Abstract
Objective
To determine the diagnostic and monitoring value of serum neurofilament light chain (NfL) in spinal muscular atrophy (SMA).
Methods
We measured serum NfL in 46 SMA patients at baseline and over 14 months of treatment with the antisense-oligonucleotide (ASO) nusinersen using the ultrasensitive single molecule array (Simoa) technology. Serum NfL levels of SMA patients were compared to controls and related to cerebrospinal fluid (CSF) NfL, blood-CSF barrier function quantified by the albumin blood/CSF ratio (Qalb) and motor scores (Hammersmith Functional Motor Scale Expanded, HFMSE; Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised, ALSFRS-R).
Results
Serum NfL levels of SMA patients were in the range of controls (p = 0.316) and did not correlate with CSF NfL (ρ = 0.302, p = 0.142) or Qalb (ρ = − 0.160, p = 0.293). During therapy, serum NfL levels were relatively stable with notable concentration changes in single SMA patients, however, within the control range. Higher NfL levels were associated with worse motor performance in SMA (baseline: HFMSE ρ = − 0.330, p = 0.025, ALSFRS-R ρ = − 0.403, p = 0.005; after 10 months: HFMSE ρ = − 0.525, p = 0.008, ALSFRS-R ρ = − 0.537, p = 0.007), but changes in motor scores did not correlate with changes in serum NfL.
Conclusion
Diagnostic and monitoring performance of serum NfL measurement seems to differ between SMA subtypes. Unlike to SMA type 1, in adolescent and adult SMA type 2 and 3 patients, neurodegeneration is not reflected by increased NfL levels and short-term therapeutic effects cannot be observed. Long-term follow-up has to be performed to see if even low levels of NfL might be good prognostic markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal muscular atrophy (SMA) is a progressive motoneuron disease caused by homozygous deletion of the SMN1 gene. Degeneration of lower motoneurons (LMN) causes spinal and bulbar muscle weakness and atrophy. Based on age of onset, achieved motor skills and life expectancy, the disease is divided into different subtypes [1]. While SMA type 1 represents the infantile-onset, acute form with most severe symptoms and death in infancy due to respiratory insufficiency [2], SMA types 2 and 3 display a chronic course with later-onset in childhood or even adolescence, survival into adulthood, and the potential for a normal lifespan, however often accompanied by severe physical disability [3]. The diagnosis of SMA is based on molecular genetic testing [4]. While previously only supportive therapeutic measures were available for SMA, in 2017 the antisense-oligonucleotide (ASO) nusinersen has been approved in Europe for the treatment of all SMA types [5] and recently, the first gene-replacement therapy (zolgensma) [6] has been approved in the US for the treatment of SMA patients less than 2 years of age. With the emergence of new therapies for neurodegenerative diseases, the need for biomarkers to track treatment response increases.
Neurofilaments (Nf) are important structural elements of neurons, particularly axons, and essential for radial growth of axons during development, maintenance of axon caliber and nerve conduction velocity [7,8,9]. Multiple studies investigated the potential of Nf levels as biomarkers in patients with neurodegenerative diseases [10]. Increased levels of Nf in both, cerebrospinal fluid (CSF) and blood, have been found e.g., in amyotrophic lateral sclerosis (ALS), a rapid progressive motoneuron disease with upper motoneuron (UMN) and LMN affection, and numerous studies showed the usefulness of Nf for diagnosis, disease progression and survival in ALS [11,12,13].
We observed high Nf levels [neurofilament light chain (NfL) and phosphorylated neurofilament heavy chain (pNfH)] in CSF in an infant with SMA type 1, which decreased below the lower limit of detection (LLOD) when treated with nusinersen [14]. The same performance of NfL in CSF was shown by Olsson et al. in 12 nusinersen treated SMA type 1 children [15]. Moreover, massively elevated pNfH levels in plasma have been observed in infants with SMA type 1 enrolled in ENDEAR (NCT02193074), a phase 3, randomized, double-blind, sham procedure-controlled study to assess the efficacy of nusinersen in infantile-onset SMA. Plasma pNfH levels inversely correlated with markers of disease severity, and showed a decline followed by a relative stabilization in SMA infants receiving nusinersen. Thus, Nf may be promising markers of disease activity and treatment response in infants with SMA type 1 [14, 16].
In a previous study, however, we did not detect elevated NfL and pNfH levels in CSF in adolescent and adult SMA type 2 and 3 patients compared to controls, neither before nor during therapy with nusinersen. Several of the adolescent and adult SMA type 2 and 3 patients had even CSF Nf level below the detection limit of the assay (ELISA) [17]. We assumed that low Nf levels in CSF in these patients may result from slow disease progression, preexisting loss of motoneurons and, in contrast to ALS, from affection of the LMN only. In ALS, in particular degeneration of the UMN and their large-caliber axons along the entire spinal cord, result in the release of a high amount of Nf in CSF [18, 19]. In SMA however, LMN axons are located in the main beyond the CSF space. Therefore, the question arises whether Nf levels in SMA might be possibly less elevated in CSF, but rather in the blood compartment.
This study aims to examine NfL in serum in adolescent and adult SMA type 2 and 3 patients in comparison to controls and during treatment with nusinersen, to evaluate its value for diagnosis and therapeutic monitoring.
Methods
The primary goals of this study were the following:
-
1.
Analysis of serum NfL levels in adolescent and adult SMA type 2 and 3 patients at baseline and during 14 months of treatment with the ASO nusinersen using the ultrasensitive single molecule array (Simoa) technology.
-
2.
Comparison of serum NfL levels in SMA patients to levels in controls. Association of serum NfL in SMA patients at baseline to CSF NfL and the blood-CSF barrier function quantified by means of the albumin blood/CSF ratio (Qalb) (see also [17]).
-
3.
Association of baseline and follow-up serum NfL levels in SMA patients to motor scores [Hammersmith Functional Motor Scale Expanded (HFMSE) and the Amyotrophic Lateral Sclerosis Functional Rating Scale—Revised (ALSFRS-R)].
Standard protocol approvals, registrations, and patient consents
The study was approved by the local ethics committees of the centers involved in Ulm, Dresden, Göttingen and Hannover (approval number at central study center at Ulm University 19/12; 2012) and all patients or their relatives (legal guardian) gave informed written consent to participate in the study.
Sampling and participants
Sample collection
Patient samples were collected since the availability of nusinersen in July 2017 to December 2018 at the following centers of the MND-NET (German Network for Motoneuron Diseases): Department of Neurology, Ulm University (Germany), Department of Neurology, Technische Universität Dresden (Germany), Department of Neurology, University Medical Center Göttingen (Germany) and Department of Neurology, Hannover Medical School (Germany).
We collected serum samples of 58 SMA patients; however, due to incomplete data sets we excluded serum samples from 12 patients, resulting in a total sample size of 46 serum samples for final analysis. Serum samples were taken and evaluated in parallel to intrathecal administration of nusinersen at baseline (start therapy, T1) and on treatment days 180 (6 months, T5), 300 (10 months, T6) and 420 (14 months, T7). CSF samples were taken and evaluated before the first intrathecal administration of nusinersen at baseline (T1).
Sample analysis
Serum was obtained from peripheral blood by centrifugation (800g, 5 min) and stored within two hours at − 80 °C. CSF was obtained by lumbar puncture. Biobanking was conducted according to current recommendations [20]. Serum NfL concentrations were measured with the single molecular array (Simoa) platform provided by Quanterix (Lexington, MA, USA) [21]. Measurements were performed on the fully automated instrument HD-1 Analyzer (Quanterix) [22] using the NfL Advantage kit from Quanterix, which employs an anti-NfL monoclonal antibody produced by UmanDiagnostics (Umeå, Sweden). The interassay coefficients of variation (CV) were < 15%. As presented before [17], ELISA kits were used for the measurement of CSF NfL (IBL, Hamburg, Germany) according to the manufacturers’ instructions. Interassay CV was < 20%. Concentrations of albumin in CSF and serum samples were determined as described earlier [17, 23]. Clinical information, including diagnostic category of any sample, was not available to the performers of the measurement.
SMA patients
Patients history and clinical data (e.g., age) were gathered before therapy started. All patients had genetically confirmed 5q-SMA (deletion in exon 7 and/or 8 in the SMN1 gene). Disease onset in SMA type 2 patients was in early childhood, in SMA type 3 patients in childhood or adolescence. Severity of symptoms was classified as per the HFMSE, a validated rating scale for SMA type 2 and 3 patients [24, 25], and the ALSFRS-R, a rating scale validated for ALS [26], which in contrast to the HFMSE includes respiratory and bulbar affection. The highest score of the HFMSE is 66 points; lower values represent a more severe stage of disease. In the ALSFRS-R a maximum of 48 points can be reached; also, here, lower values represent a more severe stage of disease. Scores were performed at baseline (T1), after five (T5), six (T6) and seven (T7) injections of nusinersen.
Control group
The control sample comprised 14 patients without neurodegenerative diseases and without inflammatory diseases of the CNS (e.g., headache disorders, syncope, vertigo and psychosomatic diseases). Only one serum sample was taken and evaluated from each control patient.
Statistical analysis
A cut-off level of 45 pg/ml was defined according to Steinacker et al. [27] and Verde et al. [13] to distinguish between elevated and non-elevated NfL levels in serum. Continuous variables were described by the mean and standard error of the mean (SEM) or median and quartiles as appropriate. Additionally, the range is presented.
To compare datasets of marker concentrations between the SMA patient group and the control group, Mann–Whitney test was used. To compare datasets of marker concentrations within the SMA patient group, the Wilcoxon signed rank test was used. Associations between marker concentrations and between the average levels of fluid analytes and motor rating scores were investigated by the Spearman rank correlation coefficient and linear regression. Because of the explorative character of this study, the results of the statistical tests should not be interpreted as confirmatory: all results of statistical tests have to be interpreted as hypothesis generating only. No adjustment for multiple testing was done. A two-sided p value ≤ 0.05 was interpreted as statistically significant. Statistical analysis was performed with the software GraphPad Prism 8.
Results
Patient characteristics
For a summary of patient (and control) characteristics at baseline, see Table 1.
No difference in age (Mann–Whitney test p = 0.3164) and sex (Fisher’s exact test p = 0.373) between the SMA patient group and the control group was observed.
Median HFMSE in all SMA patients (N = 46) at baseline (T1) was 8 (IQR 2.75–28.5, range 0.0–66.0), after 6 months of treatment (T5, N = 41) HFMSE was 7 (IQR 4.0–33.0, range 0.0–66.0), after 10 months of treatment (T6, N = 24) HFMSE was 9 (IQR 0.0–40.75, range 0.0–66.0) and after 14 months of treatment (T7, N = 9) HFMSE was 31 (IQR 4.0–55.5, range 0.0–66.0).
Median ALSFRS-R in all SMA patients (N = 46) at baseline (T1) was 33 (IQR 27.0–37.25, range 16.0–48.0), after 6 months of treatment (T5, N = 41) ALSFRS-R was 33 (IQR 27.0–38.0, range 16.0–48.0), after 10 months of treatment (T6, N = 24) ALSFRS-R was 33 (IQR 23.5–40.0, range 16.0–48.0) and after 14 months of treatment (T7, N = 9) ALSFRS-R was 36 (IQR 26.5–44.0, range 20.0–48.0).
It should be pointed out here, that this study does not primarily address the evaluation of efficacy of nusinersen in these patients. In addition, it should be noted that the total number of patients decreases over time, as treatment was not initiated at the same time for all patients. Since treatment of SMA type 3 patients started earlier in many centers, HFMSE score is correspondingly higher at later examination dates (e.g., T7).
Serum NfL levels at baseline
Serum NfL was expressed at low levels in SMA patients and controls and there was no difference in the serum NfL levels of SMA patients and controls (Table 1 and Fig. 1a, Con vs. SMA 2/3 p = 0.6687, vs. SMA 2 p = 0.2744, vs. SMA 3 p = 0.8501). Furthermore, there was no difference in SMA 2 and SMA 3 patients (p = 0.4993).
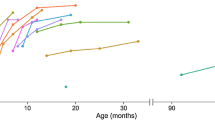
a Boxplots of serum neurofilament light chain (NfL) levels in healthy controls (Con) compared to SMA type 2 and SMA type 3 patients as well as to all SMA patients (SMA 2/3) at baseline. Given are the median concentrations, 25% and 75% percentile, and 5% and 95% whiskers; circles represent outliers. b, c Scatter plots and linear regression lines for baseline CSF NfL levels (left Y-achsis) and albumin CSF/serum ratio (Qalb, right Y-achsis) against the serum NfL concentrations for SMA 2 and SMA 3, respectively. d Serum NfL levels in individual SMA 2 (upper panel) and SMA 3 patients (lower panel) measured after the first (0 days, T1), the fifth (day 180, T5), sixth (day 300, T6), and seventh (day 420, T7) injection of nusinersen. The red lines connect the median NfL levels. e Mean serum NfL levels with SEM at T1, T5, T6, and T7 for all SMA patients and for the SMA 2 and SMA 3 subgroups. Statistical analysis (Wilcoxon test) revealed no significant changes between sampling dates except for the given one. f, g Scatter plots and linear regression lines for serum NfL against HFMSE scores (SMA 2, T5 and T6) and against ALSFRS-R scores (SMA 2/3, T1 and T6), respectively. h Higher HFMSE and ALSFRS-R scores for SMA 2/3 patients with serum NfL levels below the median (13.6 pg/ml) compared to SMA 2/3 patients with serum NfL above the median. Given are mean levels and SEM. i Comparison of serum NfL levels in SMA 2/3 patients who improved (score change > 0) from T1 to T5 and T1 to T7 with serum NfL levels in patients who showed no increase in motor scores (score change ≤ 0) from T1 to T5 or T1 to T7. Given are mean levels and SEM
Serum NfL did not correlate with the SMA patients’ age of life (SMA 2/3: ρ = 0.1989, p = 0.1851). Also, we saw no correlation for SMA patients of serum NfL with Qalb (N = 45) (ρ = − 0.1602, p = 0.293) or CSF NfL (N = 25) (ρ = 0.3021, p = 0.1422). Similar results were obtained for the SMA 3 subgroup (serum NfL vs CSF NfL: ρ = 0.8192, p = 0.8192, vs. Qalb: ρ = − 0.2352, p = 0.2473) (Fig. 1b), and for Qalb for the SMA 2 subgroup (ρ = 0.1276, p = 0.6027). CSF and serum NfL levels, however, were positively correlated in SMA 2 (ρ = 0.8167, p = 0.018) (Fig. 1c).
[Since CSF pNfH levels in SMA patients were mostly below the detection limit of the assay (median pNfH 63 pg/ml) (17), no further correlation to serum NfL values was calculated].
Serum NfL levels within the SMA group after injections of nusinersen
Serum samples after five injections of nusinersen (T5) were available from 41 patients, a second follow-up serum sample after six injections (T6) was available from 24 and a third follow-up (T7) sample from 9 SMA patients.
For one SMA 3 patient who had 20 pg/ml serum NfL at baseline, we determined a very high level of 218 pg/ml at T5 (Fig. 1d, lower panel). Re-examination of the sample confirmed high level of NfL (193 pg/ml). A closer look at the patient’s clinical characteristics revealed no noteworthy features. The patient T5 measure was excluded as an outlier from the following calculations.
We found that NfL correlated with the age of SMA patients at T5 (ρ = 0.6328, p < 0.0001) as well as T6 (ρ = 0.5649, p = 0.004).
Looking at the individual changes of the serum NfL level revealed substantial heterogeneity for both SMA 2 (Fig. 1e) and SMA 3 (Fig. 1d).
Compared to controls, NfL levels in all SMA patients as well as in type 2 and type 3 subgroups, did not differ after five (T5) (all: p = 0.2533, SMA 2: p = 0.1108, SMA 3: p = 0.5882), six (T6) (all: p = 0.5749; SMA 2: p = 0.9885, SMA 3: p = 0.3825), or seven (all: p = 0.7343, SMA 2: p = 0.1559, SMA 3: p = 0.659) injections of nusinersen.
For all SMA patients and for the SMA 2 and SMA 3 subgroups, NfL values did not differ when comparing baseline levels with follow-up levels or levels in successive visits except for a significant decrease in the SMA 2 subgroup after five nusinersen injections (T5) (Fig. 1e, SMA 2/3: T1–T5 p = 0.086, T1–T6 p = 0.2643, T1–T7 p = 0.6523, T5–T6 p = 0.6082, T6–T7 p = 0.9102; SMA 2: T1–T5 p = 0.011, T1–T6 p = 0.1934, T1–T7 p = 0.5, T5–T6 p > 0.9999, T6–T7 p = 0.5, SMA 3: T1–T5 p = 0.9346, T1–T6 p = 0.7148, T1–T7 p = 0.4375, T5–T6 p = 0.6257, T6–T7 p > 0.9999).
Association of serum NfL with HFMSE and ALSFRS-R in SMA patients
Serum NfL levels were negatively associated with SMA patients’ motor performance. Spearman analysis of serum NfL in relation to HFMSE for SMA 2/3 showed a correlation at baseline (ρ = − 0.33, p = 0.0251) and after six injections with nusinersen (ρ = − 0.5252, p = 0.0084). Looking at SMA 2 and SMA 3 separately, we saw a significant correlation at T5 and T6 (ρ = − 0.5959, p = 0.0133 and ρ = − 0.8636, p = 0.0024, shown as example in Fig. 1f) and at T1 and T5 (ρ = − 0.4085, p = 0.0383 and ρ = − 0.4949, p = 0.0163, respectively. Plotting NfL against HFMSE however, showed that the correlation analysis results are notably influenced by the significant number of patients with low (> 10) HFMSE and consequently linear regression analyses failed to be significant with the exception of significant results for SMA 2 at T5 and T6 (Fig. 1f).
Correlation analysis for NfL and ALSFRS-R revealed a significant association at T6 for SMA 2/3 (ρ = − 0.5367, p = 0.0069) and also for SMA 2 (ρ = − 0.6973, p = 0.0301) and SMA 3 (ρ = − 0.5815, p = 0.0317) subgroups. At baseline, NfL correlated with ALSFRS-R for all SMA patients and for SMA 2 (ρ = − 0.4031, p = 0.005 and ρ = − 0.5593, p = 0.0103, respectively). At T5, higher NfL correlated with lower ALSFRS-R scores for SMA 3 (ρ = − 0.459, p = 0.0276). Linear regression was significant for SMA 2/3 and SMA 3 at T1 (r2 = 0.1551, p = 0.0068 and r2 = 0.1819, p = 0.1819) and T6 (r2 = 0.1785, p = 0.0397 and r2 = 0.3352, p = 0.0301) and for SMA 2 at T1, T5, and T6 (r2 = 0.2723, p = 0.0183; r2 = 0.2381, p = 0.0469; r2 = 0.5206, p = 0.0185, respectively). For an example graph, see Fig. 1g.
Dividing SMA patients according to their serum NfL levels (above median, below median) and comparing HFMSE and ALSFRS-R scores showed that higher levels of serum NfL were associated with a worse mean performance and vice versa (Fig. 1h).
We analyzed also whether there is an association of changes in serum NfL and changes in the motor scores, or whether NfL levels correlate with future change of motor scores, and found no significant correlation. However, looking at those patients who improved within the observation period, we found that they had lower levels of serum NfL at baseline compared to the patients who showed no increase in motor scores (Fig. 1i).
Discussion
We investigated NfL in serum of adolescent and adult later-onset SMA patients at baseline and during 14 months of treatment with the ASO nusinersen using the ultrasensitive single molecule array (Simoa) technology.
Serum NfL levels of SMA patients were in the low range of controls and were relatively stable during therapy with nusinersen with notable concentration changes in single SMA patients, however, within the control range. These results are contrary to current data on Nf in children with SMA type 1 [14,15,16], but consistent with our previous findings of Nf in CSF in adolescent and adult SMA type 2 and 3 patients. We speculated if in SMA, NfL (ratio) might be higher in the blood than in the CSF compartment, however, our data suggest that the performance of serum NfL in adult and adolescent SMA type 2 and 3 patients is similar to that of CSF NfL, although we found no correlation between serum NfL and CSF NfL levels (except for the SMA 2 subgroup). In SMA type 1 children, extremely high plasma pNfH levels were described with median values of 15,400 pg/ml (range 2390–50,100 pg/ml), which are in contrast to the low serum NfL levels in adolescent and adult type 2 and 3 SMA patients, even taking into account, that different Nf proteins were measured in the blood compartment ((pNfH vs NfL), longer half-life of pNfH compared to NfL as phosphorylation leads to degradation resistance [28]) and different assays (enzyme-linked lectin assay vs Simoa) were used [16].
Higher serum NfL levels were associated with worse motor performance of SMA patients in our study, but changes in motor scores did not correlate with changes in serum NfL. Contrary to these results, our previous findings showed elevated CSF NfL (> 1000 pg/ml) at baseline in two adult SMA type 3 patients with comparatively well-preserved motor function [17]. However, these results do not inevitably exclude each other, as all measured serum NfL levels in SMA patients were within the range of controls and we saw no baseline NfL value in SMA patients above the cut-off level of 45 pg/ml, which was defined according to Steinacker et al. [27] and Verde et al. [13] to distinguish between elevated and non-elevated NfL levels in serum. In addition, we also found no relation between changes in CSF Nf levels and changes in motor scores in our previous study [17]. Again, these findings are in contrast to findings in SMA type 1 children, which showed an inverse correlation of plasma pNfH levels and markers of disease severity (e.g., measured with the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-Intend) [29]) [16].
Thus, we assume that performance of Nf levels seems to differ between SMA subtypes. It is of note, that elevated Nf in infantile-onset SMA may to some extend also be caused by physiological mechanism like programmed cell death and reinnervation processes during neuromuscular maturation in early childhood [16, 30, 31]. In addition, there are currently no data of Nf levels in patients with later-onset SMA in the early stages of the disease. However, our data show that Nf release is reduced in chronical stages of later-onset SMA compared to the acute stage of infantile-onset SMA. In line with our data, low serum NfL levels were also found in spinal and bulbar muscular atrophy (SBMA), a genetic, adult-onset, slow progressive LMN disease [32] and even in ALS, NfL are lower in patients with slow disease progression compared to patients with fast progression [33]. However, even if later-onset SMA in adolescent and adult patients shows a slower course of disease, progressive muscle weakness and loss of motor function occur in all SMA types and all ages [34]. Thus, serum NfL levels do not seem to be a suitable diagnostic and monitoring marker in these patients as neurodegeneration is not displayed by altered NfL levels, and changes in motor scores during nusinersen therapy did not correlate with changes in NfL levels in short-term observation. Though, long-term follow-up investigations may represent a considerable subject for future studies to examine whether even low levels of NfL may represent a valuable prognostic marker in adolescent and adult SMA type 2 and 3 patients.
It is of note that we solely focused on NfL in this study, but not on other potential biomarkers such as the SMN protein or the SMN transcript, respectively, skeletal muscle parameters, tau protein as a further marker of neuronal loss or glial markers. Regarding treatment monitoring, determination of full-length SMN protein and transcript levels would be obvious; but alterations in peripheral blood or CSF during therapy may not reflect the real effect in the target tissues (e.g., spinal cord). Skeletal muscle parameters e.g. creatine kinase (CK) indicating muscle damage subsequently to neuronal loss may be interesting regarding their therapy monitoring performance particular in SMA type 3 patients as the highest CK levels in SMA were detected in this patient group [35]. Although tau is considered to be a predominantly cortical marker, elevated concentrations in CSF were found in SMA type 1 children [14, 15]. If this might indicate that children with SMA may also suffer from cortical neuronal damage as suggested and whether this is true for adolescent and adult later-onset SMA patients as well, needs further investigation. The same applies for glial proteins such as the glial fibrillary acidic protein (GFAP) which could provide information on whether increased astrogliosis is associated with SMA [15].
Conclusion
We have to conclude that the diagnostic and monitoring performance of serum NfL measurement seems to differ between SMA subtypes. Unlike to SMA type 1, in adolescent and adult SMA type 2 and 3 patients, neurodegeneration is not reflected by increased NfL levels and short-term therapeutic effects cannot be observed. Long-term follow-up has to be performed to see if even low levels of NfL might represent valuable prognostic markers in these patients.
References
Lunn MR, Wang CH (2008) Spinal muscular atrophy. Lancet 371(9630):2120–33
De Sanctis R, Coratti G, Pasternak A, Montes J, Pane M, Mazzone ES et al (2016) Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord 26(11):754–759
Rudnik-Schöneborn S, Hausmanowa-Petrusewicz I, Borkowska J, Zerres K (2001) The predictive value of achieved motor milestones assessed in 441 patients with infantile spinal muscular atrophy types II and III. Eur Neurol 45(3):174–181
Ogino S, Wilson RB (2002) Genetic testing and risk assessment for spinal muscular atrophy (SMA). Hum Genet 111(6):477–500
Wurster CD, Ludolph AC (2018) Nusinersen for spinal muscular atrophy. Ther Adv Neurol Disord 11:1756285618754459
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW et al (2017) Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 377(18):1713–1722
Yuan A, Rao MV, Veeranna KD, Nixon RA (2012) Neurofilaments at a glance. J Cell Sci 125(14):3257–3263
Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL (1987) Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci USA 84(10):3472–3476
Kriz J, Zhu Q, Julien JP, Padjen AL (2000) Electrophysiological properties of axons in mice lacking neurofilament subunit genes: disparity between conduction velocity and axon diameter in absence of NF-H. Brain Res 885(1):32–44
Skillbäck T, Farahmand B, Bartlett JW, Rosén C, Mattsson N, Nägga K et al (2014) CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 83(21):1945–1953
Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM et al (2016) Neurofilaments in the diagnosis of motoneuron diseases: A prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87(1):12–20
Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, Van Damme P et al (2018) Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 90(1):e22–30
Verde F, Steinacker P, Weishaupt JH, Kassubek J, Oeckl P, Halbgebauer S et al (2019) Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 90(2):157–164
Winter B, Guenther R, Ludolph AC, Hermann A, Otto M, Wurster CD (2019) Neurofilaments and tau in CSF in an infant with SMA type 1 treated with nusinersen. J Neurol Neurosurg Psychiatry 90(9):1068–1069
Olsson B, Alberg L, Cullen NC, Michael E, Wahlgren L, Kroksmark A-K et al (2019) NFL is a marker of treatment response in children with SMA treated with nusinersen. J Neurol 266(9):2129–2136
Darras BT, Crawford TO, Finkel RS, Mercuri E, De Vivo DC, Oskoui M et al (2019) Neurofilament as a potential biomarker for spinal muscular atrophy. Ann Clin Transl Neurol 6(5):932–944
Wurster CD, Günther R, Steinacker P, Dreyhaupt J, Wollinsky K, Uzelac Z et al (2019) Neurochemical markers in CSF of adolescent and adult SMA patients undergoing nusinersen treatment. Ther Adv Neurol Disord 12:1756286419846058. https://doi.org/10.1177/1756286419846058
Brettschneider J, Petzold A, Süßmuth SD, Ludolph AC, Tumani H (2006) Axonal damage markers in cerebrospinal fluid are increased in ALS. Neurology 66(6):852–856
Menke RAL, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A et al (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann Clin Transl Neurol 2(7):748–755
Otto M, Bowser R, Turner M, Berry J, Brettschneider J, Connor J et al (2012) Roadmap and standard operating procedures for biobanking and discovery of neurochemical markers in ALS. Amyotroph Lateral Scler 13(1):1–10
Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L et al (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol 28(6):595–599
Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG et al (2016) The Simoa HD-1 Analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom 21(4):533–547
Jesse S, Brettschneider J, Süssmuth SD, Landwehrmeyer BG, Von Arnim CAF, Ludolph AC et al (2011) Summary of cerebrospinal fluid routine parameters in neurodegenerative diseases. J Neurol 258(6):1034–1041
O’Hagen JM, Glanzman AM, McDermott MP, Ryan PA, Flickinger J, Quigley J et al (2007) An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromuscul Disord 17(9–10):693–697
Glanzman AM, O’Hagen J, McDermott M, Martens W, Flickinger J, Riley S et al (2011) Validation of the expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol 26(12):1499–1507
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B et al (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function: BDNF ALS study group (phase III). J Neurol Sci 169(1–2):13–21
Steinacker P, Huss A, Mayer B, Grehl T, Grosskreutz J, Borck G et al (2017) Diagnostic and prognostic significance of neurofilament light chain NF-L, but not progranulin and S100B, in the course of amyotrophic lateral sclerosis: data from the German MND-net. Amyotroph Lateral Scler Frontotemporal Degener 18(1–2):112–119
Lee S, Sunil N, Shea TB (2011) C-terminal neurofilament phosphorylation fosters neurofilament-neurofilament associations that compete with axonal transport. Cytoskeleton 68(1):8–17
Glanzman AM, Mazzone E, Main M, Pelliccioni M, Wood J, Swoboda KJ et al (2010) The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability The CHOP INTEND is a reliable measure of motor skills in patients with SMA-I and neuromuscular disorders presenting in infancy. Neuromuscul Disord 20(3):155–161
Oppenheim RW (1989) The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci 12(7):252–255
Pérez-García MJ, Kong L, Sumner CJ, Tizzano EF (2016) Developmental aspects and pathological findings in spinal muscular atrophy. In: Spinal muscular atrophy: disease mechanisms and therapy. Elsevier Inc. pp 21–42
Lombardi V, Querin G, Ziff OJ, Zampedri L, Martinelli I, Heller C et al (2019) Muscle and not neuronal biomarkers correlate with severity in spinal and bulbar muscular atrophy. Neurology 92(11):E1205–E1211
Poesen K, Van Damme P (2018) Diagnostic and prognostic performance of neurofilaments in ALS. Front Neurol 9:1167
Wadman RI, Wijngaarde CA, Stam M, Bartels B, Otto LAM, Lemmink HH et al (2018) Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c–4. Eur J Neurol 25(3):512–518
Rudnik-Schoneborn S, Lutzenrath S, Borkowska J, Karwanska A, Hausmanowa-Petrusewicz I, Zerres K (1998) Analysis of creatine kinase activity in 504 patients with proximal spinal muscular atrophy types I–III from the point of view of progression and severity. Eur Neurol 39(3):154–162
Acknowledgements
The authors thank all patients for participating in this study. Thanks for technical assistance to Dagmar Schattauer, Sandra Hübsch, Alice Pabst and Mehtap Bulut-Karac.
Funding
The study was supported in part by grants from the German Federal Ministry of Education and Research [project FTLDc 01GI1007A, MND-Net 01GI0704]; PreFrontAls [01ED1512]; the ALS association; the Thierry Latran Foundation; and the Charcot Foundation for ALS Research. AH is supported by the Hermann und Lilly Schilling-Stiftung.
Author information
Authors and Affiliations
Consortia
Contributions
CDW design and conceptualized study. Major role in the acquisition of data. Analyzed the data. Wrote manuscript. PS design and conceptualized study. analyzed serum samples. Performed statistical analysis. Wrote manuscript. RG design and conceptualized study. Major role in the acquisition of data. Revised the manuscript for intellectual content. JCK major role in the acquisition of data. Revised the manuscript for intellectual content. PL Revised the manuscript for intellectual content. ZU major role in the acquisition of data. SW major role in the acquisition of data. KW major role in the acquisition of data. BW revised the manuscript for intellectual content. AO major role in the acquisition of data. OS-K major role in the acquisition of data. RAS analyzed serum samples. ACL design and conceptualized study. Interpreted the data. Revised the manuscript for intellectual content. SP major role in the acquisition of data. Revised the manuscript for intellectual content. AH design and conceptualized study. Revised the manuscript for intellectual content. MO design and conceptualized study. Analyzed and interpreted the data. Revised the manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: CDW has received honoraria from Biogen as an Advisory board member and for lectures and as a consultant from Hoffmann-La Roche. She also received travel expenses from Biogen. PS, SW, KW and MRAS report no disclosures. RG has received honoraria from Biogen as an Advisory board member. JCK has received financial research support form TEVA Pharmaceuticals and honoraria as speaker/consultant for AbbVie, Allergan, Biogen, Ipsen and AveXis/Novartis. PL has received financial research support from TEVA Pharmaceuticals and honoraria as speaker/consultant for AbbVie, Atheneum Partners, BIAL, Desitin, Licher MT, Medtronic, Novartis. ZU has received honoraria from Biogen as a consultant. BW has received honoraria from Biogen for a lecture. AO has received honoraria from Biogen as a consultant. OSK has received honoraria as a speaker/consultant and/or funding for travel expenses from the Deutsche Gesellschaft für Muskelkranke (DGM e.V.), Novartis, Biogen GmbH, the Jain Foundation. She received academic research support by Ludwig-Maximilians-University of Munich, FoeFoLe program, Reg.-No. 747, 11/11–05/13, and MHH Young Faculty Program, 2018–2020 as well as research support by foundations and societies by the Friedrich-Baur-GmbH, Burgkunstadt 12/13–12/15 and the Deutsche Gesellschaft für Muskelkranke (DGM e.V.), 2019–20. ACL received financial research support from AB Science, Biogen Idec, Cytokinetics, GSK, Orion Pharma, Novartis, TauRx Therapeutics Ltd. and TEVA Pharmaceuticals. He also has received honoraria as a consultant from Mitsubishi, Orion Pharma, Novartis, Teva and as an Advisory board member of Biogen and Hoffmann-La Roche. SP has received honoraria as speaker/consultant from Biogen Idec, Novartis, Cytokinetics, TEVA Pharmaceuticals, Desitin. AH has received honoraria from Biogen and Desitin as a consultant. MO received honoraria as consultant from Biogen, Axon and Fujirebio.
Ethical standards
The study was approved by the local ethics committees of the centers involved in Ulm, Dresden, Göttingen and Hannover (approval number at central study center at Ulm University 19/12; 2012) and all patients or their relatives (legal guardian) gave informed written consent to participate in the study.
Rights and permissions
About this article
Cite this article
Wurster, C.D., Steinacker, P., Günther, R. et al. Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J Neurol 267, 36–44 (2020). https://doi.org/10.1007/s00415-019-09547-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09547-y