2, sizes of pons (p < 0.0001) and MCP (p = 0.0002) decreased significantly in MRI 2, and the HCB sign was significantly more frequent on MRI 2 (p = 0.0313).
Linear correlations (Spearman test r: correlation coefficient) were found between DD and (1) severity of vermis atrophy (MRI 1 (r = 0.6293; p = 0.0006) and MRI 2 (r = 0.4317; p = 0.0448), (2) atrophy of the cerebellar hemisphere (MRI 1 (r = 0.4170; p = 0.0341) and MRI 2 (r = 0.5939; p = 0.0036)), and (3) size of pons (MRI 2 r = − 0.5071; p = 0.0160), and of MCP (MRI 2 r = − 0.4847; p = 0.0223). Although neither pons, nor midbrain and nor MCP atrophies significantly correlated with DD, size of these structures tended to decrease with DD, except for midbrain (Fig. 3).
Correlation between MRI features and clinical or paraclinical data
No correlations were found between presence of MRI features and autonomic failure [MRI 1 (p = 0.3147), MRI 2 (p = 0.5264)], autonomic dysfunction [MRI 1 (p = 1), or extrapyramidal signs (MRI 1 (p = 0.4449), MRI 2 (p = 0.2684)].
Presence of vermis atrophy was correlated with UPDRS III score/DD ratio on MRI 1 (p = 0.0430) and on MRI 2 (p = 0.0244), while no other significant correlation was found between MRI features and the SARA, SDFS and UPDRSIII scores although theses scales tended to worsen with DD (supplementary material: Figs. 4, 5, 6). Furthermore no correlation was found between presence of MRI features and dopaminergic denervation on Datscan [MRI 1 (p = 0.5008), MRI 2 (p = 1)]. A nigrostriatal dopaminergic denervation was found in seven patients without any clinical signs of parkinsonism.
Discussion
This study is, to our knowledge, the first to prospectively report sensibility, specificity, PPV and NPV of HCB sign, MCP hyperintensity, putaminal hypointensity, and hyperintense putaminal rim for the diagnosis of MSA-C. MCP hyperintensity and HCB sign have the highest specificity and PPV and, in spite of a low sensibility, they seem relevant for the diagnosis of MSA-C in case of SLOCA (p < 0.0001) (Table 3). HCB sign was previously described in SCA 1, SCA 2 and in SCA 3 (Machado–Joseph) [14,15,16]. However, a misdiagnosis between SCA and MSA-C is probably rare, due to different associated signs (especially dysautonomia in MSA-C and ocular signs in some SCA) and to a slower evolution for SCA. Since specificity and PPV of HCB sign and MCP hyperintensity appeared higher than those of pons and MCP atrophy (which are included in MSA criteria), and since they are more easy to detect, we suggest that they should be added into the list of additional features of possible MSA-C [3].
It is important to emphasize that these signal abnormalities should be searched for, on suitable MRI sequences. Hypointensity of putamen, which demonstrated increased putaminal iron content, should be searched for on T2*-weighted sequences [17], and hyperintense lateral rim of putamen, which presence is significant only on 1.5 T scanner [11], on T2 FLAIR-weighted sequences. For HCB sign which has high specificity and PPV, there is no clear recommendation [18, 19], but our findings suggest that T2-PD sequences should be recommended (Table 4).
Previous studies showed that putamen hypointensity cannot differentiate MSA nor from other parkinsonian syndromes [20, 21], nor from isolated cerebellar ataxia (ILOCA) [10]. In our series we confirm that in front of a patient with SLOCA, an isolated putaminal hypointensity is not sufficient for the diagnosis of MSA-C. However, especially in patients with ILOCA, a later progression to MSA criteria cannot be excluded, and in case of putaminal hypointensity, a strict follow-up should be proposed to search for MSA-C.
As demonstrated previously [22], pons and MCP atrophies were significantly more frequent in MSA-C patients. We showed that these atrophies can be precocious in the course of MSA-C and that probability of their presence is closed to 1 after 6 years of disease duration (Fig. 2). This last point was to our knowledge never reported. Nevertheless, due to a low sensitivity and PPV at baseline, these features are not the best for an early diagnosis of MSA-C in patients with SLOCA.
Probability of midbrain atrophy in MSA-C patients was under 0.4 at 10 years in our series. Contrary to Pradhan et al. [23], we suggest that midbrain atrophy cannot be used to differentiate MSA-C in patients with SLOCA, and so whatever the stage of the disease.
Previously, several studies found a linear correlation between DD and cerebellar atrophy [23, 24] and brainstem volume [24], but only a tendency for the HCB sign [25, 26] and for putaminal findings [26] to be more frequent when DD is longer. Herein, we confirmed linear correlations between DD and morphological features (atrophy or decrease of size), and found no correlation between DD and MRI signal changes (HCB sign, MCP and putaminal signal changes).
These last results raised the question whether some MRI signs can be correlated with presence of specific clinical signs in patients with MSA-C. Previous studies showed that clinical cerebellar signs were correlated with infratentorial MRI abnormalities [6, 10, 27], while contradictory results were obtained for correlation between parkinsonian signs and putamen hypointensity [6, 10, 28]. To our knowledge, our study was the first to search for correlations between abnormalities on conventional MRI and the three main signs of MSA (dysautonomia, cerebellar ataxia, and parkinsonism). While obviously, SARA, SDFS, and UPDRS III tended to worsen with DD (supplementary material: Figs. 4, 5, 6) the only correlation which could be established was between the severity of UPDRS III progression (scale corrected by DD) and the presence of vermis atrophy. One explanation could be that UPDRS III score has been worsened by associated cerebellar signs such as slowness or clumsiness, which can increase UPDRS III axial and tapping scores. However, no correlation with the SARA score has been found, and, to our opinion, this correlation with UPDRS III needs to be confirmed and further elucidated. In accordance with pathophysiology of dysautonomia in MSA [29] no correlation was found between brain MRI abnormalities and autonomic failure or autonomic dysfunction.
The more remarkable finding was, at baseline, the presence of MRI abnormalities in patients who had not yet developed any autonomic dysfunction or failure, parkinsonism or pyramidal signs. While the usefulness of MRI criteria for an earlier diagnosis has been reported in MSA-P [30], our study demonstrate that some MRI features which are not included in the second consensus statement may precede dysautonomia and parkinsonism in MSA-C.
A presynaptic dopaminergic denervation has been demonstrated by 123I-Ioflupane-CIT-SPECT (DaTscan), both in MSA-P [31] and MSA-C [32]. This dopaminergic denervation precedes the occurrence of clinical parkinsonism [33], and presynaptic nigrostriatal dopaminergic denervation on SPECT or PET is one of the additional feature for the diagnosis of possible MSA-C. Munoz et al. [33] have found that most, but not all, MSA-C patients without parkinsonism had subclinical nigrostriatal dopaminergic denervation, but that no correlation could be made neither with cerebellar disability, nor with pontine atrophy nor with DD. In our series, we demonstrated the absence of correlation between nigrostrial denervation and each of the other MRI features found in MSA-C. These findings suggest that in MSA-C nigrostriatal dopaminergic denervation and MRI features may occur independently, and that functional and morphological imaging provide complementary information for the diagnosis of MSA-C.
Several limits of our study should be pointed out. First, and as in most clinical studies, none of our patients had a pathological confirmation of MSA. However, the disease duration to the end of the 1-year follow-up could be considered as long enough for a reliable clinical diagnosis. Second, the number of patient in each group could appear small: however, it was large enough to provide significant results and furthermore, we can emphasize that a prospective, standardized follow-up is rare in diseases such as SLOCA. Another limitation is that, we did not used Unified Multiple System Atrophy Rating Scale (UMSARS) but this scale did not appear appropriate for other SLOCA that MSA-C. Lastly, it could be raised that MSA diagnosis was made with the second consensus statement criteria which include some of the MRI features which were also assessed in our study (atrophy of pons and MCP). However, all patients had, at the end of the prospective follow-up, the clinical criteria for possible or probable MSA, and atrophy of pons and MCP were not necessary to the diagnosis at this stage. Moreover, the most important findings in our study are related to HCB sign and MCP hyperintensity which are not included in the second consensus statement criteria.
In summary, we showed that, in patients who will experience probable MSA-C, MRI abnormalities can precede the main diagnosis criteria which are dysautonomia and parkinsonism, and that in front of a patient with SLOCA, presence of atrophy, but also of signal abnormalities in pons, MCP or putamen should be carefully searched for in suitable sequences, in order not to miss an early diagnosis of MSA. Since HCB sign and MCP hyperintensity have higher specificity and PPV than those of pons and MCP atrophy, we propose that they should be added into the list of additional features of possible MSA-C.
References
Gebus O, Montaut S, Monga B, Wirth T, Cheraud C, Rego AC, Zinchenko I, Carré G, Hamdaoui M, Hautecloque G, Nguyen-Them L, Lannes B, Chanson JB, Lagha-Boukbiza O, Fleury MC, Devys D, Nicolas G, Rudolf G, Bereau M, Mallaret M, Renaud M, Acquaviva M, Koenig M, Koob M, Kremer S, Namer IJ, Cazeneuve C, -Laguna A, Tranchant C, Anheim M (2017) Deciphering the causes of sporadic late-onset cerebellar ataxias: a prospective study with implications for diagnostic work. J Neurol 264(6):1118–1126
Hadjivassiliou M, Martindale J, Shanmugarajah P, Grünewald RA, Sarrigiannis PG, Beauchamp N, Garrard K, Warburton R, Sanders DS, Friend D, Duty S, Taylor J, Hoggard N (2017) Causes of progressive cerebellar ataxia: prospective evaluation of 1500 patients. J Neurol Neurosurg Psychiatry 88(4):301–309
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71(9):670–676
Lewinski F, Werner C, Jörn T, Mohr A, Sixel-Döring F, Trenkwalder C (2007) T2*-weighted MRI in diagnosis of multiple system atrophy: a practical approach for clinicians. J Neurol 254(9):1184–1188
Righini A, Antonini A, Ferrarini M, de Notaris R, Canesi M, Triulzi F, Pezzoli G (2002) Thin section MR study of the basal ganglia in the differential diagnosis between striatonigral degeneration and Parkinson disease. J Comput Assist Tomogr 26(2):266–271
Lee EA, Cho HI, Kim SS, Lee WY (2004) Comparison of magnetic resonance imaging in subtypes of multiple system atrophy. Parkinsonism Relat Disord 10(6):363–368
Bhattacharya K, Saadia D, Eisenkraft B, Yahr M, Olanow W, Drayer B, Kaufmann H (2002) Brain magnetic resonance imaging in multiple-system atrophy and Parkinson disease: a diagnostic algorithm. Arch Neurol 59(5):835–842
Nicoletti G, Fera F, Condino F, Auteri W, Gallo O, Pugliese P, Arabia G, Morgante L, Barone P, Zappia M, Quattrone A (2006) MR imaging of middle cerebellar peduncle width: differentiation of multiple system atrophy from Parkinson disease. Radiology 239(3):825–830
Seppi K, Schocke MFH, Prennschuetz-Schuetzenau K, Mair KJ, Esterhammer R, Kremser C, Muigg A, Scherfler C, Jaschke W, Wenning GK, Poewe W (2006) Topography of putaminal degeneration in multiple system atrophy: a diffusion magnetic resonance study. Mov Disord Off J Mov Disord Soc 21(6):847–852
Bürk K, Bühring U, Schulz JB, Zühlke C, Hellenbroich Y, Dichgans J (2005) Clinical and magnetic resonance imaging characteristics of sporadic cerebellar ataxia. Arch Neurol 62(6):981–985
Lee W-H, Lee C-C, Shyu W-C, Chong P-N, Lin S-Z (2005) Hyperintense putaminal rim sign is not a hallmark of multiple system atrophy at 3T. AJNR Am J Neuroradiol 26(9):2238–2242
-Metz M, Naumann M, Csoti I, Solymosi L (2001) Measurement of the midbrain diameter on routine magnetic resonance imaging: a simple and accurate method of differentiating between Parkinson disease and progressive supranuclear palsy. Arch Neurol 58(7):1076–1079
Anheim M, Lagier-Tourenne C, Stevanin G, Fleury M, Durr A, Namer IJ, Denora P, Brice A, Mandel JL, Koenig M, Tranchant C (2009) SPG11 spastic paraplegia. A new cause of juvenile parkinsonism. J Neurol 256(1):104–108
Murata Y, Yamaguchi S, Kawakami H, Imon Y, Maruyama H, Sakai T, Kazuta T, Ohtake T, Nishimura M, Saida T, Chiba S, Oh-I T, Nakamura S (1998) Characteristic magnetic resonance imaging findings in Machado-Joseph disease. Arch Neurol 55(1):33–37
Namekawa M, Honda J, Shimazaki H (2015) “Hot Cross Bun” sign associated with SCA1. Intern Med 54(7):859–860
Bürk K, Skalej M, Dichgans J (2001) Pontine MRI hyperintensities (“the cross sign”) are not pathognomonic for multiple system atrophy (MSA). Mov Disord Off J Mov Disord Soc 16(3):535
Sugiyama A, Ito S, Suichi T, Sakurai T, Mukai H, Yokota H, Yonezu T, Kuwabara S (2015) Putaminal hypointensity on T2*-weighted MR imaging is the most practically useful sign in diagnosing multiple system atrophy: a preliminary study. J Neurol Sci 349(1–2):174–178
Deguchi K, Ikeda K, Kume K, Takata T, Kokudo Y, Kamada M, Touge T, Honjo N, Masaki T (2015) Significance of the hot-cross bun sign on T2*-weighted MRI for the diagnosis of multiple system atrophy. J Neurol 262(6):1433–1439
Kasahara S, Miki Y, Kanagaki M, Kondo T, Yamamoto A, Morimoto E, Okadaa T, Itoc H, Takahashic H, Togashi K (2012) “Hot cross bun” sign in multiple system atrophy with predominant cerebellar ataxia: A comparison between proton density-weighted imaging and T2-weighted imaging. Eur J Radiol 81(10):2848–2852
Schrag A, Good CD, Miszkiel K, Morris HR, Mathias CJ, Lees AJ, Quinn NP (2000) Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology 54(3):697–702
Schrag A, Kingsley D, Phatouros C, Mathias CJ, Lees AJ, Daniel SE, Quinn NP (1998) Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry 65(1):65–71
Lin DJ, Hermann KL, Schmahmann JD (2016) The diagnosis and natural history of multiple system atrophy, cerebellar type. Cerebellum 15(6):663–679
Pradhan S, Tandon R (2017) Relevance of non-specific MRI features in multiple system atrophy. Clin Neurol Neurosurg 159:29–33
Burk K (2004) MRI-based volumetric differentiation of sporadic cerebellar ataxia. Brain 127(1):175–181
Abe K, Hikita T, Yokoe M, Mihara M, Sakoda S (2006) The “cross” signs in patients with multiple system atrophy: a quantitative study. J Neuroimaging 16(1):73–77
Horimoto Y, Aiba I, Yasuda T, Ohkawa Y, Katayama T, Yokokawa Y, Goto A, Ito Y (2002) Longitudinal MRI study of multiple system atrophy—when do the findings appear, and what is the course? J Neurol 249(7):847–854
Konagaya M, Konagaya Y, Iida M (1994) Clinical and magnetic resonance imaging study of extrapyramidal symptoms in multiple system atrophy. J Neurol Neurosurg Psychiatry 57(12):1528–1531
Wakai M, Kume A, Takahashi A, Ando T, Hashizume Y (1994) A study of parkinsonism in multiple system atrophy: clinical and MRI correlation. Acta Neurol Scand 90(4):225–231
Wenning GK, Tison F, Shlomo Y, Daniel SE, Quinn NP (1997) Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12(2):133–147
Mestre TA, Gupta A, Lang AE (2016) MRI signs of multiple system atrophy preceding the clinical diagnosis: the case for an imaging-supported probable MSA diagnostic category. J Neurol Neurosurg Psychiatry 87(4):443–444
Antonini A, Benti R, De Notaris R, Tesei S, Zecchinelli A, Sacilotto G, Meucci N, Canesi M, Mariani C, Pezzoli G, Gerundini P (2003) 123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci 24(3):149–150
Pirker W, Asenbaum S, Bencsits G, Prayer D, Gerschlager W, Deecke L, Brücke T (2000) [123I]beta-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord Off J Mov Disord Soc 15(6):1158–1167
Muñoz E, Iranzo A, Rauek S, Lomeña F, Gallego J, Ros D, Santamaría J, Tolosa E (2011) Subclinical nigrostriatal dopaminergic denervation in the cerebellar subtype of multiple system atrophy (MSA-C). J Neurol 258(12):2248–2253
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has been approved by the local ethical committee.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2020_9702_MOESM1_ESM.pdf
Figure 1: Scale for the Assessment and Rating of Ataxia (SARA) with disease duration in group 1. Each point is the average value obtained when considering all the data obtained for MSA-C patients (PDF 10 kb)
415_2020_9702_MOESM2_ESM.pdf
Figure 2: Spinocerebellar Degeneration Functional Score (SDFS) with disease duration in group 1. Each point is the average value obtained when considering all the data obtained for MSA-C patients. (PDF 9 kb)
415_2020_9702_MOESM3_ESM.pdf
Figure 3: Unified Parkinson’s Disease Rating Scale III (UPDRS III) with disease duration in group 1. Each point is the average value obtained when considering all the data obtained for MSA-C patients. (PDF 10 kb)
Rights and permissions
About this article
Cite this article
Carré, G., Dietemann, J.L., Gebus, O. et al. Brain MRI of multiple system atrophy of cerebellar type: a prospective study with implications for diagnosis criteria. J Neurol 267, 1269–1277 (2020). https://doi.org/10.1007/s00415-020-09702-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09702-w