Abstract
Background
Progressive multifocal leukoencephalopathy (PML) is a subacute onset demyelinating disease caused by JC virus and characterized by multifocal involvement of the subcortical white matter and cerebellar hemispheres or peduncles on magnetic resonance imaging (MRI). However, non-HIV PML patients with brain lesions limited to the cerebellum and brainstem have not been well characterized.
Methods
We report a 68-year-old man with systemic lupus erythematosus under treatment with immunosuppressants who developed non-HIV PML with brain lesions limited to the cerebellum and brainstem and successfully treated with a combination of mefloquine and mirtazapine. We performed a literature review to characterize patients with non-HIV PML with brain lesions limited to the cerebellum and brainstem.
Results
Eight cases with non-HIV brainstem/cerebellar form PML were identified including our case. All cases had compromised status related underlying diseases. Four (50%) had a good prognosis. Five cases were treated, including 3 with favourable outcomes. Between the good prognosis group (n = 4) and the poor prognosis group (n = 4), treatment status for PML and the interval between the initial manifestation and diagnosis did not differ. Among those who performed contrast-enhanced brain imaging, lesion enhancement was related to good prognosis (good prognosis group vs. poor prognosis group; 100% vs. 0%).
Conclusion
PML should be considered in the differential diagnosis of brain lesions limited to the cerebellum and brainstem in immunocompromised patients. The presence of immune response against JC virus and inflammatory reactions may indicate good prognosis in non-HIV brainstem/cerebellar form PML
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive multifocal leukoencephalopathy (PML) is a subacute-onset demyelinating disease caused by JC virus (JCV) that usually affects individuals with immunosuppressed conditions and is characterized by multifocal involvement of the subcortical white matter and cerebellar hemispheres or peduncles on magnetic resonance imaging (MRI). The clinical course shows subacute progression, and the prognosis is poor, with a 3-month mortality rate of 20–50% in untreated patients [12, 13].
PML is classified into HIV-associated and non-HIV-associated PML, with the latter caused by various conditions, such as malignancy, chemotherapy, and immunosuppressants for autoimmune disease [35]. Recently, the increased use of monoclonal antibody therapy for multiple sclerosis or haematologic diseases has been attributed to an increase in the prevalence of non-HIV-associated PML [35]. PML typically involves the cerebral white matter, the brainstem and cerebellum, but rarely involves the spinal cord [10]. Clinical symptoms vary according to the lesions [33]. No treatment for PML has been established, but immunosuppressants should be discontinued if possible. Other case reports have described the efficacy of mirtazapine, a noradrenergic and specific serotonergic antidepressant [3], and mefloquine, an antimalarial agent, for PML [21, 26]. Because PML can show various clinical presentations and MRI findings, its differential diagnosis includes a variety of diseases, such as glioma, malignant lymphoma, multiple sclerosis and neuropsychiatric lupus as well as infectious encephalitis [29].
Here, we present a patient with systemic lupus erythematosus (SLE) under treatment with immunosuppressants. The patient developed non-HIV-associated PML that presented as brain lesions limited to the cerebellum and brainstem. Following brain biopsy, oral tacrolimus therapy was discontinued and combined treatment with mefloquine and mirtazapine improved the patient’s symptoms.
Case report
A 68-year-old male patient presented to our department with difficulty in speech, vertigo and an unsteady gait lasting for 4 months. At the age of 59 years old, the patient was diagnosed with rheumatoid arthritis. At the age of 65 years old, he developed joint pain, rash in hands, canker sores, facial erythema, a discoid rash and photodermatosis and had positive results for serum anti-nuclear antibody and dsDNA-IgG antibody, leading to a diagnosis of SLE. Treatment with prednisolone and tacrolimus was started. His body temperature was 36.8 °C, blood pressure was 147/72 mmHg, and pulse rate was 68 beats/min. The patient was alert and cognitively intact. He showed right gaze-evoked nystagmus without limitation of extraocular movement. He had paresthesia in his right face and slurred speech, but facial weaknesses was not observed. Neither motor weakness nor sensory impairments were noted. Tendon reflexes were normal, and pathological reflexes were negative. A finger-to-nose test showed dysmetria and clumsiness in the right hand. Truncal ataxia and lateropulsion to the right were evident.
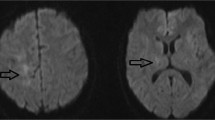
Electrocardiography was normal. Laboratory data showed mild renal dysfunction (serum creatinine: 1.12 mg/dL; eGFR: 51.1 ml/min/1.73 m2) and increased HbA1c levels (7.0%). WBC count was preserved (7200/µL), but CD4-positive lymphocyte was decreased (452/µL). Anti-ss DNA antibody and anti-ds DNA antibody tests were positive (Table 1). Tumour markers, such as CEA and SCC, were not elevated. An anti-HIV antibody test was negative. Levels of serum sIL-2R, terminal deoxynucleotidyl transferase, ACE, and anti-AQP4 antibodies were within normal ranges. An anti-ribosomal P antibody test was negative. Cerebrospinal fluid (CSF) examination did not show pleocytosis or increased protein levels. IL-6 and β2MG levels were elevated. Qualitative analysis of JCV DNA by PCR in CSF was negative (Table 2). Cytology of CSF showed lymphocyte infiltration, and there were no malignant cells. Contrast-enhanced chest and abdominal computed tomography showed no mass lesions or swollen lymph nodes. Diffusion-weighted and fluid-attenuated inversion recovery MRI of the brain showed high signal intensities extending from the right pons to the right cerebellar hemisphere via the right middle cerebellar peduncle with slight enhancement on gadolinium-enhanced T1-weighted MRI (Fig. 1).
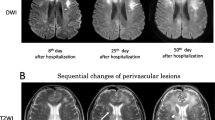
Brain MRI. Diffusion-weighted (a) and fluid-attenuated inversion recovery (b) magnetic resonance images of the brain showing high signal intensities extending from the right pons to right cerebellar hemisphere via the right middle cerebellar peduncle. Gadolinium-enhanced T1-weighted images (c, d) showing slight enhancement in the corresponding lesions
Upon admission, oral tacrolimus therapy (3 mg/day) was discontinued, and the prednisolone dose was gradually decreased. Gait disturbance progressed, and he was even unable to stand up 7 days after admission. At the same time, right facial palsy appeared. A follow-up brain MRI performed on day 8 revealed enlargement of brain lesions in the right pons and cerebellum. On day 15, stereotactic brain biopsy was performed in the right cerebellum, and lymphoid cell infiltration was observed in the frozen section. From day 16, intravenous methylprednisolone (1 g/day) was administered for 3 days, and this led to temporary improvement of vertigo and unsteadiness, but these symptoms subsequently relapsed. Histopathological specimens revealed infiltration of plasma cells, lymphoid cells, and abundant macrophages in the damaged area (Fig. 2). Epstein-Barr early RNA in situ hybridization (EBER-ISH) showed positive label in B lymphocytes, suggesting acute demyelinating encephalomyelitis with Epstein-Barr virus reactivation. However, immunostaining for JCV capsid protein revealed intranuclear viral inclusions in mildly enlarged glial nuclei. JCV-infected glial cells were detected by immunohistochemistry with anti-JCV VP2/VP3 antibody [34]. Based on these pathological findings, a diagnosis of PML was made. Additionally, quantitative JCV DNA PCR of CSF was found to be positive. Following combined therapy with mefloquine and mirtazapine, the patient achieved marked improvement in clinical symptoms, and he became able to walk independently (Fig. 3). Six months later, JCV DNA PCR turned negative. Over a 9-month follow-up, no relapse occurred and his clinical symptoms were stable.
Pathological specimens. Histopathological specimens showing infiltration of inflammatory cells in the damaged area of the white matter on HE staining (a), and a glial cell with mildly-enlarged nuclei were present, suggesting JCV-infected cells. Plasma cells and lymphoid cells were also infiltrating. b Epstein-Barr early RNA in situ hybridization (EBER-ISH) staining was positive in B lymphocytes (c). JCV-infected glial cells were detected by immunohistochemistry with anti-JCV VP2/VP3 antibody [34] (d)
Clinical course of the patient. Upon admission, oral tacrolimus therapy (3 mg/day) was discontinued, and the prednisolone dose was gradually decreased. Gait disturbance progressed, and he was unable to stand up on Day 7. At the same time, right facial palsy appeared. A follow-up brain MRI performed on Day 8 revealed enlargement of the brain lesions in the right pons and cerebellum. On Day 15, stereotactic brain biopsy from the right cerebellum was performed, and atypical lymphocytes were observed in the frozen section. From Day 16, intravenous methylprednisolone (1 g/day) was administered for 3 days, and this led to temporary improvement of vertigo and unsteadiness, but these symptoms subsequently relapsed. Following a diagnosis of PML, combined therapy with mefloquine and mirtazapine resulted in marked improvement in his clinical symptoms. PSL prednisolone; mPSL methylprednisolone; DEX dexamethasone
Discussion
Here, we report a patient with SLE under treatment with immunosuppressants who developed non-HIV-associated PML that presented as brain lesions limited to the cerebellum, brainstem and middle cerebellar peduncle. Differential diagnoses of middle cerebellar peduncle lesions in our patient included glioma, malignant lymphoma or demyelinating diseases (such as multiple sclerosis), CNS lupus, and Behcet’s disease [22, 37]. In the present case, because the patient’s clinical symptoms progressively deteriorated, brain biopsy was performed before the results of quantitative JCV DNA PCR of CSF were available. The findings in JCV-infected glial cells, which were immunoreactive for JCV capsid protein on brain biopsy, and positive results from quantitative JCV DNA PCR of CSF later led to a diagnosis of PML. Thus, unilateral brainstem and cerebellar lesions, when found in immunosuppressive patients, should lead to consideration of PML. To treat non-HIV-associated PML, the first choice is reconstruction of the patient’s immune system by quitting immunosuppressants. However, autoimmune diseases and demyelinating diseases require enhanced immunosuppression therapy. Misdiagnosis and inappropriate treatment can, therefore, exacerbate disease, and a correct and early diagnosis is, therefore, important.
PML was thought a fatal, intractable demyelinating disease caused by JC virus; it belongs to the polyoma virus family. Latent infection with JCV is not rare, and 50–90% of the population is seropositive for JCV [24]. In compromised patients, reactivated JCV affects oligodendrocytes and causes leukoencephalopathy [12]. Previously, PML was considered a rare disease that mainly occurred in compromised hosts, such as those with HIV infection, hematologic disease, posttransplantation status, and chemotherapy or immunosuppression therapy [13]. In contrast, today, more attentions have focused on PML, and an increase in the number of patients being treated with various monoclonal antibodies, such as natalizumab and rituximab has been well documented [12].
In the established PML diagnostic criteria, PML is diagnosed based on clinical features, imaging findings and CSF PCR for JCV. According to the diagnostic criteria, our patient was classified as “definite PML” [8]. A histopathological diagnosis requires confirmation of JCV protein labelling by immunostaining and JCV DNA PCR-positive by biopsy specimens. PML typically involves the subcortical white matter but can also involve the infratentorial cerebellar white matter [12], similar to what was observed in our patient. Parr et al. [27] reported that 24% of PML patients with or without HIV status showed cerebellar lesions, and 20% of patients showed brainstem lesions. On brain MRI, the characteristic findings of PML are diffuse asymmetrical, unclear boundary lesions with T1-weighted low-intensity and T2-weighted high-intensity signals [29, 32]. A crescent-shaped cerebellar sign without dentate nucleus involvement has been described in PML patients [31] and was observed in our patient. Additionally, concomitant gadolinium enhancement suggests immune reconstitution inflammatory syndrome (IRIS). Natalizumab-related PML and IRIS-PML show enhancement lesions on contrast-enhanced MRI [12, 29]. Histopathological features of PML include oligodendrocytes with amphophile intranuclear inclusions and demyelination and softening of the white matter, and similar pathological features were also observed in our patient.
Brainstem and cerebellar involvement are not rare in PML, but PML lesions limited to the cerebellum and brainstem are less frequently presented in non-HIV patients in the literature. In Table 2, we summarize 8 cases of non-HIV infratentorial-onset PML, including our patient [4, 5, 16, 17, 25, 28, 30]. All cases had compromised status related to various underlying diseases, such as chronic renal failure, idiopathic CD4 lymphocytopenia and malignant lymphoma. Out of 8 patients, 4 had a good prognosis. Five cases were treated, including 3 with favourable outcomes. In our patient, the interval between the initial symptom onset and PML diagnosis was 5 months, and this was not significantly shorter than that observed in other cases. When comparing the good prognosis group (cases 3, 4, 7 and 8) and the poor prognosis group (cases 1, 2, 5 and 6), treatment for PML and the interval between the initial manifestation and diagnosis were found to be unlikely to contribute to the patients’ clinical outcomes. For example, case 3 had idiopathic CD4+ T lymphocytopenia and was diagnosed with PML several months after the initial manifestations. The patient received no specific treatment for PML because of the patient’s overall good condition, and his clinical symptoms and MRI findings had markedly improved one year later. In contrast, case 6 had diabetes, hypertension and chronic renal failure and died 3 months after onset despite the relatively early diagnosis of PML based on MRI findings and positive JCV PCR on CSF (approximately 2 months after initial manifestation) and treatment with intravenous methylprednisolone and mirtazapine. These observations suggest that the clinical prognosis of the cerebellum and brainstem form of non-HIV PML may depend on the patients’ general status with regard for comorbid conditions, and comorbid chronic renal failure could indicate a poor prognosis in this series. JCV DNA levels > 3.64 log copies/mL [11] and the presence of JCV–specific cytotoxic T-lymphocytes [20] have been associated with shorter survival in PML, whereas in HIV-related PML patients, lesion enhancement on computed tomographic scan or MRI has been related with the recovery of neurological function [9]. This finding may be applicable to non-HIV PML patients with cerebellum and brainstem involvement showing lesion enhancement on MRI, indicating a good recovery. Among those who performed contrast-enhanced brain imaging, lesion enhancement was related to favorable prognosis (good prognosis group (cases 4, 7 and 8) vs. poor prognosis group; 100% vs. 0%; Table 2). Unlike HIV-associated PML cases, the presence of immune response against JC virus and inflammatory reactions, indicated by contrast-enhancing lesions on neuroimaging and increase in CD 4+ T -cell counts, may contribute to favorable clinical course in non-HIV PML cases [14]. Akagawa et al. [1] reported 2 non-HIV related PML patients with autoimmune diseases on immunosuppressants. The 2 patients were treated with mefloquine, mirtazapine and risperidone and showed good recovery, which was attributed to preserved immune responses against JC virus indicated by gadolinium enhancement at lesions on brain MRI and balanced infiltration of CD 8 + and CD 4 + on brain biopsy, respectively.
The three-month mortality rate of PML in untreated patients has been reported to be 20–50% [12], and the probability of survival at 1 year was 52% for HIV-positive PML and 58% for HIV-negative PML patients [20]. In HIV-related PML, highly active antiretroviral therapy contributed to higher 1-year survival rates [2]. However, for non-HIV PML other than immediate discontinuation of causative immunosuppressants, therapeutic options and their evidence basis are limited.
In patients with non-HIV and biological agent-associated PML, after discontinuation of immunosuppression therapy, plasma exchange was well tolerated [12]. However, plasma exchange may accelerate development of natalizumab-associated PML-IRIS [36] and unlikely efficacious in rituximab-associated PML [12]. In addition to those therapies, mefloquine and mirtazapine are other treatment options [21]. Mirtazapine is a noradrenergic and specific serotonergic antidepressant that prevents combining between the virus and oligodendrocytes [3]. Mefloquine shows an anti-JCV effect in vitro, but its mechanism remains unexplained. Several [7, 15, 18] but not all [19, 23] were successfully treated with mefloquine. The effect of mefloquine may depend on the recovery of cell-mediated immunity and genetic polymorphism in the MDR1 P-glycoprotein, which causes differences in the BBB transport of mefloquine and may, therefore, play a role in this disease [6]. Because no sufficient evidence has accumulated regarding the use of mefloquine and mirtazapine for non-HIV-associated PML, further clinical trials are warranted.
In conclusion, we describe a patient with non-HIV-associated PML with brain lesions limited to the cerebellum and brainstem who had SLE and was on immunosuppression therapy. Although qualitative analysis of JCV DNA PCR in CSF was negative, JCV was confirmed positive in specimens obtained in brain biopsy, and this led to a diagnosis of PML. Early initiation of treatment with mirtazapine and mefloquine improved the patient’s clinical symptoms. In compromised patients with isolated brainstem and cerebellar lesions, PML should be included in the differential diagnosis. The presence of immune response against JC virus and inflammatory reactions may indicate good prognosis in non-HIV brainstem/cerebellar form PML.
References
Akagawa Y, Ueno A, Ikeda J, Ishii W, Shishido-Hara Y, Sekijima Y (2018) Two patients with progressive multifocal leukoencephalopathy with immune response against JC virus showing good long-term outcome by combination therapy of mefloquine, mirtazapine, and risperidone. Rinsho Shinkeigaku 58:324–331
Antinori A, Cingolani A, Lorenzini P, Giancola ML, Uccella I, Bossolasco S, Grisetti S, Moretti F, Vigo B, Bongiovanni M, Del Grosso B, Arcidiacono MI, Fibbia GC, Mena M, Finazzi MG, Guaraldi G, Ammassari A, d'Arminio Monforte A, Cinque P, De Luca A, Italian Registry Investigative Neuro ASG (2003) Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA). J Neurovirol 9(Suppl 1):47–53
Anttila SA, Leinonen EV (2001) A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev 7:249–264
Aotsuka Y, Uzawa A, Nishimura K, Kojima K, Yamaguchi M, Makino T, Nakamichi K, Saijo M, Kuwabara S (2016) Progressive multifocal leukoencephalopathy localized in the cerebellum and brainstem associated with idiopathic CD4(+) T lymphocytopenia. Intern Med 55:1645–1647
Arai Y, Tsutsui Y, Nagashima K, Shinmura Y, Kosugi T, Wakai M, Nishikage H, Yamamoto J (2002) Autopsy case of the cerebellar form of progressive multifocal leukoencephalopathy without immunodeficiency. Neuropathology 22:48–56
Barraud de Lagerie S, Comets E, Gautrand C, Fernandez C, Auchere D, Singlas E, Mentre F, Gimenez F (2004) Cerebral uptake of mefloquine enantiomers with and without the P-gp inhibitor elacridar (GF1210918) in mice. Br J Pharmacol 141:1214–1222
Beppu M, Kawamoto M, Nukuzuma S, Kohara N (2012) Mefloquine improved progressive multifocal leukoencephalopathy in a patient with systemic lupus erythematosus. Intern Med 51:1245–1247
Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, Bartt R, Major EO, Nath A (2013) PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology 80:1430–1438
Berger JR, Levy RM, Flomenhoft D, Dobbs M (1998) Predictive factors for prolonged survival in acquired immunodeficiency syndrome-associated progressive multifocal leukoencephalopathy. Ann Neurol 44:341–349
Bernal-Cano F, Joseph JT, Koralnik IJ (2007) Spinal cord lesions of progressive multifocal leukoencephalopathy in an acquired immunodeficiency syndrome patient. J Neurovirol 13:474–476
Bossolasco S, Calori G, Moretti F, Boschini A, Bertelli D, Mena M, Gerevini S, Bestetti A, Pedale R, Sala S, Sala S, Lazzarin A, Cinque P (2005) Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin Infect Dis 40:738–744
Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A (2010) Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol 6:667–679
Brooks BR, Walker DL (1984) Progressive multifocal leukoencephalopathy. Neurol Clin 2:299–313
Du Pasquier RA, Koralnik IJ (2003) Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial? J Neurovirol 9(Suppl 1):25–31
Hirayama M, Nosaki Y, Matsui K, Terao S, Kuwayama M, Tateyama H, Yoshida M, Hashizume Y (2012) Efficacy of mefloquine to progressive multifocal leukoencephalopathy initially presented with parkinsonism. Clin Neurol Neurosurg 114:728–731
Irie T, Kasai M, Abe N, Seto K, Naohara T, Kawamura K, Higa T, Sano K, Takahashi H, Nagashima K (1992) Cerebellar form of progressive multifocal leukoencephalopathy in a patient with chronic renal failure. Intern Med 31:218–223
Ito D, Yasui K, Hasegawa Y, Nakamichi K, Katsuno M, Takahashi A (2016) Progressive multifocal leukoencephalopathy with bilateral middle cerebellar peduncle lesions confirmed by repeated CSF-JC virus tests and coexistence of JC virus granule cell neuronopathy. Report of a case. Rinsho Shinkeigaku 56:481–485
Kishida S, Tanaka K (2010) Mefloquine treatment in a patient suffering from progressive multifocal leukoencephalopathy after umbilical cord blood transplant. Intern Med 49:2509–2513
Kobayashi Z, Akaza M, Numasawa Y, Ishihara S, Tomimitsu H, Nakamichi K, Saijo M, Morio T, Shimizu N, Sanjo N, Shintani S, Mizusawa H (2013) Failure of mefloquine therapy in progressive multifocal leukoencephalopathy: report of two Japanese patients without human immunodeficiency virus infection. J Neurol Sci 324:190–194
Marzocchetti A, Tompkins T, Clifford DB, Gandhi RT, Kesari S, Berger JR, Simpson DM, Prosperi M, De Luca A, Koralnik IJ (2009) Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 73:1551–1558
Moenster RP, Jett RA (2012) Mirtazapine and mefloquine therapy for progressive multifocal leukoencephalopathy in a patient infected with human immunodeficiency virus. Am J Health Syst Pharm 69:496–498
Morales H, Tomsick T (2015) Middle cerebellar peduncles: Magnetic resonance imaging and pathophysiologic correlate. World J Radiol 7:438–447
Morimoto A, Ueno H, Fujii H, Nakamura T, Nakamichi K, Saijo M, Yukitake M, Matsumoto M (2013) Ineffective mefloquine therapy in progressive multifocal leukoencephalopathy complicated with malignant lymphoma: finding and usefulness of susceptibility-weighted imaging. Rinsho Shinkeigaku 53:843–847
Nakamichi K, Mizusawa H, Yamada M, Kishida S, Miura Y, Shimokawa T, Takasaki T, Lim CK, Kurane I, Saijo M (2012) Characteristics of progressive multifocal leukoencephalopathy clarified through internet-assisted laboratory surveillance in Japan. BMC Neurol 12:121
Nishigori R, Warabi Y, Shishido-Hara Y, Nakamichi K, Nakata Y, Komori T, Isozaki E (2019) Inflammatory cerebellar PML with a CD4/CD8 ratio of 2.9 showed a favorable prognosis in a patient with rheumatoid arthritis: a case report. Intern Med. https://doi.org/10.2169/internalmedicine.3038-19
Ohnuki E, Asayama S, Asayama T, Nakamichi K, Saijo M, Kosaka S (2016) A case of progressive multifocal leukoencephalopathy with chronic renal failure, whose JC virus in cerebrospinal fluid disappeared after mefloquine-mirtazapine dual therapy. Rinsho Shinkeigaku 56:705–708
Parr J, Horoupian DS, Winkelman AC (1979) Cerebellar form of progressive multifocal leukoencephalopathy (PML). Can J Neurol Sci 6:123–128
Phan-Ba R, Lommers E, Tshibanda L, Calay P, Dubois B, Moonen G, Clifford D, Belachew S (2012) MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry 83:224–226
Rocha AJ, Littig IA, Nunes RH, Tilbery CP (2013) Central nervous system infectious diseases mimicking multiple sclerosis: recognizing distinguishable features using MRI. Arq Neuropsiquiatr 71:738–746
Rueger MA, Miletic H, Dorries K, Wyen C, Eggers C, Deckert M, Faetkenheuer G, Jacobs AH (2006) Long-term remission in progressive multifocal leukoencephalopathy caused by idiopathic CD4+ T lymphocytopenia: a case report. Clin Infect Dis 42:e53–56
Sahraian MA, Radue EW, Eshaghi A, Besliu S, Minagar A (2012) Progressive multifocal leukoencephalopathy: a review of the neuroimaging features and differential diagnosis. Eur J Neurol 19:1060–1069
Shah R, Bag AK, Chapman PR, Cure JK (2010) Imaging manifestations of progressive multifocal leukoencephalopathy. Clin Radiol 65:431–439
Shishido-Hara Y (2010) Progressive multifocal leukoencephalopathy and promyelocytic leukemia nuclear bodies: a review of clinical, neuropathological, and virological aspects of JC virus-induced demyelinating disease. Acta Neuropathol 120:403–417
Shishido-Hara Y, Higuchi K, Ohara S, Duyckaerts C, Hauw JJ, Uchihara T (2008) Promyelocytic leukemia nuclear bodies provide a scaffold for human polyomavirus JC replication and are disrupted after development of viral inclusions in progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol 67:299–308
Tan CS, Koralnik IJ (2010) Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 9:425–437
Tan IL, McArthur JC, Clifford DB, Major EO, Nath A (2011) Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 77:1061–1067
Uchino A, Sawada A, Takase Y, Kudo S (2004) Symmetrical lesions of the middle cerebellar peduncle: MR imaging and differential diagnosis. Magn Reson Med Sci 3:133–140
Acknowledgements
We thank the patient for his participation in this study. We wish to thank Dr. Nobuaki Funata, Department of Pathology, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital for his help with pathological diagnosis.
Funding
This work was partly supported by a Grant-in-Aid for the Research Committee of Prion Disease and Slow Virus Infection, Research on Policy Planning and Evaluation for Rare and Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (Grant Number H29-Nanchitou (Nan)-Ippan-036) and by JSPS KAKENHI (Grant Number 17K09768).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript and contributed to the design of the study and interpretation of data. MH, KS and HF drafted the manuscript. MH, TU, HM, SA, SK, Y S–H, KN and MS contributed to the diagnosis and treatment of the patient. TU, Y S–H, TN and KH revised the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no potential conflicts of interest in relation to this article.
Ethical approval
Not applicable.
Informed consent
Written informed consent was obtained from the patient included in the study.
Rights and permissions
About this article
Cite this article
Hamaguchi, M., Suzuki, K., Fujita, H. et al. Successful treatment of non-HIV progressive multifocal leukoencephalopathy: case report and literature review. J Neurol 267, 731–738 (2020). https://doi.org/10.1007/s00415-019-09629-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09629-x