Abstract
Clinical trials need to specify which specific gait characteristics to monitor as mobility measures for each neurological disorder. As a first step, this study aimed to investigate a set of measures from daily-life monitoring that best discriminate mobility between people with multiple sclerosis (MS) and age-matched healthy control subjects (MS-Ctl) and between people with Parkinson’s disease (PD) and age-matched healthy control subjects (PD-Ctl). Further, we investigated how these discriminative measures relate to the disease severity of MS or PD. We recruited 13 people with MS, 21 MS-Ctl, 29 people with idiopathic PD, and 20 PD-Ctl. Subjects wore 3 inertial sensors on their feet and the lumbar back for a week. The Area Under Curves (AUC) from the receiver operator characteristic (ROC) plot was calculated for each measure to determine the objective measures that best separated the MS and PD groups from their respective control cohorts. Adherence wearing the sensors was similar among groups for 58–66 h of recording (p = 0.14). Quantity of mobility (activity measures, such as a median number of strides per gait bout, AUC = 0.93) best discriminated mobility impairments in MS from MS-Ctl. In contrast, quality of mobility (such as turn angle, AUC = 0.90) best discriminated mobility impairments in PD from PD-Ctl. Mobility measures with AUC > 0.80 were correlated with MS and PD clinical scores of disease severity. Thus, measures characterizing mobility impairments differ for MS versus PD during daily life suggesting that mobility measures for clinical trials and clinical practice need to be specific to each neurological disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The maintenance of mobility is crucial for active aging, allowing individuals to lead dynamic and independent lives, and it is linked to quality of life. Mobility impairments are very common in patients with neurological disorders, leading to elevated risk of falls and reduced quality of life [1,2,3]. Specific types of mobility impairments differ depending upon the neurological disorder. For example, weakness, spasticity, and ataxia impairments characterize mobility impairments in people with multiple sclerosis (MS), whereas bradykinesia, shuffling, freezing, and difficulties in turning characterize mobility impairments in people with Parkinson’s disease (PD). Gait speed slows in individuals with any neurological disorder or aging [4]. However, slow gait is a general, non-specific characteristic of impaired mobility and may not be the most discriminative impairment for each neurological disorder.
Recently, the use of mobile health technologies has made it possible to quantify mobility outside the clinic and during real-life [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Various researchers have shown that mobile health technologies can be used to augment the standard clinical assessment based on active monitoring, such as performing prescribed/predefined tests at home or based on passive monitoring, during routine, daily life tasks in the community [25]. Key challenges in using mobile health technologies to monitor mobility in daily life are an excessive number of measures and a lack of consensus on the most useful measures for each neurological disorder [25]. Hence, there is an unmet need to determine the specific mobility measures that can objectively and continuously monitor mobility during daily life that are representative of different disorders.
In this study, we focused on the following aspects of mobility [26] in daily life: quality and quantity of gait and turning. We derived mobility measures using passive monitoring of natural walking and turning during unrestricted daily activities with at least 8 h/day over a week using mobile health technologies. To reduce the excessive number of mobility measures, previous studies have used factor analysis or principal component analysis to identify separate domains of mobility. For example, lower body, upper body, and turning have been previously shown to be relatively independent during clinical gait testing [27]. In addition, activity and variability domains have been used to characterize mobility in the laboratory and during daily life [16, 24, 28, 29]. Hence, we grouped our mobility measures into five domains: upper body, lower body, turning, activity, and variability. The measures incorporating all the domains of mobility, except activity, are referred to as “quality of mobility”, and measures within the activity domain are referred to as “quantity of mobility”. Determining which mobility measures best discriminate mobility impairments related to the severity of MS and PD compared to corresponding age-matched healthy control cohorts is needed to monitor the response to therapy [16, 18, 30,31,32].
The objectives of the present study were to investigate: (1) which specific measures of gait and turning during real-life monitoring best discriminate mobility characteristics in people with MS and PD from their age-matched control cohorts and (2) the extent to which objective gait and turning measures are related to clinical scores of disease severity (clinical concurrent validity). We hypothesized that the most discriminative aspects of mobility would differ for MS and PD and the worse these mobility measures, the more severe the disease.
Methods
Participants
Thirteen people with MS (Patient-Reported Expanded Disability Status Scale: 4.27 ± 0.61 (mean ± SD); Multiple Sclerosis Walking Scale: 28.69 ± 9.53; and Modified Fatigue Index Scale: 37.92 ± 17.01), 21 MS-Ctl healthy controls, 29 people with PD (Unified Parkinson’s disease Rating Scale part III total score tested ON medication: 34.66 ± 11.02; and Hoehn and Yahr stage: 2.07 ± 0.45 with I (n = 1), II (26), III (1), and IV (1)) and 20 PD-Ctl healthy controls participated in the study. Inclusion criteria for PD were a diagnosis of idiopathic Parkinson’s disease from movement disorders neurologist with the United Kingdom Parkinson’s disease Society Brain Bank criteria, Hoehn and Yahr scores of II-IV, and complaints about mobility. Inclusion criteria for MS were a confirmed diagnosis of relapsing–remitting or progressive MS, a mild-to-moderate MS-associated disability (EDSS score ≤ 6.0) confirmed by a neurologist specialist and complaints about mobility. Exclusion criteria for all subjects included the inability to follow protocol instructions, other factors affecting gait such as musculoskeletal disorders, uncorrected vision or vestibular problems, or inability to stand or walk in the home without an assistive device. The experimental protocol was approved by the Institutional Review Board of the Oregon Health and Science University. All the participants provided informed written consent.
Data collection
Subjects were asked to wear three inertial sensors (Opals by APDM, Inc., Portland, OR, USA), one on top of each foot and one over the lower lumbar area (at top of pelvis with an elastic belt that clipped together) for a week of continuous monitoring for at least 8 h/day. Each Opal sensor contains a tri-axial accelerometer, gyroscope, and magnetometer sampling at 128 Hz. The Opals are lightweight (< 25 g), have a battery life of more than 12 h, and includes 8 GB of storage, that can record over 30 days of data. Subjects removed the sensors at night and recharged the batteries. Data were stored in the internal memory of the Opals. Subjects mailed back the sensors using a pre-paid box after completion of a week of data collection. Data were uploaded to a secure cloud-based database upon return of the devices and downloaded to a local computer for further processing.
In the laboratory, clinical scores provided estimates of the severity of MS or PD. Subjects with MS walked 25 feet as quickly, but safely, as possible from one marked line on the floor to another, similar to a Timed 25- Foot Walk test [33, 34]. Further, we collected patient-reported outcomes: Patient-Reported Expanded Disability Status Scale (PREDSS) [35, 36], Multiple Sclerosis Walking Scale (MSWS-12) [37], and the Modified Fatigue Index Scale (MFIS) [38]. Subjects with PD were tested in the ON levodopa state with the Movement Disorder Society-Unified Parkinson’s disease Rating Scale (MDS-UPDRS) [39] Part III Motor Signs, including the postural instability and gait disability (PIGD) subscore.
Mobility measures
The algorithms used for detecting gait bouts and extracting spatial and temporal measures of gait and turning were detailed previously ([40], under review). In summary, the algorithm first searches for possible bouts of walking using a time-domain approach to inertial sensor data from the feet and for turns based on yaw rotational orientation of the pelvis. Second, individual steps are combined into potential bouts of walking, as long as the duration from one step to the next step is no longer than 2.5 s. Finally, each possible bout that contains at least three steps and is at least 3 s in duration is processed with the commercial gait analysis algorithms included in Mobility Lab (APDM, Inc., Portland, Oregon) [41–43]. Our gait analysis algorithm uses the Unscented Kalman Filter to fuse information from the accelerometers, gyroscopes, and magnetometers to precisely estimate the orientation and position trajectory of each foot between quiet stance periods [44, 45]. This approach reduces the problem of tracking over a long period of time. For the results reported in this paper, we only included stride pairs during periods of straight walking, and we excluded walking during turns, which were characterized independently. For turning measures, we used a previously published algorithm to detect and characterize each turn [13]. Briefly, a candidate turn was defined as a trunk rotation around the vertical plane with a minimum of 40°/sec, and a start and end of the turn was defined with a threshold of 15°/sec. Only turns with durations between 0.5 and 10 s, and turn angles of 40° or more were considered. Relative turn angles were obtained by integrating the angular rate of the lumbar sensor about the vertical axis. A turn may occur within straight walking bouts. If a turn was present in a bout, and if a stride is even partially in a turn, it is excluded from the straight walking bout. Only step time was used to determine how many steps were in a turn.
In total, we extracted 46 mobility measures and grouped them into five domains similar to previous factor analysis: 10 lower body, 3 upper body, 7 turning, 6 activity, and 20 variability [27–29]. We evaluated the variability of each measure from all the gait strides and turns across all days as the coefficient of variation (standard deviation divided by the mean, CV). The detailed description of the definition of mobility measure is given in Appendix 1.
Statistical analysis
The normality of data was determined with Shapiro–Wilk tests and parametric analysis was used, unless otherwise stated. Independent t tests or Mann–Whitney U tests (if not normally distributed) were used to compare differences between groups. One-way Analysis of Variance or Kruskal–Wallis rank-sum test (if not normally distributed) was used to compare the total time duration of recording across all groups.
To investigate which specific measures best discriminate mobility characteristics in MS from MS-Ctl group, and PD from PD-Ctl group, we first calculate Receiver Operating Characteristic (ROC) curves [46] and computed the Area Under Curve (AUC) [47], and then ordered them based on the highest to lowest AUC value. Since MS-Ctl are expected to be younger than PD-Ctl, we also computed AUC to observe the effect of age. Box plots were used to show the distribution of all top three mobility measures discriminating PD from PD-Ctl and MS from MS-Ctl groups.
Clinical concurrent validity of the top mobility measures discriminating MS and PD groups was determined by relating the weekly average mobility measures to the clinical scores of the severity of the neurological disease. Specifically, Spearman’s correlation was used to assess the relation between mobility measures and severity of MS (PREDSS, MFIS, MSWS, and gait speed from timed 25-foot walk test) and severity of PD (such as UDPRS Part III, and PIGD sub-score of the UPDRS Part III). All statistical analysis was performed using R Version 1.1.456 software. The statistical significance was set to p < 0.05.
Results
Group characteristics and adherence
Age, height, and weight were similar between the MS and MS-Ctl and between the PD and PD-Ctl groups. The total number of turns/hour were statistically significant but not the total number of bouts/hour between groups. Table 1 shows the demographics and activity characteristics of subjects who participated in this study. Adherence to the weekly recordings for each subject group averaged 65.23 ± 7.34 (mean ± SD) hours in MS, 66.34 ± 16.06 h in MS-Ctl, 66.35 ± 13.60 h in PD, and 58.34 ± 14.89 h in PD-Ctl of continuous data. The Kruskal–Wallis rank-sum test on groups showed no significant differences in total hours of mobility recording per week (p = 0.14), supporting the feasibility of the approach.
Mobility measures discriminating people with MS from MS-Ctl
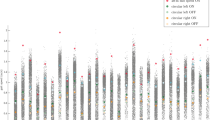
Nine Mobility measures were most discriminative in differentiating mobility of the MS group from the MS-Ctl group, each with an AUC ≥ 0.80 (Fig. 1). Gait quantity measures summarizing activity (fewer median number of strides per bout and fewer number of strides per hour as shown in Fig. 2) were most discriminative in MS. In addition, gait quality measures related to speed and temporal components (such as percentage of the gait cycle in a single limb, swing phase, and percentage of the gait cycle in double support) also showed AUC > 0.80.
Boxplot of top three mobility measures discriminating mobility in people with MS from MS-Ctl and PD from PD-Ctl. Each mobility measure is calculated by taking an average of all strides across a week. Based on normality check test results, p values between groups were calculated using either independent t test or Mann–Whitney U test indicated by superscripta (if not normally distributed)
Mobility measures discriminating people with PD from PD-Ctl
Five mobility measures were most discriminative in differentiating mobility of the PD group from the PD-Ctl group, with an AUC ≥ 0.80 (Fig. 1). Various gait quality measures related to bradykinesia, shuffling gait (such as smaller turn angles and increased variability of swing phase, and decreased pitch of the foot at initial contact as shown in Fig. 2) performed better in discriminating mobility characteristics in people with PD from PD-Ctl than the quantity of mobility measures that discriminated mobility in people with MS from MS-Ctl, such as the median number of strides per bout, and the number of strides/hour. These results were also apparent from the comparison of AUC values of specific mobility measures in Fig. 1.
Mobility measures discriminating MS-Ctl from PD-Ctl
MS-Ctl group was younger than PD-Ctl group, specifically, the mean age for MS-Ctl was 46.43 ± 11.06 years, and for PD-Ctl was 64.44 ± 7.52 years. Out of the total 46 mobility measures, five individual mobility measures (variability in a number of steps in a turn, turn duration, cadence, step duration, and stride duration) best differentiated mobility of the MS-Ctl group from the PD-Ctl group, with an AUC ≥ 0.80 (Figure S1). Gait speed AUC was 0.78. These measures characterize the slowness of walking and turning due to aging. Therefore, to minimize the contribution from the aging, we used age-matched control subjects for each neurological disorder.
Clinical concurrent validity of mobility measures
Figure 3 shows a correlation of top mobility measures discriminating each neurological disorder with clinical scores. For people with MS, the PREDSS only ranged from 3.5 to 6 in our cohort but significant correlations were found for percentage cycle in double support (Fig. 3), percentage cycle in swing (p = 0.022), pitch of the foot at initial contact (p = 0.031), and variability of the foot at toe-off (p = 0.028) (not shown in Figure). Further, the MFIS showed a moderate negative correlation (r = − 0.53) with the median number of strides per bout consistent with shorter gait bout lengths in those with complaints of fatigue; this correlation, however, not statistically significant (p = 0.063). Gait speed from the 25-foot walk time in the laboratory was also correlated with gait speed over a week of daily monitoring. However, none of the top mobility measures showed a significant correlation with MSWS-12 score.
For people with PD, the top three discriminative mobility measures (turn angle, swing variability, and pitch of the foot at initial contact) were significantly correlated with the PIGD subscore of the MDS-UPDRS Part III as seen in Fig. 3. In addition, pitch of the foot at initial contact variability (p = 0.018), and stride length (p = 0.018) were also significantly correlated with the PIGD subscore. However, none of the top mobility measures were significantly correlated with UPDRS Parts III total score.
Discussion
Our findings demonstrate that different measures over a week of daily monitoring discriminated mobility in people with MS than mobility in people with PD. Specifically, smaller median number of strides in the bout was the most discriminative measure in people with MS whereas smaller turn angle was the most discriminative in people with PD. Both quantity and quality of the mobility discriminated well mobility of people with MS from MS-Ctl, however, only quality of mobility discriminated well people with PD from PD-Ctl, and not quantity.
Activity measures, and specifically median number of valid strides in a bout and number of valid strides per hour were smaller in MS than MS-Ctl, and were the most discriminative measures characterizing mobility impairment in MS. In fact, lower levels of physical activity and fewer daily step counts have previously been observed in individuals with MS compared to healthy control subjects (see refs. in Block et al. [5],Giggins et al. [48]. Furthermore, lower physical activity levels, specifically, fewer steps per day have been shown to be strongly related to EDSS, MSWS-12,Timed 25-Foot Walk test, and 6-min Walk test [49, 50]. Thus, it is not surprising that gait speed during passive monitoring in daily life and temporal measures of gait associated with gait speed (double support and swing time as a percentage of the gait cycle, cadence, step duration and stride length) were also very discriminative measures characterizing gait impairments in MS.
In contrast to people with MS, turning, and specifically smaller turn angles, best characterized mobility disability in people with PD during unsupervised daily mobility. In fact, turning performance has been observed to be greatly compromised in people with PD leading to a reduced quality of life and injurious falls [17, 51]. Specifically, turning characteristics are even more sensitive to early, unmedicated PD than straight-ahead, linear gait characteristics in a clinical Timed Up and Go Test [27]. Turning in individuals with PD is characterized by long turning durations, more steps to complete the turn, and slower peak and average speed compared to age-matched, healthy controls in daily life [13, 15]. Turning, especially turning in place 360° is also a trigger for freezing of gait so it is possible that people with PD avoid large turns to avoid freezing. Indeed, a significantly smaller turn angle in freezers was observed in a study of 94 non-freezer and freezer PD subjects passively observed during 3 days of daily life [24]. Also, a recent longitudinal study in community dwelling old adults found that fewer turns in daily life differentiate future recurrent fallers from non-fallers [51]. After the turn angle, the swing (percentage of the gait cycle) variability measure was the most discriminative to PD. Specifically, higher swing variability was observed in people with PD compared to PD-Ctl. In fact, higher variability in various gait measures was observed previously in patients with PD compared to healthy control subjects in the laboratory [52] and in free living conditions [8, 15, 16]. Further, a variability of mobility measures (such as stride-time variability) has been shown to be related to fall risk in PD [12]. Thus, it is not surprising that turn angle, swing CV during passive monitoring in daily life and measures characterizing freezing or shuffling (such as pitch angle of foot at initial contact) were also very discriminative measures characterizing mobility impairments in PD.
Although previous studies have found that people with PD had fewer steps/day than age-matched controls [10, 53], we did not find a significant difference (p = 0.35) in a number of strides/hour between PD (82.11 ± 43.75) and PD-Ctl groups (98.25 ± 66.83). Further, our total number of steps per day is far less than what has been reported in the literature. Several possibilities may explain this discrepancy: (1) Our number of steps/day did not include turns while turns were included in previous studies. Considering an average of ~ 80–100 turns/hour and ~ 3–5 steps per turn, if we add turning, our total number of steps will increase drastically. (2) Lord et al. [10] considered at least one step to qualify as a stepping bout, whereas we considered at least 3 steps to qualify as a bout. (3) We only considered valid strides in each bout for our analysis to avoid the effects due to potential false positive steps. (4) Algorithm differences defining a valid bout and a valid step could result in very different number of steps (or strides) per day. Hence, for activity measures, we recommend caution while comparing our results with consumer-grade activity trackers (such as activPAL™, Fitbit).
Mobility measures discriminating the older (PD-Ctl) from the younger (MS-Ctl) groups (see supplementary Figure S1), specifically, slow turns, slow cadence and slow gait speed with accompanying changes in gait temporal characteristics may be related to slowness due to aging and/or various comorbidities (such as arthritis, heart disease) [54, 55]. The mobility measures distinguishing the MS-Ctl versus PD-Ctl control groups were distinct from the top mobility measures characterizing the mobility impairments in MS and PD. Hence, we used age-matched, control cohorts for the two different neurological disorders.
Mobility measures may be useful to track the progression of neurological disease severity. We used the relationship between mobility measures with patient-reported outcomes and clinician-reported outcomes representing disease severity as a type of concurrent (clinical) validity or meaningfulness of the measures. The median number of strides per bout over the week was correlated with the self-reported fatigue scale, MFIS for people with MS. The turning and gait shuffling mobility measures over the week were correlated with the UPDRS PIGD sub-score that represents a clinician’s evaluation of balance and gait for PD. These results demonstrate the correlation between clinical scores related to the severity of neurological disease and mobility measures. Future studies need to determine the test–retest reliability and sensitivity of the top mobility measures to disease progression in daily life to be useful as digital biomarkers for clinical trials.
There are several limitations to the current study. First, the turn algorithm used in this study was defined by a threshold on a vertical rotational rate of the pelvis (15°/s) [13]. This can be challenging when turns are nearly as slow and small as the angular velocities and angles of trunk motion during gait. Too high a threshold to detect the onset and offset of turns could result in smaller turn angles rather than longer turn durations with the initial and final rate of change of trunk rotation is very slow. Second, we performed all mobility analysis by taking the mean of each measure for all the strides over a week for each subject and thus gave equal weight to each stride. But in reality, gait speed and other measures vary for gait bouts of different lengths [16] and people with MS might have fewer long gait bouts because of fatigue. Hence, future work will focus on analyzing the effect of bout length on each mobility measure and how gait bout length affects the discriminatory power of each mobility measure. Thirdly, we ranked mobility measures characterizing mobility impairments in MS and PD based on AUC, but we cannot assume this ranking would be identical across all cohorts. Future work with larger cohorts is needed to investigate if these findings would generalize to other individuals. Lastly, in this paper, we report the most discriminative mobility measures for a specific group of people with MS and a group with PD, but to apply this knowledge in clinical practice, each individual’s gait characteristics are needed to tailor interventions specific for each individual’s specific mobility disability. Future studies need to develop age-related normative values for passive monitoring of mobility. Future work would also explore how to characterize diurnal fluctuations of mobility throughout the day.
Conclusion
Quantity and quality of the mobility both discriminated well people with MS from MS-Ctl, however, only quality of mobility discriminated well people with PD from PD-Ctl, and not quantity. Several of these mobility measures from a week of passive monitoring in daily life were also related to clinical and patient-reported outcomes of neurological severity, providing clinical concurrent validity. Thus, the most sensitive objective measures of daily life mobility for clinical trials and clinical practice need to be specific for each neurological disease.
References
Nutt JG, Marsden CD, Thompson PD (1993) Human walking and higher-level gait disorders, particularly in the elderly. Neurology 43(2):268–279
Snijders AH, Van De Warrenburg BP, Giladi N, Bloem BR (2007) Neurological gait disorders in elderly people: clinical approach and classifi cation. Lancet Neurol 6(1):63–74
Baker JM (2018) Gait disorders. Am J Med 131(6):602–607
Studenski S et al (2011) Gait speed and survival in older adults. JAMA 305(1):50–58
Block VAJ, Pitsch E, Tahir P, Cree BAC, Allen DD, Gelfand JM (2016) Remote physical activity monitoring in neurological disease: a systematic review. PLoS ONE 11(4):e0154335
Hale LA, Pal J, Becker I (2008) Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch Phys Med Rehabil 89(9):1765–1771
Chastin SFM, Baker K, Jones D, Burn D, Granat MH, Rochester L (2010) The pattern of habitual sedentary behavior is different in advanced Parkinson’s disease. Mov Disord 25(13):2114–2120
Weiss A, Sharifi S, Plotnik M, Van Vugt JPP, Giladi N, Hausdorff JM (2011) Toward automated, at-home assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabil Neural Repair 25(9):810–818
Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE (2012) Capturing ambulatory activity decline in Parkinson’s disease. J Neurol Phys Ther 36(2):51–57
Lord S, Godfrey A, Galna B, Mhiripiri D, Burn D, Rochester L (2013) Ambulatory activity in incident Parkinson’s: more than meets the eye? J Neurol 260(12):2964–2972
Weiss A et al (2013) Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-day accelerometer recordings. Neurorehabil Neural Repair 27(8):742–752
Weiss A, Herman T, Giladi N, Hausdorff JM (2014) Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days. PLoS ONE 9(5):e96675
El-Gohary M et al (2014) Continuous monitoring of turning in patients with movement disability. Sensors (Switzerland) 14(1):356–369
Wallen MB, Franzen E, Nero H, Hagstromer M (2015) Levels and patterns of physical activity and sedentary behavior in elderly people with mild to moderate parkinson disease. Phys Ther 95(8):1135–1141
Mancini M et al (2015) Continuous monitoring of turning in Parkinson’s Disease: rehabilitation potential. Neuro Rehabil 37(1):3–10
Del Din S, Godfrey A, Galna B, Lord S, Rochester L (2016) Free-living gait characteristics in ageing and Parkinson’s disease: impact of environment and ambulatory bout length. J Neuroeng Rehabil 13(1):1–12
Mancini M et al (2016) Continuous monitoring of turning mobility and its association to falls and cognitive function: a pilot study. J Gerontol Ser A Biol Sci Med Sci 71(8):1102–1108
Bernad-Elazari H, Herman T, Mirelman A, Gazit E, Giladi N, Hausdorff JM (2016) Objective characterization of daily living transitions in patients with Parkinson’s disease using a single body-fixed sensor. J Neurol 263(8):1544–1551
De Lima ALS et al (2017) Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS ONE 12(12):1–15
Adams JL et al (2017) Multiple wearable sensors in Parkinson and Huntington disease individuals: a pilot study in clinic and at home. Digit Biomark 1(1):52–63
Lipsmeier F et al (2018) Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov Disord 33(8):1287–1297
Arora S et al (2018) Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology 91(16):e1528–e1538
Zhan A et al (2018) Using smartphones and machine learning to quantify Parkinson disease severity the mobile Parkinson disease score. JAMA Neurol 75(7):876–880
Mancini M, Weiss A, Herman T, Hausdorff JM (2018) Turn around freezing: community-living turning behavior in people with Parkinson’s disease. Front Neurol 9:1–9
Del Din S, Godfrey A, Mazzà C, Lord S, Rochester L (2016) Free-living monitoring of Parkinson’s disease: lessons from the field. Mov Disord 31(9):1293–1313
Boucą-Machado R, Maetzler W, Ferreira JJ (2018) What is functional mobility applied to Parkinson’s disease? J Parkinsons Dis 8(1):121–130
Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB (2010) The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson’s disease. J Neurol Neurosurg Psychiatry 81(2):171–176
Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L (2013) Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol Ser A 68(7):820–827
Morris R, Hickey A, Del Din S, Godfrey A, Lord S, Rochester L (2017) A model of free-living gait: a factor analysis in Parkinson’s disease. Gait & Posture 52:68–71
Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR (2013) Quantitative wearable sensors for objective assessment of Parkinson’s disease. Mov Disord 28(12):1628–1637
Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A (2015) Digital health revolution: is it time for affordable remote monitoring for Parkinson’s disease? Front Neurol 6(34):1–3
Espay AJ et al (2019) A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov Disord Clin Pract 34(5):657–663
Fischer JS, Rudick RA, Cutter GR, Reingold SC, Ms N, Clinical S (1999) The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. Mult Scler J 5(4):244–250
Motl RW, Cohen JA, Benedict R, Phillips G, Larocca N (2017) Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler J 23(5):704–710
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis : an expanded disability status scale (EDSS). Neurology 33(11):1444–1453
Collins CDE et al (2016) A comparative analysis of patient-reported expanded disability status scale tools. Mult Scler J 22(10):1349–1358
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ (2003) Measuring the impact of MS on walking ability the 12-item MS walking scale ( MSWS-12). Neurology 60(1):31–36
Flachenecker P, Ku T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P (2002) Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler J 8(6):523–526
Goetz CG et al (2008) Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170
Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Nutt JG, El-Gohary M, Lapidus JA, Horak FB, Curtze C, Digital biomarkers of mobility in Parkinson’s disease during daily living. (under review)
Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB (2011) Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci, Suppl 1, 007. https://doi.org/10.4172/2155-9538.S1-007
Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C (2017) Validity and repeatability of inertial measurement units for measuring gait parameters. Gait & Posture 55:87–93
Morris R, Stuart S, McBarron G, Fino PC, Mancini M, Curtze C (2019) Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol Meas 40(9):095003
Wan EA, van der Merwe R (2000) The unscented kalman filter for nonlinear estimation. In: Proceedings of the IEEE 2000 Adaptive Systems for Signal Processing, Communications, and Control Symposium (Cat. No.00EX373), pp 153–158
van der Merwe R (2004) Sigma-point kalman filters for probabilistic inference in dynamic state-space models. Oregon Health & Science University (Doctoral dissertation, OGI School of Science & Engineering at Oregon Health & Science University)
Fawcett T (2006) An introduction to ROC analysis. Pattern Recognit Lett 27(8):861–874
Turck N et al (2011) pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform 8:12–77
Giggins OM, Clay I, Walsh L (2017) Physical activity monitoring in patients with neurological disorders: a review of novel body-worn devices. Digit Biomark 4:14–42
Cavanaugh JT, Gappmaier VO, Dibble LE, Gappmaier E (2011) Ambulatory Activity in Individuals With Multiple Sclerosis. Journal of neurologic physical therapy 4103:26–33
Erin SM, Motl RW, Gliottoni RC (2009) The effect of walking mobility on the measurement of physical activity using accelerometry in multiple sclerosis. Clin Rehabil 23(3):248–258
Leach JM, Mellone S, Palumbo P, Bandinelli S, Chiari L (2018) Natural turn measures predict recurrent falls in community-dwelling older adults: a longitudinal cohort study. Sci Rep 8(1):1–9
Hausdorff JM, Cudkowicz ME, Firtion R (1998) Gait variability and basal ganglia disorders: stride-to-S tride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord 13(3):428–437
Busse ME, Pearson OR, Van Deursen R, Wiles CM (2004) Quantified measurement of activity provides insight into motor function and recovery in neurological disease. J Neurol Neurosurg Psychiatry 75(6):884–888
Prince F, Hkbert R, Winter A (1997) Gait in the elderly. Gait & Posture 5:128–135
Pirker W, Katzenschlager R (2017) Gait disorders in adults and the elderly. Wien Klin Wochenschr 129(3–4):81–95
Acknowledgements
We thank our participants for generously donating their time to participate and Graham Harker for helping with data collection. This study was supported by the National Multiple Sclerosis Society Mentor Fellowship (MB0027), and National Institutes of Health grants from the National Institute on Aging (#R44AG055388 and #R43AG044863).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Drs. McNames, El-Gohary, and Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. Dr. Horak also consults with Biogen, Neuropore, Sanofi, and Takeda. This potential conflict has been reviewed and managed by OHSU.
Ethical standards
This study was conducted in accordance with the standards and approved by local human subjects ethics committees, and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects provided informed written consent prior to their inclusion in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shah, V.V., McNames, J., Mancini, M. et al. Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living. J Neurol 267, 1188–1196 (2020). https://doi.org/10.1007/s00415-020-09696-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09696-5