Abstract
Background and purpose
Focal epilepsy of unknown cause (FEUC) is an under-investigated topic despite its remarkable frequency. We aimed to report the long-term follow-up findings along with the drug-response, 5 year remission rates and diagnostic changes to give an insight about the heterogeneous characteristics of FEUC.
Methods
Demographic, clinical, neurophysiological and imaging data of 196 patients diagnosed as FEUC according to ILAE criteria, with a minimum 5-year follow-up were evaluated in a tertiary epilepsy center. The drug resistance, 5 years of remission and relapse rates were investigated and the subgroups were compared statistically.
Results
The rate of drug resistance was 21.8% and status epilepticus (p < 0.001), abnormal neurological examination (p = 0.020), seizure onset before 10 years (p = 0.004) and a high initial seizure frequency (p = 0.006) were significant predictors of drug resistance. The rates of terminal 5-year remission, 5-year remission ever and relapse were 39.9%, 44.26% and 24.04%, respectively. There were 13 patients (6.6%) with a changed final diagnosis. Drug resistance (p = 0.004), pathological EEG (p = 0.034) and status epilepticus (p = 0.021) were negative variables for achieving remission. The lobar localization of seizures was not a predictor of remission or relapse. Onset after 10 years of age had a higher probability of achieving a 5-year remission according to Kaplan–Meier curves (p < 0.001).
Conclusions
Focal epilepsy of unknown cause has a benign electroclinical subgroup with favorable long-term course, lower drug resistance and higher 5 years of terminal remission and remission ever rates, when appropriately treated. Our findings might be valuable in terms of counseling and management of patients with FEUC at the first referral to epilepsy clinics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Focal epilepsy of unknown cause (FEUC) or with its former name cryptogenic focal epilepsy is an under-investigated topic despite its remarkable frequency and importance for the patients who will face anxiety about their prognosis labeled as “unknown”. There are only a handful of papers reporting prognosis of these patients. FEUC is a term that refers to localization-related epilepsy with an unknown, still uncovered etiology [1, 2]. The cause is presumed to be symptomatic but no observable abnormality can be detected on magnetic resonance imaging (MRI) and also by other diagnostic investigations including genetic, metabolic and autoimmune reasons. About 40% of adult-onset epilepsies are diagnosed as FEUC, thus they constitute an important subgroup among all types of epilepsies [1, 2]. Data about the etiology and prognosis of FEUC accumulate with time and the number of patients diminishes with the improvement of imaging techniques and diagnostic tools like genetic and autoimmune studies [3,4,5,6,7,8,9]. However, there is a lack of studies about the long-term clinical course, prognostic markers and final diagnosis of FEUC patients.
In this study, we aimed to report the long-term prognosis, drug-response, 5-year remission rates, and diagnostic changes during the follow-up in patients diagnosed with FEUC in an established epilepsy center. Our second aim was to distinguish some characteristics of FEUC shared among subgroups like age at onset, antiepileptic drug resistance etc., to give an insight about the heterogeneous nature of FEUC and to highlight the importance of correct diagnosis to apply appropriate management plan.
Methods
Patient selection
A total of 196 patients aged between 16–75 years, regularly followed-up in our epilepsy center, diagnosed as FEUC according to the criteria of International League Against Epilepsy (ILAE) were included to the study [10]. Other inclusion criteria involved at least one normal 1.5 or 3 T MRI performed with a specific epilepsy protocol and a minimum of one EEG recorded in our laboratories, available for re-evaluation. Exclusion criteria comprised noncompliance of the patient, short follow-up time (less than 5 years), absence of appropriate neuroimaging and other necessary biochemical, metabolic, serological and immunological laboratory investigations. When a clinical suspicion is considered, we additionally performed lumbar puncture analysis and excluded patients with diagnostic cerebrospinal fluid results for a given etiology. We also excluded those patients diagnosed with psychogenic non-epileptic seizures at the beginning of our study.
Patients with FEUC were classified into subgroups according to the electroclinical lobar localization, from which the seizures originated as follows; temporal, frontal, parieto-occipital, multifocal and unknown origin of FEUC. Temporal lobe epilepsy with unknown cause (TLE-UC) is further subdivided into lateral and mesial subtypes. The traditional lobar localization of the patients was defined upon the concordant semiology of the seizures, ictal EEG and interictal EEG data and PET findings, respectively [11] (Fig. 1).
Flow chart of the study. AE autoimmune epilepsy (diagnosed with anti-neuronal antibodies), TLE temporal lobe epilepsy, PLE parietal lobe epilepsy, OLE occipital lobe epilepsy, FEUC focal epilepsy of unknown cause, F frontal, T temporal, PO parieto-occipital, P parietal, JAE juvenile absence epilepsy, AED-R antiepileptic drug resistance, MTLE-HS mesial temporal lobe epilepsy with hippocampal sclerosis, Sz seizure
The local ethics committee has approved the retrospective study protocol.
Clinical and EEG data collection
We collected the following data from the medical records: the demographic characteristics such as age, sex, age of onset, age at diagnosis, duration of epilepsy, habits of alcohol and illicit drug use, history of complicated birth, head injury; febrile seizure and status epilepticus, neurological and psychiatric comorbid diseases, other comorbid systematic diseases (thyroid disease, autoimmune diseases, etc.), family history of epilepsy, parental consanguinity.
The initial EEG and all other follow-up EEGs were reviewed by two experienced clinical neurophysiologists (BB and NB). EEGs were recorded with scalp electrodes placed according to the International 10–20 system with bipolar and reference montages. Standard activating procedures were performed in all participants. We also reviewed video-EEG data in these patients if available. The standard definitions for EEG activities were used [12].
Other investigated clinical data included seizure types, ictal semiology, types of aura, initial antiepileptic therapy regimen, current antiepileptic therapy, initial type of seizures, initial frequency of seizures, current frequency of seizures, 5-year remission, relapses and, antiepileptic drug resistance and final diagnosis of the patient. Aura was defined as an ictal phenomenon preceding an observable seizure; when isolated, it may represent a focal sensory aware seizure as well [13]. Drug-resistant epilepsy was defined as using two properly chosen and well-tolerated antiepileptic drugs at adequate doses according to suitable antiepileptic drug schedules and achieving seizure-freedom [14]. Remission was defined as being seizure-free for any type of seizure for a minimum of 5 years with or without antiepileptic medication during the course of epilepsy [15]. Relapse was defined as having seizures after a minimum 5 years of seizure-freedom.
We also performed serological, immunological and genetic tests to explore a possible symptomatic etiology and excluded those patients with auto-antibody positivity or genetic mutations at the end of the follow-up period. Additionally, we investigated the patients who underwent epilepsy surgery and recorded the type of surgery, neuro-pathological diagnosis, post-op EEG examinations and outcome of the surgery according to the Engel classification [16].
Neuroimaging data
We excluded the patients without any MRI after 2009 because 1.5 T MRI was not available in our institution before this date.
MRI studies were performed with a 1.5-T scanner (Magnetom Siemens Symphony, Erlangen, Germany) with thin coronal in addition to sagittal and axial planes including T1, T2, and fluid-attenuated inversion recovery (FLAIR) images to visualize mesial temporal regions optimally. We proceed with investigation of mesial temporal lobe structures with the following series: T2 W paracoronal series [repetition time (TR): 4900, echo time (TE): 104, field-of-view (FOV): 230, matrix: 256 × 512, flip angle: 150°, slice thickness: 3 mm, recording time: 4.31 min], paracoronal FLAIR (TR: 8080, TE: 111, TI: 2500, FOV: 230, matrix: 179 × 256, flip angle: 150°, slice thickness: 3 mm, recording time: 4.31 min) and paracoronal multiplanar reconstruction (TR: 1900, TE: 3.3, FOV: 250, matrix: 179 × 256, flip angle: 15°, slice thickness: 3 mm, recording time: 5.42 min) series. Images taken at conditions in compliance with 1.5 T MRI epilepsy protocol and were reviewed by an experienced neuroradiologist [7, 17]. Most of the patients also underwent 3 T MRI to clarify the etiology of seizures, according to clinical needs.
We also collected positron emission tomography (PET) and single photon emission computerized tomography (SPECT) data from the medical files of included patients, when available. These investigations were reviewed by a nuclear medicine specialist who was blind to clinical features.
Statistical analysis
Descriptive statistics were applied for the clinical and demographic features. The findings of FEUC patients with and without drug resistance were compared with χ2 test, Fisher’s exact test and t test, where appropriate. The potential risk factors for predicting drug resistance which showed a significant association (p < 0.05) in univariate analysis were investigated by binary logistic regression analysis as well. We additionally performed ROC analysis to determine a cutoff value for age of seizure onset using ROC analysis method and chose 10 years as a cutoff value (p = 0.01, sensitivity 80%, specificity 58%). As the main outcome measure was long-term seizure remission (> 5 years), time to seizure remission in relation with age of seizure onset was analyzed by the Kaplan–Meier method to illustrate the likelihood of remission. To the purpose of performing Kaplan–Meier analysis we divided age of seizure onset as < 10 years and ≥ 10 years of age, as the age of onset before 10 years was one of the significant factors for having drug resistance in our statistical analysis. The log-rank test was performed to compare survival between patient subgroups. IBM SPSS Statistics V.22 was used and the level of significance was set at p < 0.05.
Results
Clinical and laboratory properties
There were 107 (58.5%) female and 76 (41.5%) male patients in the cohort with FEUC, after exclusion of 13 patients with a final changed diagnosis. The mean follow-up duration was 18.98 ± 11.18 years.
The mean age, age of onset, age of diagnosis and disease duration of the included patients were 39.12 ± 13.26, 18.86 ± 13.10, 20.30 ± 13.19 and 20.25 ± 11.21 years, respectively. The mean number of EEG examinations of the patients were 5.74 and the mean number of MRIs applied was 2.01 ± 1.07.
The initial seizure type was focal to bilateral tonic–clonic (fTC) in 124 patients (67.8%), focal seizure with impaired awareness (FSIA) in 48 patients (26.2%) and focal onset aware seizures (FoAS) in 11 patients (6.0%). Initial seizure frequency was ≥ 1/month in 80 patients (56.3%) and < 1/month in 103 patients (43.7%).
The initial EEG examinations were normal in 73 patients (39.9%) and pathological (including epileptiform (n: 61, 55.45%) and nonepileptiform (n: 49, 44.55%) discharges in 110 patients (60.1%). During the long-term follow-up, only 18 patients’ interictal EEG examinations persisted to be normal (9.8%) whereas 129 patients (70.5%) developed epileptiform discharges (sharp waves, spikes, polyspikes) and 36 patients (19.7%) had focal nonspecific findings (slow wave activity) in their EEG examinations. Moreover, 40 patients (21.85%) had generalized EEG discharges (epileptiform (n: 18) or nonspecific generalized (n: 22) additionally.
At the end of the 5 years of follow-up period, 23 patients with FEUC (12.6% of the cohort) had non-epileptic psychogenic seizures in addition to their proven epileptic seizures.
Patients with a change in the final diagnosis
After inclusion with the FEUC diagnosis and a minimum of 5 years follow-up in our center, there were 13 patients (6.6%) with a changed final diagnosis at the end of the follow-up period. Their latter diagnoses and the basis of the new diagnoses were as follows:
Eleven patients with previously undetected symptomatic focal epilepsy (a) 5 with hippocampal sclerosis [In 3 of them hippocampal sclerosis was not detected in the initial 1.5 T MRI but diagnosed with 3 T MRI, the remaining two were diagnosed with post-operative neuro-pathological analysis, only], (b) 6 with other structural lesions (1 temporal cortical dysplasia diagnosed with the help of MR-spectroscopy, 1 small post-traumatic left parietal encephalomalacia; 1 left temporal uncal cavernoma and 2 with lesions of unknown etiology detected with 3 T MRI not seen in 1.5 T and the last patient with type 1A occipital cortical dysplasia diagnosed with post-operative pathology, only).
One patient with autoimmune epilepsy diagnosed with immunological analysis with N-methyl-d-aspartate receptor (NMDA-R) auto-antibody positivity,
One patient with genetic epilepsy (diagnosed as juvenile absence epilepsy with video-EEG recordings of absence seizures after many years).
Four of these patients were operated and three of them (one patient with cortical dysplasia type-1 and 2 patients who had temporal neuronal gliosis in sector CA4) were post-op seizure-free, whereas another one did not improve and had post-op Engel 4 grade (neuropathological reports of CA1 and partial CA2 gliosis).
In the end of the follow-up these 13 patients were excluded from further analysis due to their changed final diagnoses (see flow chart in Fig. 1).
Antiepileptic drug responses
The majority of the patients were under monotherapy (n = 98, 53.6%) at the beginning of the follow-up period and the seizures were under control with a single antiepileptic drug. During the follow-up period; 16 patients (8.7%) became drug-free, 51 patients were under carbamazepine (27.86%), 14 patients (7.65%) under oxcarbazepine, 13 patients (7.1%) under levetiracetam, 9 patients (4.91%) under lamotrigine and 5 patients (2.73%) were under valproic acid monotherapy and remaining 40.9% of the patients were receiving polytherapy.
Of these 183 patients with FEUC, 40 patients (21.8%) had drug resistance. The lobar distribution of patients with FEUC according to drug resistance is shown in Fig. 1. The demographic and clinical properties of FEUC patients with and without drug resistance are summarized in Table 1, comparatively.
A binary logistic regression analysis was performed and a model was developed including the following significant clinical features; status epilepticus, having a pathological EEG, abnormal neurological examination, high initial seizure frequency and seizure onset < 10 years of age. Having a status epilepticus history (p < 0.001), abnormal neurological examination (p = 0.020), seizure onset before 10 years of age (p = 0.004) and a high initial seizure frequency (more than one seizure per month) (p = 0.006) were found statistically significant between patients with and without drug resistance, as shown in Table 2.
Five-year remission and relapse rates
During the follow-up, 73 (39.9%) of the 183 patients had a terminal 5-year remission, 11 (15.06%) of these patients were drug-free whereas 49 (67.12%) were under monotherapy. Eighty-one patients (44.26%) had a 5-year remission ever and 44 patients (24.04%) had experienced one or more relapses during the follow-up.
The statistical analysis of clinical and demographic factors which could be related with 5-year terminal remission showed that; antiepileptic drug resistance (p = 0.004), presence of at least one pathological EEG (p = 0.034) and a history of status epilepticus (p = 0.021) were significantly higher in patients with FEUC without a 5-year remission.
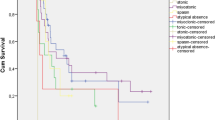
The cumulative rate of achieving a 5-year terminal remission related with the age of seizure onset was analyzed by Kaplan–Meier curve, comparing seizure onset before (early onset) and after (late onset) 10 years of age. Patients with seizures starting after 10 years of age had a higher probability of achieving a 5-year remission compared to the patients with seizure onset before 10 years of age (log rank test significance, p < 0.001) (Fig. 2).
Analysis of the lobar distribution for the patients with 5 years of terminal remission, 5-year remission ever and relapses are given in Fig. 3. The remission and relapse rates were found to be similarly distributed among the lobar origin of seizures, statistically.
Lobar distribution of the patients with FEUC with 5 years of terminal remission, 5-year remission ever and relapses. EUC epilepsy of unknown cause, FEUC focal epilepsy of unknown cause, FLE-UC frontal lobe epilepsy of unknown cause, LTLE-UC lateral temporal lobe epilepsy of unknown cause, MTLE-UC mesial temporal lobe epilepsy of unknown cause, POLE-UC parietal lobe epilepsy of unknown cause
Amongst the 183 patients which constituted our main study cohort, two patients only were eligible for epilepsy surgery; one was post-op seizure-free whereas the other patient had post-op Engel 3; they both had neuro-pathology reports as undefined.
Discussion
The present long-term study of a large cohort with FEUC highlighted that this under-investigated group showed a relatively low rate of drug resistance (21.8%). The drug resistance in FEUC was predicted by a history of status epilepticus, abnormal neurological examination, early age of seizure onset and a high initial seizure frequency like in other epileptic populations. Moreover, we demonstrated that even in a tertiary epilepsy center, a considerable number of patients (6.6%) with a prior diagnosis of FEUC might switch to a different diagnosis during long-term follow-up which emphasizes the significance of continuing diagnostic investigations in establishing the cause of epilepsy in these patients [10]. Hence, it is crucial to reevaluate these patients with new diagnostic tools such as improved neuroimaging, video-EEG monitoring, genetic, serologic and immunologic methods; epilepsy surgery specimen may also change the final diagnosis in most of the cases [18,19,20,21].
Considering that our center is a reference center for epilepsy, it is important to note that the rate of drug resistance in our FEUC patients at the end of the long-term follow-up was lower than expected when compared to the general epilepsy population (between 30 and 40%) [22,23,24,25,26]. The etiology of epilepsy was known to be associated with the risk of drug resistance in many studies despite the diversities in the classification criteria creating controversial results [27]. In a study classifying focal epilepsy into symptomatic and “probably symptomatic” (mostly defining patients with FEUC) groups, 57.8% versus 39.2% of the patients had drug resistance. Thus the “probably symptomatic” group showed lower drug resistance rates with a more favorable course in this study with shorter follow-up than ours [28]. In a retrospective study, 94 (40%) out of 234 patients with lesional symptomatic epilepsy were reported as drug-resistant and majority of these patients had hippocampal sclerosis as the causative lesion for their seizures [29]. Kwan et al. reported, on the other hand, similarly higher drug resistance rates in both symptomatic (43%) and cryptogenic (FEUC) (39%) epilepsies than generalized epilepsies (26%) in their prospective study including a sample of 525 patients [25]. A plausible explanation for this higher drug resistance rates in FEUC might be that their study design did not allow some diagnostic investigations by epileptologists and lack of some new methods such as genetic, advanced neuroimaging and immunologic investigations at the time of the study. Remarkably, symptomatic epilepsies and FEUC were reported to have higher chance of developing drug resistance than idiopathic generalized epilepsies as previous studies have shown [30,31,32]. Our results strikingly demonstrate that drug resistance in some patients with FEUC is even closer to the drug resistance rates in idiopathic/genetic epilepsies which implies a more favorable outcome and benign course even in a series from a tertiary epilepsy center.
Determinants of antiepileptic drug response
Early identification of the predictive factors for developing drug resistance is crucial to foresee long-term outcome and potential treatment options in FEUC [33]. The identified risk factors have a lot of discrepancies across the studies due to the variable definitions of drug resistance and lack of consistency between study cohorts. Although the predictors of drug resistance have been previously investigated in genetic and symptomatic epilepsies and some significant factors like the lobar origin of seizures along with some specific etiologies such as hippocampal sclerosis and cortical developmental disorders have been reported, there are no studies focused exclusively on patients with FEUC with long-term follow-up [28, 34,35,36].
We disclosed that early seizure onset, higher initial seizure frequency, a history of status epilepticus, abnormal neurological examination and having a minimum of one pathological EEG associated with drug resistance in univariate analysis, in line with the previously reported studies [22, 37, 38]. Furthermore, multivariant analysis confirmed that, status epilepticus history, abnormal neurological examination findings, seizure onset before 10 years of age and a high initial seizure frequency (more than one per month) were the most significant predictors of drug resistance in our cohort with FEUC.
Early age at epilepsy onset has been described as one of the risk factors for drug resistance in many of the previous reports, consistently [34, 39,40,41,42,43,44,45]. In a sample of 605 children with epilepsy (25.79% idiopathic epilepsy, 28.43% FEUC), an association between the age of seizure onset and prognosis was suggested in patients with FEUC, as earlier seizure onset being related with a more unfavorable prognosis [42]. Likewise, higher initial seizure frequency, abnormal neurological examination, status epilepticus history are other reported predictors of drug resistance reported by the previous studies with shorter follow-up [28, 46]. As all these factors were found to be significant mostly in symptomatic focal epilepsies, it was tempting to speculate that the predictive value of these factors, might point out to the presence of an underlying symptomatic etiology which could not be uncovered yet in our patient group with drug resistance with current technologies.
On the other hand, the role of EEG findings in predicting the outcome of epilepsy is still vague [47, 48]. According to some reports, pathological EEG findings like epileptiform discharges or focal slowing (nonspecific findings) were found to be related with the risk of developing drug resistance [49, 50]. Although majority of our patients with drug resistance had pathological EEG findings, it was not established as one of the significant predictors of drug resistance in the final regression analysis.
Determinants of long-term seizure remission and outcome
It is of great importance to foresee the probability of achieving seizure freedom in FEUC patients as these patients will continue to live with the burden of having an elusive diagnosis as “unknown”. Having a drug resistance is widely accepted to be related with a poor prognosis, and an important negative predictor for entering remission regardless of the etiological diagnosis of epilepsy [51, 52]. The results of our study confirmed the role of drug resistance as an important negative variable for achieving a terminal long-term remission as expected, besides other clinical and demographic factors such as; presence of at least one pathological EEG and a history of status epilepticus. Both of the last two variables may reflect the presence of an underlying severe brain dysfunction with more extensive involvement of hyperexcitable epileptogenic networks which might negatively affect the long-term remission chance and create an unfavorable outcome in some patients with FEUC [15, 31, 48, 53,54,55].
In a large Italian prognostic study reporting the long-term prognosis of epilepsy and identifying the prognostic factors in 1006 newly diagnosed children and adults with different etiologies; factors related with a 5 years of remission were reported as having one or two seizures at diagnosis, having a generalized epilepsy, no additional psychiatric diseases and being treated with one or two antiepileptic medications [56]. Our results reporting the role of drug resistance and the related factors such as high initial seizure frequency are line with the results of this study.
In further analysis by Kaplan–Meier method, age of seizure onset after 10 years was demonstrated to be a strong predictive factor for entering a terminal long-term remission in our sample. Aguglia et al., reported older age of onset as an independent prognostic predictor of seizure freedom in their cohort including non-lesional and hippocampal sclerosis-related temporal lobe epilepsy and suggested a positive relationship with older age of seizure onset and higher remission rates [57]. In another population-based study in children, younger age of onset was found to be related with higher drug resistance and a worse outcome where the unknown etiology of epilepsy was associated with less drug-resistance rates [58]. Our results in adult population with long-term data are in harmony with these studies.
The 5-year terminal remission rates (39.9%) and 5 years of remission ever rates (44.26%) in our FEUC group were encouraging when compared to previously reported rates in other focal epilepsy groups [4]. It is worth to emphasize that 15.06% of these patients were even drug-free and a notable rate of patients were only under monotherapy (67.12%). In the study of Kwan and Brodie, a rate of 45% with terminal remission was reported in cryptogenic focal epilepsies but the definition of remission was limited to 1 year of seizure-freedom [25]. Gasparini et al., reported that a quarter of their patients with cryptogenic focal epilepsy entered a 5 year of terminal remission regardless of age of onset [5]. Another important finding in our study was that one fourth of these patients, experienced one or more relapses during the course. Unlike other focal epilepsies with lower relapse rates [59, 60], the magnitude of our relapse rate is surprising and resembles the higher relapse rates in idiopathic/genetic epilepsies suggesting the possibility of an underlying genetic etiology [15]. Berg et al., showed that children with FEUC had a repeating relapse and remission cycle and higher relapse rates [61].
In a large prognostic study including a variety of different etiologies; the most common prognostic pattern was reported as relapsing remitting course with a high relapse rate of 52.5% where the relapse rate was reported as 49.2% in patients with an unknown etiology [56]. However, in another study, a pattern of relapse and remission could not be demonstrated in majority of the patients with FEUC and the relapse rates were reported as 10% with higher first remission rates at the final follow-up [4] The discrepancies between these studies might be the result of a clinical heterogeneity within FEUC where patients with higher remission and relapse rates with a benign course are related with a possibly genetic etiology whereas the others might be related with a rather uncovered symptomatic etiology [4].
Interestingly, the lobar origin of seizures was not demonstrated to be a predictor of remission or relapse in our group. We observed statistically similar terminal remission, remission ever and relapse rates between different lobar origins of seizures. This observation is in line with other prognostic studies [46, 62]. A plausible explanation for this observation might be a shared genetic pathophysiology which resembles the complex genetic mechanisms underlying familial epilepsies, where the different members of the same family might have seizures originating from different cortical regions [63]. We believe complex genetic mechanisms might, in the future, explain the similar prognostic rates in terms of remission and relapse among different lobar localizations in our sample with FEUC. Therefore, the integration of genetic testing into everyday clinical practice might be valuable for diagnostic research to uncover etiology of FEUC and to give proper prognostic information to the patients in a near future [64].
Strengths and limitations
Our study has some strengths; it is a study focusing on an ignored topic with long-term follow-up of minimum 5 years in the inclusion step, based on a large sample of compliant patients with FEUC in an established tertiary epilepsy center. In addition, all patients are well investigated and followed-up with regular visits in our center by experienced epileptologists. There are also some limitations of our study; first, this study was performed in a tertiary referral center which might create a selection bias. In spite of this limitation, the drug resistance and remission rates of our FEUC sample were favorable implying that our results are less likely to be negatively affected from this selection bias and giving hope for some patients with FEUC. Second, because of the retrospective nature of our study, there might be some missing data involving the pre follow-up period of our patients which might affect our results.
Conclusion
Our study emphasized that the initial diagnosis of FEUC always needs further diagnostic evaluation such as genetic testing, immunological studies, advanced imaging which might illuminate the underlying hidden etiology in at least 6.6% of patients even in a tertiary center. We conclude that when appropriately treated, FEUC has a benign subgroup with favorable course, lower drug resistance and higher 5 years of terminal remission and remission ever rates comparing to other focal epilepsies. In this regard, we believe that our data might be valuable in terms of patient counseling, treatment decisions, and management in patients with FEUC at the first referral to epilepsy clinics. Clinicians must be aware of the related factors regarding drug resistance and long-term remission in FEUC and enlighten the patients at the pretreatment phase to give an insight to their epilepsy and diminish the anxiety of having an unclarified diagnosis. Further well-designed prospective researches are needed in this aspect to confirm our results.
Change history
26 December 2019
The original version of this article unfortunately contained a mistake.
References
Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde BW, Engel J, French J, Glauser TA, Mathern GW, Moshé SL, Nordli D, Plouin P, Scheffer IE (2010) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 51:676–685
Shorvon SD (2011) The etiologic classification of epilepsy. Epilepsia 52:1052–1057
Berg AT (2001) Epidemiology in epilepsy. Epilepsy Curr 1:56–56
Wirrell EC, Grossardt BR, So EL, Nickels KC (2011) A population-based study of long-term outcomes of cryptogenic focal epilepsy in childhood: cryptogenic epilepsy is probably not symptomatic epilepsy. Epilepsia 52:738–745
Gasparini S, Ferlazzo E, Beghi E, Tripepi G, Labate A, Mumoli L, Leonardi CG, Cianci V, Latella MA, Gambardella A, Aguglia U (2013) Family history and frontal lobe seizures predict long-term remission in newly diagnosed cryptogenic focal epilepsy. Epilepsy Res 107:101–108
Lambrecq V, Marchal C, Michel V, Guehl D, Burbaud P, Rougier A (2013) Clinical features of late-onset partial cryptogenic epilepsy: toward an idiopathic temporal epilepsy? Epilepsy Behav 28:168–171
Ekizoglu E, Tuzun E, Woodhall M, Lang B, Jacobson L, Icoz S, Bebek N, GursesC GA, Waters P, Vincent A, Baykan B (2014) Investigation of neuronal autoantibodies in two different focal epilepsy syndromes. Epilepsia 55:414–422
Gozubatik-Celik G, Ozkara C, Ulusoy C, Gunduz A, Delil S, Yeni N, Tuzun E (2017) Anti-neuronal autoantibodies in both drug responsive and resistant focal seizures with unknown cause. Epilepsy Res 135:131–136
Kesim YF, Uzun GA, Yucesan E, Tuncer FN, Ozdemir O, Bebek N, Ozbek U, Iseri SA, Baykan B (2016) Screening LGI1 in a cohort of 26 lateral temporal lobe epilepsy patients with auditory aura from Turkey detects a novel de novo mutation. Epilepsy Res 120:73–78
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshé SL, Nordli DR, Perucca E, Tomson T, Wiebe S, Zhang YH, Zuberi SM (2017) ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58:512–521
Fisher RS, Cross JH, D'Souza C, French JA, Haut SR, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E, Scheffer IE, Schulze-Bonhage A, Somerville E, Sperling M, Yacubian EM, Zuberi SM (2017) Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 58:531–542
Kane N, Acharya J, Benickzy S, Caboclo L, Finnigan S, Kaplan PW, Shibasaki H, Pressler R, van Putten MJAM (2017) A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin Neurophysiol Pract 2:170–185
Blume WT, Lüders HO, Mizrahi E, Tassinari C, van Emde BW, Engel J Jr (2001) Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia 42:1212–1218
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51:1069–1077
Sillanpää M, Schmidt D, Saarinen MM et al (2017) Remission in epilepsy: how long is enough? Epilepsia 58:901–906
Engel J Jr (1987) Outcome with respect to epileptic seizures. In: Engel J Jr (ed) Surgical treatment of the epilepsies. Raven Press, New York, pp 535–571
Vanli-Yavuz EN, Erdag E, Tuzun E, Ekizoglu E, Baysal-Kirac L, Ulusoy C, Peach S, Gundogdu G, Sencer S, Sencer A, Kucukali CI, Bebek N, Gurses C, Gokyigit A, Baykan B (2016) Neuronal autoantibodies in mesial temporal lobe epilepsy with hippocampal sclerosis. J Neurol Neurosurg Psychiatry 87:684–692
Koepp MJ, Woermann FG (2005) Imaging structure and function in refractory focal epilepsy. Lancet Neurol 4:42–53
Wang ZI, Alexopoulos AV, Jones SE, Jaisani Z, Najm IM, Prayson RA (2013) The pathology of magnetic-resonance-imaging-negative epilepsy. Mod Pathol 26:1051–1058
Vale FL, Effio E, Arredondo N, Bozorg A, Wong K, Martinez C, Downes K, Tatum WO, Benbadis SR (2012) Efficacy of temporal lobe surgery for epilepsy in patients with negative MRI for mesial temporal lobe sclerosis. J Clin Neurosci 19:101–106
Kogias E, Altenmüller DM, Klingler JH, Schmeiser B, Urbach H, Doostkam S (2017) Histopathology of 3 Tesla MRI-negative temporal lobe epilepsies. J Clin Neurosci 47:273–277. https://doi.org/10.1016/j.jocn.2017.10.012
Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V (2018) The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia 59:2179–2193
Del Felice A, Beghi E, Boero G, La Neve A, Bogliun G, De Palo A, Specchio LM (2010) Early versus late remission in a cohort of patients with newly diagnosed epilepsy. Epilepsia 51:37–42
Silva-Alves MS, Secolin R, Carvalho BS, Yasuda CL, Bilevicius E, Alvim MK, Santos RO, Maurer-Morelli CV, Cendes F, Lopes-Cendes I (2017) A prediction algorithm for drug response in patients with mesial temporal lobe epilepsy based on clinical and genetic information. PLoS ONE 4:e0169214. https://doi.org/10.1371/journal.pone.0169214
Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319
Dasarı A, Bansal D, Gudala K (2017) Brivaracetam add-on therapy for epilepsy: evidence based meta-analysis and metaregression of randomized controlled trials. Neurol Sci Neurophysiol 34:1–15
Berg AT, Scheffer IE (2011) New concepts in classification of the epilepsies: entering the 21st century. Epilepsia 52:1058–1062
Gilioli I, Vignoli A, Visani E, Casazza M, Canafoglia L, Chiesa V, Gardella E, La Briola F, Panzica F, Avanzini G, Canevini MP, Franceschetti S, Binelli S (2012) Focal epilepsies in adult patients attending two epilepsy centers: classification of drug-resistance, assessment of risk factors, and usefulness of "new" antiepileptic drugs. Epilepsia 53:733–740
Park KM, Shin KJ, Ha SY, Park J, Kim SE, Kim SE (2014) Response to antiepileptic drugs in partial epilepsy with structural lesions on MRI. Clin Neurol Neurosurg 123:64–68
Arroyo S, Brodie MJ, Avanzini G, Baumgartner C, Chiron C, Dulac O, French JA, Serratosa JM (2002) Is refractory epilepsy preventable? Epilepsia 43:437–444
Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B (2001) Early development of intractable epilepsy in children: a prospective study. Neurology 56:1445–1452
Voll A, Hernández-Ronquillo L, Buckley S, Téllez-Zenteno JF (2015) Predicting drug resistance in adult patients with generalized epilepsy: a case-control study. Epilepsy Behav 53:126–130
Orozco-Hernández JP, Quintero-Moreno JF, Marín-Medina DS, Valencia-Vásquez A, Villada HC, Lizcano A, Martínez JW (2018) Multivariable prediction model of drug resistance in adult patients with generalized epilepsy from Colombia: a case-control study. Epilepsy Behav 88:176–180
Souirti Z, Sghir A, Belfkih R, Messouak O (2016) Focal drug-resistant epilepsy: progress in care and barriers, a Morroccan perspective. J Clin Neurosci 34:276–280
French JA (2007) Refractory epilepsy: clinical overview. Epilepsia 48:3–7
Tang F, Hartz AMS, Bauer B (2017) Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol 8:301
Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ (2007) Predictors of pharmacoresistant epilepsy. Epilepsy Res 75:192–196
Alexandre V Jr, Capovilla G, Fattore C, Franco V, Gambardella A, Guerrini R, La Briola F, Ladogana M, Rosati E, Specchio LM, Striano S, Perucca E, SOPHIE Study Group (2010) Characteristics of a large population of patients with refractory epilepsy attending tertiary referral centers in Italy. Epilepsia 51:921–925
Labate A, Aguglia U, Tripepi G, Mumoli L, Ferlazzo E, Baggetta R, Quattrone A, Gambardella A (2016) Long-term outcome of mild mesial temporal lobe epilepsy: a prospective longitudinal cohort study. Neurology 86:1904–1910
Nicolson A, Appleton RE, Chadwick DW, Smith DF (2004) The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsies. J Neurol Neurosurg Psychiatry 75:75–79
Holmes GL, Engel J (2001) Predicting medical intractability of epilepsy in children: how certain can we be? Neurology 56:1430–1431
Ochoa-Gómez L, López-Pisón J, Fuertes-Rodrigo C, Fernando-Martínez R, Samper-Villagrasa P, Monge-Galindo L, Peña-Segura JL (2016) Prognosis of non-symptomatic epilepsy in relation to their age of onset, monitored at a neuropediatric section of regional reference over a period of three years. Rev Neurol 62:145–151
Camfield P, Camfield C (2003) Childhood epilepsy: what is the evidence for what we think and what we do? J Child Neurol 18:272–287
Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B, Ebrahimi N (2001) Defining early seizure outcomes in pediatric epilepsy: the good, the bad and the in-between. Epilepsy Res 43:75–84
Casetta I, Granieri E, Monetti VC, Gilli G, Tola MR, Paolino E, Govoni V, Iezzi E (1999) Early predictors of intractability in childhood epilepsy: a community-based case–control study in Copparo, Italy. Acta Neurol Scand 99:329–333
Semah F, Picot MC, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M (1998) Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology 51:1256–1262
Seneviratne U, Cook M, D’Souza W (2012) The prognosis of idiopathic generalized epilepsy. Epilepsia 53:2079–2090
Sun Y, Seneviratne U, Perucca P, Chen Z, Tan MK, O'Brien TJ, D'Souza W, Kwan P (2018) Generalized polyspike train: an EEG biomarker of drug-resistant idiopathic generalized epilepsy. Neurology 91:1822–1830
Sander JW, Sillanpaa M (2008) The natural history and prognosis of epilepsy. In: Engel J, Pcdley TA (eds) Epilepsy: a comprehensive textbook. Lippincott Williams & Wilkins, Philadelphia, pp 69–96
Mohanraj R, Brodie MJ (2013) Early predictors of outcome in newly diagnosed epilepsy. Seizure 22:333–344
Asadi-Pooya AA, Sperling MR (2015) Age at onset in patients with medically refractory temporal lobe epilepsy and mesial temporal sclerosis: impact on clinical manifestations and postsurgical outcome. Seizure 30:42–45
Arts WF, Geerts AT, Brouwer OF, Boudewyn Peters AC, Stroink H, van Donselaar CA (1999) The early prognosis of epilepsy in childhood: the prediction of a poor outcome. The Dutch study of epilepsy in childhood. Epilepsia 40:726–734
Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA (2007) Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol 62:382–389
Berg AT, Levy SR, Novotny EJ, Shinnar S (1996) Predictors of intractable epilepsy in childhood: a case–control study. Epilepsia 37:24–30
Marini C, King MA, Archer JS, Newton MR, Berkovic SF (2003) Idiopathic generalised epilepsy of adult onset: clinical syndromes and genetics. J Neurol Neurosurg Psychiatry 74:192–196
Beghi E, Beretta S, Carone D, Zanchi C, Bianchi E, Pirovano M, Trentini C, Padovano G, Colombo M, Cereda D, Scanziani S, Giussani G, Gasparini S, Bogliun G, Ferrarese C, PRO-LONG Study Group (2019) Prognostic patterns and predictors in epilepsy: a multicentre study (PRO-LONG). J Neurol Neurosurg Psychiatry. 90:1276–1285
Aguglia U, Beghi E, Labate A, Condino F, Cianci V, Mumoli L, Gasparini S, Quattrone A, Gambardella A (2011) Age at onset predicts good seizure outcome in sporadic non-lesional and mesial temporal sclerosis based temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 82:555–559
Aaberg KM, Bakken IJ, Lossius MI, Lund Søraas C, Tallur KK, Stoltenberg C, Chin R, Surén P (2018) Short-term seizure outcomes in childhood epilepsy. Pediatrics 141:e20174016
Berg AT, Testa FM, Levy SR (2011) Complete remission in nonsyndromic childhood-onset epilepsy. Ann Neurol 70:566–573
Camfield PR, Camfield CS (2017) Intractable seizures after a lengthy remission in childhood-onset epilepsy. Epilepsia 58:2048–2052
Berg AT, Lin J, Ebrahimi N, Testa FM, Levy SR, Shinnar S (2004) Modeling remission and relapse in pediatric epilepsy: application of a Markov process. Epilepsy Res 60:31–40
Sillanpaa M, Schmidt D (2009) Early seizure frequency and aetiology predict long-term medical outcome in childhood-onset epilepsy. Brain 132:989–998
Epi4K Consortium (2017) Phenotypic analysis of 303 multiplex families with common epilepsies. Brain 140:2144–2156
Afawi Z, Oliver KL, Kivity S, Mazarib A, Blatt I, Neufeld MY, Helbig KL, Goldberg-Stern H, Misk AJ, Straussberg R, Walid S, Mahajnah M, Lerman-Sagie T, Ben-Zeev B, Kahana E, Masalha R, Kramer U, Ekstein D, Shorer Z, Wallace RH, Mangelsdorf M, MacPherson JN, Carvill GL, Mefford HC, Jackson GD, Scheffer IE, Bahlo M, Gecz J, Heron SE, Corbett M, Mulley JC, Dibbens LM, Korczyn AD, Berkovic SF (2016) Multiplex families with epilepsy: success of clinical and molecular genetic characterization. Neurology 86:713–722
Funding
This study was supported by the Istanbul University Research Fund (Project No: BAP-2018-31114).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Atalar, A.Ç., Vanlı-Yavuz, E.N., Yılmaz, E. et al. Long-term follow-up of a large cohort with focal epilepsy of unknown cause: deciphering their clinical and prognostic characteristics. J Neurol 267, 838–847 (2020). https://doi.org/10.1007/s00415-019-09656-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09656-8