Abstract
Background
Spinal cord (SC) involvement correlates with poor prognosis in patients with multiple sclerosis (MS). Nevertheless, there is no consensus on the use of SC-MRI at follow-up, mainly because of the belief that SC lesions are nearly always symptomatic.
Objectives
The aim of the present study was to investigate the frequency of asymptomatic SC combined unique activity (CUA, new/enlarging T2 or gadolinium-positive [Gd+] lesions) on MRI in a cohort of patients diagnosed with clinically isolated syndrome (CIS) or relapsing–remitting MS (RRMS).
Methods
We retrospectively investigated all scans showing SC-CUA in patients with CIS or RRMS referred to a single Italian MS centre. We determined whether they were symptomatic and whether they had associated brain radiological activity.
Results
In 340 SC-MRI scans with SC-CUA (230 patients), SC-CUA was asymptomatic in 31.2%; 12.1% of SC-CUA had neither clinical activity nor brain radiological activity (44.5% and 25.4%, respectively, considering only follow-up SC-CUA). At multivariate analysis asymptomatic SC-CUAs were associated with older age at onset (34.0 ± 10.37 vs 31.0 ± 9.99 years, p = 0.006), non-spinal onset (76.4 vs 47.4%, p < 0.001), lower EDSS score at MRI (1.8 ± 0.93 vs 2.4 ± 1.28, p = 0.001) and lower number of Gd+ SC lesions (0.1 ± 0.33 vs 0.3 ± 0.54, p = 0.04), compared to symptomatic SC-CUAs.
Conclusions
A substantial proportion of our patients had SC-CUA without clinical symptoms and/or without concomitant brain MRI activity. In these patients, SC-CUA was the only sign of disease activity, suggesting that regular SC-MRI follow-up is required for reliable assessment of radiological activity and may improve the management of patients with MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinal cord (SC) involvement is a fundamental aspect of multiple sclerosis (MS) pathology and its relevance both as a diagnostic pillar and a prognostic factor is well recognized. The presence of SC lesions (SCLs), both in the early phase and during the disease course, increases the risk of disability progression and evolution to the secondary progressive stage [1,2,3,4]. Furthermore, higher baseline SCL load significantly increases the risk of conversion to clinically definite MS and long-term disability in patients with clinically isolated syndrome (CIS) or radiologically isolated syndrome [5, 6]. Therefore, recent guidelines recommend MRI imaging of the whole spinal cord (SC-MRI) at diagnosis, especially in patients who do not fulfil brain MRI criteria for dissemination in space [7, 8]. However, the role of SC-MRI in evaluating disease evolution and response to disease-modifying treatments (DMTs) is not well established. This is due partially to technical limitations in SC visualization and to economical constraints. In addition, whereas new brain lesions are often clinically silent and correlate well with future relapse risk [9], many MS experts consider the occurrence of asymptomatic new SCLs to be rare. Therefore, SC-MRI is not recommended for routine follow-up in MS [7, 8, 10]. Currently, the importance of asymptomatic SCLs during MS monitoring is debated [11,12,13,14,15].
The aim of the present study was to determine the frequency of asymptomatic SC combined unique activity (SC-CUA), defined as the occurrence of new/enlarging T2 or gadolinium-positive (Gd+) SCLs, with or without concomitant brain MRI activity, among all scans with SC-CUA in a cohort of relapsing–remitting (RR) MS patients at a single MS centre, and identify clinical and radiological characteristics associated with asymptomatic SC-CUA.
Methods
Study design
This was a single centre, observational, retrospective study.
Patients and methods
We considered all available MRI scans performed on a 3 T MR scanner in the Parma University Hospital on patients with a diagnosis of CIS or RRMS according to McDonald criteria [16] referred to the Parma MS centre from disease onset onward. SC and corresponding brain MRIs were included if the SC-MRI scans exhibited at least 1 CUA, defined as new/enlarging T2 or Gd+ SCLs compared to previous SC-MRI or at disease onset. SC-CUA was classified as symptomatic when associated with Expanded Disability Status Scale (EDSS) worsening suggestive of SC involvement or a relapse (excluding relapses clearly not involving the SC, such as optic neuritis or brain stem symptoms). Patients with incomplete data were excluded.
We collected clinical and radiological data associated with each SC-CUA scan and compared the data from patients who were symptomatic vs asymptomatic when the scan was performed, to identify possible predictors of active SCLs that were “clinically silent”.
We obtained local ethics committee approval, in accordance with the ethical standards stated in the 1964 Declaration of Helsinki and its later amendments. Each patient gave written informed consent prior to participation.
Data collection
All data were prospectively recorded in electronic medical records (iMED 6.1). An ad hoc questionnaire was compiled for each MRI scan that showed evidence of SC-CUA, including demographic information (age, sex), clinical data (age at MS onset, MS type, disease duration, EDSS, MS onset location, ongoing DMT, number of relapses and EDSS worsening suggestive of SC involvement since previous SC-MRI or since disease onset for first scans) and radiological features (number, location and extension of new/enlarging T2 or Gd+ SCLs, number of new/enlarging T2 or Gd+ brain lesions at a brain MRI scan performed ± 6 months from the SC imaging). All data were retrospectively reported in an electronic database.
Neurological examination was performed at baseline, then at least every 6 months, rating the EDSS score and collecting data about the occurrence of new relapses. Relapses were defined as “acute or subacute episodes of new or increasing neurologic dysfunction followed by full or partial recovery, in the absence of fever or infection”, lasting ≥ 24 h based on history and neurological examination [17]. EDSS worsening was defined by a ≥ 0.5-point increase in EDSS score from previous SC-MRI or MS onset. We considered only relapses or EDSS worsening that was suggestive of SC involvement, excluding relapses clearly not involving the SC.
All included MRI scans were performed using a 3 T MR scanner in Parma University Hospital, following a protocol in accordance with the guidelines from The Italian Neurological and Neuroradiological Societies [18]. All exams were reviewed by two experienced neuroradiologists (G.C. and S.G.), who were blinded to the patients’ identity and clinical features.
MRI protocol
The 3 T MR scanner (Discovery MR750; GE Healthcare, Milwaukee, WI) was equipped with a 4-channel phased-array spine coil. The SC-MRI protocol for MS included: Sagittal two-dimensional (2D) T2-weighted fast spin echo, Axial 2D T2*-weighted MERGE (“Multiple Echo Recombined Gradient-Echo”) images and Dixon sequences for high-resolution Sagittal and Axial 2D T1-weighted fat-suppressed images following contrast agent administration. For the same signal-to-noise ratio, the voxel size was 0.4 × 0.4 × 2.5 mm in the sagittal plane and 0.3 × 0.3 × 2.5 mm in the axial plane.
The brain MRI protocol for MS included: Sagittal three-dimensional (3D) CUBE fluid attenuated inversion recovery (FLAIR) and double inversion recovery (DIR) T2-weighted sequences, Axial GRE T2*weighted images, Axial diffusion weighted images (DWI), Axial FSE T1-weighted pre and post-Gd images. Voxel sizes for 3D images were between 1.0 and 1.2 mm and for 2D images were of 2.0 mm.
T2-hyperintense and contrast enhancing SCLs were counted on Axial T2-weighted MERGE images, on Sagittal T2-weighted images and on post-Gd Sagittal and Axial T1-weighted images.
All brain and SC lesions were counted on the images of the first examination and confirmed by a second senior observer (GC). All follow-up examinations were reviewed with the same method for new or enlarging lesions. Significant artefacts were excluded before lesion counting.
SC-CUA was defined as new/enlarging T2 or Gd+ SCLs.
Outcomes
-
Frequency of asymptomatic (aSC+) and symptomatic (sSC+) SC-CUA among all scans with evidence of SC-CUA
-
Frequency of asymptomatic (aSC+B−) and symptomatic (sSC+B−) SC-CUA without concomitant brain-CUA at MRI.
Statistical analysis
Descriptive statistics was used in reporting demographic and clinical data, and the radiological characteristics of all SC-CUAs (the number of new/enlarged T2 and Gd+ lesions, proportion of scans including at least 1 lesion with longitudinal extension ≥ 3 segments, proportion of scans including at least 1 lesion with transversal involvement of > 1 spinal column, frequency of involvement of cervical and thoracic regions, number of new/enlarging T2 or Gd+ lesions on concomitant brain MRI).
We also investigated the frequency of asymptomatic (aSC+) and symptomatic (sSC+) SC-CUA and the frequency of asymptomatic (aSC + B−) and symptomatic (sSC+ B−) SC-CUA without concomitant brain MRI activity. We reported mean ± SD age, disease duration and EDSS at MRI and mean ± SD number of relapses since previous SC-MRI or since MS onset. For patients who had multiple MRI scans, we used the mean of continuous variables that change at every scan (i.e., age, disease duration, EDSS, number of relapses since previous MRI or MS onset). Sex, mean age at MS onset and anatomical location of MS onset (spinal vs non-spinal) were analysed for all patients.
Additional variables were analysed for all scans with SC-CUA and for scans from patients experiencing a first SC-CUA. The frequency of aSC+, sSC+, aSC+ B− and sSC+ B- was also calculated on the subgroup of follow-up scans with more than 1 SC-CUA from patients who had a follow-up MRI.
Univariate and multivariate analyses were performed with generalized linear models using a generalized estimating equation to compare demographic and clinical features between the two groups of aSC + and sSC + ; only variables that were significantly different or showed a trend at univariate analysis (mean age at MRI, mean age at MS onset, mean EDSS score, anatomical location of MS onset, SC regions involvement (cervical or thoracic vs both), number of new/enlarging T2 and of Gd-enhancing SCLs) were considered in the multivariate analysis.
The same analysis was conducted on the group of RRMS patients (excluding CIS) as a sensitivity analysis.
All the analyses were performed using the statistical package SPSS IBM version 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.); a significance level of 0.05 was considered.
Results
SC-CUA: clinical and demographic features
We identified 340 scans with SC-CUA (as defined) in 230 predominantly female patients with mean ± SD age at MRI of 37.7 ± 10.61 years, 93.8% of scans were from patients who had a RR course and 60.6% were receiving DMT. Demographic and clinical characteristics are described in Table 1. We registered EDSS worsening since previous SC-MRI in 27.1% of cases (almost all associated with relapses); at MRI examination 50.6% were receiving a first-line DMT (interferon beta, glatiramer acetate, teriflunomide or dimethylfumarate), 10.0% a second line (natalizumab, fingolimod, alemtuzumab or immunosuppressants), while 39.4% were not receiving a DMT.
The results were similar when only the first scans with SC-CUA (n = 230) were considered: 91.7% of cases had a RR course, while 8.3% were CIS; we registered EDSS worsening since previous SC-MRI in 26.5% of cases; at MRI examination 47.8% of cases were receiving a first-line DMT (interferon beta, glatiramer acetate, teriflunomide or dimethylfumarate), 6.5% a second line (natalizumab, fingolimod, alemtuzumab or immunosuppressants), while 45.7% were not receiving a DMT.
Our sample of 230 patients included 141 who had only 1 SC-CUA MRI scan, 70 patients with 2 scans, 18 patients with 3 scans, while only 1 patient had 5 SC-CUA scans. In those patients who presented more than 1 scan with SC-CUA (n = 89), the mean time interval between the first and the second MRI was 35.99 ± 38.73 months.
SC-CUA: radiological features
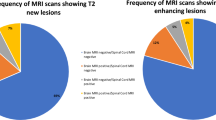
We found new/enlarging T2 SCLs in 99.8% of the 340 SC-CUA scans (55.9% with 1 lesion, 20.9% 2 lesions, 10.9% 3 lesions, 5.3% 4 lesions, 3.8% 5 lesions, 1.8% 6 lesions and 1.2% with more than 6 lesions). Gd enhancement was present in 20.0% of cases (n = 68), 18.2% with 1 lesion, while only 3 cases with more than 1 lesion (Fig. 1). All Gd-enhancing SCLs were new/enlarged T2 lesions, while we did not observe any case of reactivation of pre-existing spinal lesion.
We found cervical SC involvement in 68.8%, thoracic in 13.8% and both cervical and thoracic in 17.4% of cases.
SCLs were found to extend at least 3 vertebral segments in 5% of cases and involved more than 1 of the SC white-matter columns in 48.5% of scans.
Similar results were found when only the first SC-CUA scans (n = 230) were considered (data not shown).
Brain MRI characteristics
Among the 340 concomitant brain MRI scans, only 215 (63.2%) were considered active (defined by the presence of at least one new/enlarged T2 or Gd+ lesion), of which 203 (94.4%) presented at least 1 new/enlarged T2 lesion and 64 (29.8%) at least 1 Gd+ lesion. Only 12 MRI scans (5.6%) showed at least 1 Gd+ lesion without new/enlarged T2 lesions.
Similar results were found when only the first SC-CUA scans (n = 230) were considered (data not shown).
SC-CUA vs brain-CUA
In a substantial proportion of the 340 scans with SC-CUA, brain MRI showed inflammatory activity as well (n = 215, 63.2%); whereas 41 of the remaining 125 cases in which brain MRI was stable (32.8%) had asymptomatic SC-CUA. Therefore 12.1% (41/340) of cases with inflammatory activity were detected only through SC-MRI follow-up (Fig. 2).
Considering only RRMS patients (n = 319) the results were very similar (data not shown).
Considering only follow-up MRI in patients with more than 1 scan showing SC-CUA (n = 110), 42.7% (n = 47) of the corresponding brain MRIs were active as well. Among the remaining 63 cases of SC-CUA with inactive brain MRI, 44.4% (28/63) were asymptomatic. Therefore, in this subgroup of scans we found a higher proportion of MS disease activity (25.4%) that was detectable only with SC-MRI follow-up compared to previous analysis.
Symptomatic vs asymptomatic SC-CUA
Among all 340 scans showing SC-CUA, 106 (31.2%) were asymptomatic, while the 234 symptomatic ones were associated with relapses (97%) or EDSS worsening (3%).
Considering only follow-up MRI scans in patients with more than 1 scan showing SC-CUA, a higher proportion of SC-CUAs were asymptomatic (44.5%, 49/110).
At univariate analysis, comparing symptomatic and asymptomatic SC-CUAs, we found that symptomatic cases were younger at MS onset (31.0 ± 9.99 vs 34.0 ± 10.37 years, p = 0.01), with a trend toward lower age at MRI (36.6 ± 10.7 vs 38.9 ± 9.88 years, p = 0.052), had more myelitis at onset (52.6% vs 23.6%, p < 0.001), and a higher mean EDSS score (2.4 ± 1.28 vs 1.8 ± 0.93, p < 0.001). Scans from patients who were symptomatic had a higher number of new/enlarging T2 (2.1 ± 1.54 vs 1.6 ± 1.07, p = 0.004) and of Gd+ SCLs (0.3 ± 0.54 vs 0.1 ± 0.33, p = 0.002) and were more likely to have involvement of both cervical and thoracic regions (21.8 vs 7.5%, p = 0.002).
Multivariate analysis confirmed most of these results (Table 2). In particular, SC-CUA was more frequently asymptomatic in patients with older age at MS onset and who presented with a non-spinal CIS at onset, while a higher EDSS and a higher number of Gd-enhancing SCLs were more often associated with clinical activity.
A sensitivity analysis conducted on the subgroup of RRMS patients (n = 319) showed similar results (Online Resource 1).
Discussion
In our sample of 340 SC-CUA scans, 31.2% were from clinically asymptomatic patients, despite radiologically documented re-exacerbation of inflammation, while 36.8% did not have concomitant brain-CUA and 12.1% were both asymptomatic and lacked brain-CUA. These percentages increased markedly when only follow-up scans showing SC-CUA (n = 110) were considered; in particular, 44.5% of SC-CUAs were asymptomatic and 25.4% presented neither clinical symptoms nor brain disease activity. Therefore, a substantial part of acute inflammation may go undetected without regular SC-MRI follow-up, increasing the risk of undertreatment. SC involvement is a crucial consideration when making treatment decisions, because of its correlation with disease progression and long-term disability [1,2,3,4]. Inflammatory activity detected at MRI constitutes a substantial part of all prognostic scores elaborated in recent years, although they focus mainly on brain MRI [9, 19].
Despite the wide acceptance of SCL load as a negative prognostic factor, MRI guidelines recommend SC-MRI only at diagnosis, mainly because many MS experts consider asymptomatic new SC lesions to be rare [7, 8]. Whereas new brain lesions are often clinically silent, new SC involvement is generally thought to be associated with new symptoms or changes at neurological examination because of the dense, highly organised anatomical structure of the SC. Moreover, evidence for the correlation between MRI activity and future relapse risk is well consolidated only for brain lesions [9, 19]. Some authors question the quality of SC-MRI data in the clinical practice setting, because of several technical limitations [12].
Therefore, the importance of asymptomatic SCLs during MS monitoring is debated [11,12,13,14,15], mainly because of their perceived low incidence. In a study evaluating serial brain and SC-MRIs of RRMS patients, Thorpe et al. found that about two-thirds of SCLs were asymptomatic, although less frequently than brain lesions. During a 12-month follow-up, only 10% of total active lesions were found in the SC and only 2 of the 59 had CUA only in the SC [14]. However, this study was carried out more than 20 years ago on a small population using equipment not comparable to current MR scanners.
A recent Swiss retrospective study evaluated the prognostic value of new asymptomatic SCLs, in association with the new brain lesions, as predictors of disease activity during a 2-year follow-up in 103 RRMS patients [13]. Asymptomatic new SCLs were reported in about 25% of clinically stable patients and, combined with asymptomatic new brain lesions, were associated with a higher relapse risk. About 10% of clinically silent patients had radiological disease activity only in the SC. However, this study provides only a partial picture reflecting a limited sample size of clinically stable patients.
More recently, an Italian MRI study compared the frequency of acute inflammatory activity, as Gd-enhancing lesions, in SC versus brain [15]. Of 1180 MRI scans with radiological activity, 25.2% showed only SC involvement, and 58.5% of those were asymptomatic. However, the authors considered only Gd-enhancing lesions as radiological activity, which represented only a modest part of MRI disease activity evaluated in clinical practice, as our data have shown. In the present study, nearly all scans with SC-CUA had new/enlarging T2 SCLs (99.8%), whereas only 20% had Gd-enhancing lesions. We did not observe enhancement of any pre-existing SCLs. Considering brain-CUA (n = 215), we recorded Gd-enhancement without the occurrence of new lesions in 5.6% of cases. Radiological MS activity on conventional MRI was mainly new/enlarged lesions, while only a small percentage of cases (12/555, 2.2%) showed reactivation of pre-existing lesions.
Cervical SC was the most involved region, consistent with previous reports and we found SCLs with a longitudinal extension ≥ 3 segments in 5% of cases [2, 20].
As expected, SC-CUA that was associated with higher EDSS scores or a higher number of Gd-enhancing SCLs at MRI was more likely to be symptomatic. Asymptomatic SC-CUA was associated with older age at onset and presented more frequently with non-spinal MS onset, compared to symptomatic SC-CUA. Our findings suggest that SC-MRI monitoring would be useful also in patients without SC involvement at MS onset, who are classically associated with a less aggressive course [2, 3]. Asymptomatic SCLs in CIS have been described as a negative predictor of long-term prognosis and conversion to clinically definite MS, especially in non-spinal CIS [2].
Our study has some limitations, such as its observational retrospective design, small sample size and the clinical practice setting. In addition, although all scans were performed on the same 3 T MR scanner, following a protocol in accordance with Italian Guidelines [18], technical limitations (e.g., partial volume effects, breathing and swallowing artefacts, cerebrospinal fluid and blood flow) make SC-MRI studies more challenging than brain MRI studies [12].
Our study also has strengths, such as the thorough assessment of radiological activity. Even with the limitations of conventional MRI techniques used in clinical practice, we evaluated both new/enlarged T2 and Gd-enhancing SCLs, rather than only Gd+ lesions as in some studies [15]. In addition, the availability of concomitant brain MRI scans allowed us to distinguish cases with only SC activity from those with concomitant brain activity.
In conclusion, our results show that a consistent percentage of patients with active SCLs remained asymptomatic. In some of these patients, asymptomatic SCLs represented the only sign of disease reactivation. Thus, regardless of brain MRI activity, SC-MRI should be performed at follow-up because the prognostic value of SC involvement, isolated or concomitant with brain lesions, is a crucial factor in making treatment decisions.
Our findings suggest that regular SC-MRI follow-up would allow more reliable assessment of radiological activity and improve the management of MS patients. However, further longitudinal studies to evaluate the prognostic relevance of asymptomatic SCLs are warranted to justify the additional burden associated with routine SC-MRI scans at follow-up.
References
Kearney H, Miller DH, Ciccarelli O (2015) Spinal cord MRI in multiple sclerosis—diagnostic, prognostic and clinical value. Nat Rev Neurol 11:327–338
Muccilli A, Seyman E, Oh J (2018) Spinal cord MRI in multiple sclerosis. Neurol Clin 36:35–57
Arrambide G, Rovira A, Sastre-Garriga J et al (2018) Spinal cord lesions: a modest contributor to diagnosis in clinically isolated syndromes but a relevant prognostic factor. Mult Scler 24:301–312
Brownlee WJ, Altmann DR, Alves Da Mota P et al (2017) Association of asymptomatic spinal cord lesions and atrophy with disability 5 years after a clinically isolated syndrome. Mult Scler 23:665–674
Sombekke MH, Wattjes MP, Balk LJ et al (2013) Spinal cord lesions in patients with clinically isolated syndrome: a powerful tool in diagnosis and prognosis. Neurology 80:69–75
Okuda DT, Mowry EM, Cree BA et al (2011) Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 76:686–692
Filippi M, Rocca MA, Ciccarelli O et al (2016) MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guideline. Lancet Neurol 15:292–303
Traboulsee A, Simon JH, Stone L et al (2016) Revised recommendations of the consortium of MS centers task force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol 37:394–401
Sormani MP, Bruzzi P (2013) MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 12:669–676
Wattjes MP, Rovira À, Miller D et al (2015) Evidence based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol 11:597–606
Cortese R, Ciccarelli O (2018) Clinical monitoring of multiple sclerosis should routinely include spinal cord imaging—yes. Mult Scler 24:1536–1537
Kearney H (2018) Clinical monitoring of multiple sclerosis should routinely include spinal cord imaging—no. Mult Scler 24:1537–1539
Zecca C, Disanto G, Sormani MP et al (2016) Relevance of asymptomatic spinal MRI lesions in patients with multiple sclerosis. Mult Scler 22:782–791
Thorpe JW, Kidd D, Moseley IF et al (1996) Spinal MRI in patients with suspected multiple sclerosis and negative brain MRI. Brain 119:709–714
Ruggieri S, Logoteta A, Tinelli E et al (2018) Measuring disease activity in multiple sclerosis: the essential role of spinal cord MRI monitoring. ECTRIMS Online Library. Ruggieri S 2018; 228465 (P621)
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173
Lublin FD, Reingold SC, Cohen JA et al (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83:278–286
Filippi M, Rocca MA, Bastianello S et al (2013) Guidelines from the Italian neurological and neuroradiological societies for the use of magnetic resonance imaging in daily life clinical practice of multiple sclerosis patients. Neurol Sci 34:2085–2093
Sormani MP, Gasperini C, Romeo M et al (2016) Assessing response to interferon-b in a multicenter dataset of patients with MS. Neurology 87:134–140
Eckstein C, Syc S, Saidha S (2011) Differential diagnosis of longitudinally extensive transverse myelitis in adults. Eur Neurol J 3:27–39
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
F. Granella has received research grants for his Institution from Biogen and Sanofi Genzyme; has served on scientific advisory boards for Biogen, Novartis, Sanofi Genzyme, Roche and Merck Serono; and has received funding for travel from Biogen, Merck Serono and Sanofi Genzyme. E. Tsantes served on scientific advisory boards for Roche and Merck & Co; has received funding for travel from Biogen, Merck & Co, Sanofi Genzyme and Roche V. Bazzurri has received funding for travel from Sanofi Genzyme, Biogen and Roche. E. Curti served on scientific advisory boards for Merck & Co and Novartis; has received funding for travel from Biogen, Merck & Co, Teva Pharmaceutical Industries, Sanofi Genzyme, Roche and Novartis. S. Graziuso and G. Crisi have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Granella, F., Tsantes, E., Graziuso, S. et al. Spinal cord lesions are frequently asymptomatic in relapsing–remitting multiple sclerosis: a retrospective MRI survey. J Neurol 266, 3031–3037 (2019). https://doi.org/10.1007/s00415-019-09526-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09526-3