Abstract
Vestibulo–ocular reflexes (VOR) are mediated by frequency-tuned pathways that separately transform the different dynamic and static aspects of head motion/position-related sensory signals into extraocular motor commands. Voltage-dependent potassium conductances such as those formed by Kv1.1 are important for the ability of VOR circuit elements to encode highly transient motion components. Here we describe the impact of the Kv1.1 channel blocker 4-aminopyridine (4-AP) on spontaneous and motion-evoked discharge of superior oblique motoneurons. Spike activity was recorded from the motor nerve in isolated preparations of Xenopus laevis tadpoles. Under static conditions, bath application of 1–10 µM 4-AP increased the spontaneous firing rate and provoked repetitive bursts of spikes. During motion stimulation 4-AP also augmented and delayed the peak firing rate suggesting that this drug affects the magnitude and timing of vestibular-evoked eye movements. The exclusive Kv1.1 expression in thick vestibular afferent fibers in larval Xenopus at this developmental stage suggests that the altered extraocular motor output in the presence of 4-AP mainly derives from a firing rate increase of irregular firing vestibular afferents that propagates along the VOR circuitry. Clinically and pharmacologically, the observed 4-AP-mediated increase of peripheral vestibular input under resting and dynamic conditions can contribute to the observed therapeutic effects of 4-AP in downbeat and upbeat nystagmus as well as episodic ataxia type 2, by an indirect increase of cerebellar Purkinje cell discharge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vestibulo–ocular reflex (VOR) is the dominating contributor to gaze stabilization during head/body motion (VOR) [1]. This reflex depends on the transformation of vestibular sensory signals into spatio-temporally adequate extraocular motor commands [2]. The neuronal pathway between inner ear hair cells and extraocular muscle fibers consists of frequency-tuned, parallel information channels [3]. The dynamic diversity of the respective cellular elements correlates with the necessity to encode and mediate signals over a wide range of head motion frequencies and acceleration profiles [4, 5]. Accordingly, the three-neuronal VOR pathway is composed of functional subgroups of cells with distinct intrinsic properties and response dynamics at each hierarchical level [3]. The dynamically different cell types form neuronal filters that are ideally suited for the encoding of particular temporal aspects of head/body movements, respectively [6].
Filter properties of vestibular neurons derive from specific sets of ion channels [7,8,9]. The highly transient firing dynamics of neuronal elements that comprise phasic VOR pathway components are caused by voltage-dependent potassium channels of the Kv1.1 type [9]. These channels are abundant in a particular subgroup of first- [8] and second-order vestibular neurons [6, 10]. Blocking Kv1.1 channels with low concentrations of 4-aminopyridine (4-AP) diminishes the transient response dynamics and assigns to these neurons more low-pass filter-like properties [6].
Clinically, 4-AP has been proven as potent therapeutic agent for symptoms associated with vestibular and cerebellar disorders, such as downbeat nystagmus [11,12,13], episodic ataxia type 2 [14, 15] and upbeat nystagmus [16]. The improvement of the clinical symptoms presumably derives from discharge regularization of vestibular/cerebellar circuit elements [17], potentially in combination with a general increase in firing rate. In upbeat nystagmus, 4-AP evidently acts by restoring visuo-ocular function to suppress the nystagmus [16]. Finally, in a single case with severe head-shaking nystagmus due to neurovascular compression, aminopyridine reduced the symptoms by likely improving action potential propagation including spike conduction along the vestibular nerve [18].
To decipher the neuronal substrates and reveal alterations of VOR performance at the cellular and circuit level following 4-AP administration, we used semi-intact preparations of Xenopus laevis tadpoles. The effect of the drug on discharge rate and dynamics of superior oblique (SO) motoneurons was tested at rest and during head/body motion. This allowed estimating the contribution of Kv1.1 channels to the transformation of vestibular sensory signals into extraocular motor commands.
Material and methods
Animals and experimental preparation
Xenopus laevis tadpoles of either sex (n = 21) at developmental stages 51–53 [19] were obtained from the in house animal breeding facility at the Biocenter-Martinsried of the Ludwig-Maximilians-University Munich. Tadpoles were maintained in tanks with non-chlorinated water (17–18 °C) at a 12/12 light/dark cycle. Experiments were performed in vitro on semi-intact preparations and comply with the "Principles of animal care", publication No. 86-23, revised 1985 of the National Institute of Health. Permission for these experiments was granted by the Regierung von Oberbayern (ROB-55.2-2532.Vet_03-17-24).
Tadpoles were anesthetized in 0.05% 3-aminobenzoic acid ethyl ester methanesulfonate (MS-222; Pharmaq Ltd. UK) in ice-cold frog Ringer solution (75 mM NaCl, 25 mM NaHCO3, 2 mM CaCl2, 2 mM KCl, 0.1 mM MgCl2, and 11 mM glucose, pH 7.4) and decapitated at the level of the upper spinal cord. The skin was removed, the skull opened from dorsal and the forebrain disconnected [20]. The remaining central nervous system, vestibular sensory periphery with afferent connections, and extraocular motoneuronal projections were functionally preserved. This allowed a natural activation of the VOR on a 6d-motion stimulator (PI H-840, Physik Instrumente, Karlsruhe, Germany). Extraocular motor units were recorded from the trochlear nerve after disconnection from the SO target muscle at the innervation site (Fig. 1a). For all experiments, preparations were placed in a Sylgard-lined recording chamber that was continuously superfused with oxygenated (Carbogen: 95% O2, 5% CO2) Ringer solution at a constant temperature of 17.0 ± 0.1 °C.
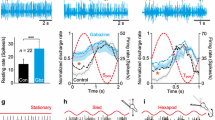
Impact of 4-aminopyridine (4-AP) on spontaneous discharge of the superior oblique (SO) motor nerve. a Schematic of a semi-intact Xenopus preparation depicting multi-unit SO nerve recordings and the direction of applied roll motion. b, c Episode of spontaneous SO nerve discharge before (control, black in b) and during bath application of 10 µmol 4-AP (red in b); box plot in c depicts multi-unit SO nerve resting rates in the absence (gray bars) and presence of 1, 5, 7 and 10 µM 4-AP (colored bars). d–f Episodes of spontaneous SO nerve discharge (top and middle trace in d) and corresponding firing rates (bottom plot in d) before (control, black) and during bath application of 10 µmol 4-AP (red); two 4-AP-related bursts are depicted at higher temporal resolution in e; box plot in f depicts the number of spike bursts (#/s) with interspike frequencies > 100 Hz (sampled in periods of > 60 s, dashed line in bottom trace in d) in the absence (gray bars) and the presence of 1, 5, 7 and 10 µM 4-AP (colored bars). Numbers in brackets in c indicate the number of preparations and also apply to f; *p < 0.05 (Wilcoxon signed-rank test) indicates the significance of difference
Electrophysiology and pharmacology
The recording chamber with the preparation affixed to the Sylgard floor was mounted in the center of the rotation axes of the 6d-motion stimulator [21]. Spontaneous and motion-evoked multi-unit spike discharge of the SO nerve was recorded extracellularly (EXT 10-2F; npi electronics; Tamm, Germany) with glass suction electrodes, digitized at 20 kHz (CED 1401, Cambridge Electronic Design, UK) and stored on computer for offline analysis. Suction electrodes were made from borosilicate glass (Science Products, Hofheim, Germany), pulled on a P-87 Brown/Flaming electrode puller. A modulation of SO nerve activity was elicited by sinusoidal rotations (1 Hz; ± 12.5°/s peak velocity) in a plane formed by the ipsilateral posterior (iPC) and contralateral anterior vertical semicircular canal (cAC) pair (Fig. 1a) [22]. The role of Kv1.1 channels in the generation of extraocular motor commands was evaluated by bath application of 4-AP (1–10 µM; Sigma) dissolved in frog Ringer solution.
Data analysis
Peri-stimulus time histograms (PSTHs) of average SO nerve firing patterns over a single head motion cycle were obtained from raw data using Spike2 (Cambridge Electronic Design, UK) scripts. Average responses were calculated from 15 cycles. The phase relation of motion-induced discharge with respect to the table position was obtained by comparing the timing of peak neuronal spike activity with the timing of the maximal table deflection. The PSTHs were further processed and analyzed statistically using Microcal Origin 6.0G (OriginLab Corp., USA). PSTHs were normalized and averaged (± SEM; standard error of the mean) for comparison. Statistical differences were calculated with the Wilcoxon signed-rank test (paired parameters; Prism, Graphpad Software, Inc, USA).
Tissue processing and immunohistochemistry
Tadpoles (n = 3) were deeply anesthetized in 0.05% MS-222 in ice-cold frog Ringer solution. Following decapitation, the dorsal portion of the head and rostral spinal cord was fixed by immersion in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) for 3 h at 4 °C. After washing three times with PBS, the tissue was embedded in 3% agarose, cryoprotected in 30% sucrose in PBS, and cut at a thickness of 20 μm on a cryostat (Leica). To detect Kv1.1 channel, anti-Kv1.1 (APC-009, 1:200, Alomone Labs) primary antibody and subclass-specific secondary antibody labeled with Alexa488 (A-11008, 1:1000, Thermo Fisher) was used. Nuclear staining was performed with 4′6-diamidino-2-phenylindoledihydrochloride (DAPI) (Sigma) to identify cell bodies. All sections were embedded in Aqua Polymount (Polyscience). Images were acquired and analyzed with an Olympus Fluoview confocal microscope with FV10-ASW 2.1 software.
Results
Spontaneous and motion-evoked discharge of SO motor units
The motoneuronal discharge at rest and during roll motion was obtained in vitro by recording multi-unit spike activity of the trochlear nerve after disconnection from its SO target muscle (Fig. 1a). The magnitude of the discharge was variable between different recordings and depended on the number of electrically accessible units within the suction electrode. In the absence of passive head/body motion (black trace in Fig. 1b) the average resting rate was ~ 25 spikes/s (25.3 ± 0.8 spikes/s; mean ± SEM; n = 21; light gray bar in Fig. 1c). Natural stimulation of vestibular endorgans was performed by sinusoidal roll motion (1 Hz; ± 12.5°/s peak velocity) in a plane formed by the iPC and cAC pair (dark blue shading in Fig. 1a). This motion caused a robust, phase-timed modulation of the multi-unit spike discharge (black trace in Fig. 2a). Firing increased during roll motion in the direction of the iPC with an average peak discharge rate of ~ 130 spikes/s (130.2 ± 5.1 spikes/s; mean ± SEM; n = 21) and a phase-lag of ~ 20° re table velocity (21.8° ± 3.9°; mean ± SEM; n = 21). This phase relation complies with previously established values and suggests both semicircular canal and otolith hair cells as the origin of the extraocular motor responses [23]. During motion in the direction of the cAC, the spike firing often ceased at the maximal roll position (black trace in Fig. 2b) with an average minimal firing rate of ~ 9 spikes/s (red arrow in Fig. 2b; 8.6 ± 1.6 spikes/s; mean ± SEM; n = 21).
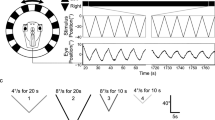
Impact of 4-aminopyridine (4-AP) on motion-evoked discharge of the superior oblique (SO) motor nerve. a SO nerve discharge (top and middle trace) during sinusoidal roll motion (blue sine wave; 1 Hz, ± 12.5°/s peak velocity) and corresponding firing rates (bottom plot) before (control, black) and during bath application of 7 µmol 4-AP (red). b Average firing rate modulation over a single cycle (dashed blue sine wave) of roll motion before (control, gray and black curves) and during bath application of 7 µM 4-AP (red and pink curves); solid curves and light shaded areas represent the mean firing rate ± SEM (n = 5 preparations); dashed gray and pink curves indicate the average discharge modulation of the typical example shown in a. c–e Box plots depicting peak discharge (c), minimal firing rate (d; see arrows in a, b) and phase relation of the response re table position (e) during sinusoidal rotation in the absence (gray bars) and presence of 1, 5, 7 and 10 µM 4-AP (colored bars). Numbers in brackets in c indicate the number of preparations and also apply to d, e; *p < 0.05 (Wilcoxon signed-rank test) indicates the significance of difference; n.s. not significant, Tpos table position
Bath application of 4-AP caused an increase of the multi-unit resting discharge (red traces in Fig. 1b, d), which was found to be statistically significant (p < 0.05; Wilcoxon signed-rank test) across all concentrations relative to the discharge rate prior to 4-AP application (Fig. 1c). The effect of 4-AP was reversible and usually lasted > 1–2 h but was not further investigated here. The augmentation of the firing rate was accompanied by a recruitment of additional motor units with very large amplitudes (arrow heads in Fig. 1b), which were absent under control conditions. Although spike shape analysis was impossible to perform due to high firing rates of the multi-unit discharge, close inspection of the spikes clearly confirmed a separate class based on spike amplitude, which only appeared after 4-AP application. These neurons likely coincide with the previously reported subgroup of large, high-dynamic extraocular motoneurons with very low resting rates in Xenopus tadpoles [20]. In addition, 4-AP altered the irregular spontaneous discharge into a pattern that consisted of short, repetitive bursts of spikes (Fig. 1d, e). These bursts contained few spikes with interspike frequencies well above 100 Hz (see black curve at bottom of Fig. 1d) and, when sampled over a period of > 60 s, were relatively rare under control conditions (gray bars in Fig. 1f). The occurrence of these bursts increased considerably after bath application of 4-AP in a dose-dependent manner (colored bars in Fig. 1f) with an average inter-burst interval of 200–250 ms (Fig. 1e) at 4-AP concentrations > 7 µM. The slight decrease of firing rate increase and burst occurrence for 4-AP concentrations > 7 µM potentially derives from a sustained depolarization of the membrane potential beyond spike threshold and a consequent dropout of action potentials in some neurons along the VOR pathway.
The robust, phase-timed modulation of the multi-unit discharge during sinusoidal roll motion stimulation persisted in the presence of 4-AP (red trace in Fig. 2a, b). However, the peak discharge increased at all concentrations of 4-AP compared to control conditions (colored bars in Fig. 2c). In addition, the burst-like firing in the presence of 4-AP was also maintained during motion stimulation, causing a rather noisy PSTH after averaging the discharge over single motion cycles (Fig. 2a, b). During roll motion in the direction of the cAC, the spike discharge did not cease, as often seen prior to drug application, but usually continued firing (arrows in Fig. 2a, b). Calculation of the respective averages over single cycles revealed a significant increase of the minimal discharge (arrow in Fig. 2b and colored bars in Fig. 2d) from ~ 9 spikes/s under control conditions to ~ 30 spikes/s in the presence of 7 µM 4-AP (32.8 ± 8.9 spikes/s; mean ± SEM; n = 5). In addition to the generally elevated firing rates, the motion-evoked responses altered the phase re velocity from ~ 20° in controls to > 45° in the presence of 4-AP (colored bars in Fig. 2e).
Immunohistochemistry of Kv1.1 in vestibular pathways
The substrate for 4-AP is a voltage-dependent potassium conductance generated by the Kv1.1 channel. Immuno-histochemical labeling with a Kv1.1 antibody successfully identified populations of vestibular afferent fibers and their associated cell bodies in the ganglion of Scarpa (Fig. 3a). Ganglion cells were found to be non-uniformly labeled, with only a specific subset of cells with particularly large somata being Kv1.1-immuno-positive (Fig. 3a, see arrowhead). Processes of Kv1.1-immuno-positive vestibular ganglion cells were observed to project peripherally and centrally. Peripheral processes appeared to project into the vestibular sensory epithelia within the inner ear (VE in Fig. 3a), while centrally projecting processes extended with the VIIIth nerve into the dorsal part of the hindbrain (Fig. 3b, c). These latter central projections terminated in topographically identified vestibular regions and likely connect with central vestibular targets, confirming the contribution of Kv1.1-expressing afferent fibers to vestibulo–motor transformations (Fig. 3d).
Kv1.1-immuno-histochemistry and outline of central vestibular and cerebellar circuits. a–c Coronal sections through a Xenopus laevis hindbrain labeled with an antibody against Kv1.1 (yellow) and counterstained with DAPI (blue); cell bodies of Scarpa’s Ganglion (ScG, arrowhead) and associated proximal and distal central processes of Kv1.1-positive cells (a) outlining projections to the vestibular sensory epithelia (VE) and fasciculation within the vestibular nerve (VNe); distal projections of Kv1.1-positive afferent fibers in the dorsal region of the hindbrain at a lower (b) and higher (c, panel box in b) magnification. d Schematic of central vestibular and cerebellar circuits; axon collaterals of irregular vestibular afferents activate local vestibular interneurons (red) and as mossy fibers (MF) activate granule cells (GC) and consequently cerebellar Purkinje cells, which in turn mediate an inhibition (red) onto a directly activated (green) central vestibular projection neurons (light brown). RF reticular formation, Ven fourth ventricle, VN vestibular nuclei. Scale bars are 100 µm in a, b and 50 µm in c
Discussion
The three major findings of this study were as follows: first, systemically applied 4-AP increased the spontaneous discharge of SO motoneurons and caused repetitious bursts of spikes under static conditions; second, during sinusoidal head motion, the peak firing rate was augmented; third, the overall higher firing rates and phase-shifted cyclic extraocular motor output in the presence of 4-AP likely derive from blocked Kv1.1 channels in thick vestibular nerve afferents. Our findings suggest propagation of the pharmacologically altered afferent firing rate properties throughout the VOR network.
Neuronal site of 4-AP action
The restriction of Kv1.1 immuno-positivity within the three-neuronal VOR pathway to large caliber vestibular nerve afferents in Xenopus tadpoles suggests that SO nerve firing rate alterations in the presence of 4-AP derive exclusively from a pharmacological block of voltage-dependent potassium conductances in these afferent fibers. The obvious lack of Kv1.1 immuno-labeled central vestibular neurons in tadpoles is at variance with the presence of large-celled Kv1.1 immuno-positive phasic second-order vestibular neurons in adult frogs [10]. This difference is likely related to different requirements for the detection of swimming-related motion dynamics of larval and adult frogs, and as such an eco-physiological adaptation of the membrane properties of central vestibular neurons [24]. The lack of Kv1.1 channels in vestibular neurons of tadpoles complies with the more tonic membrane properties of these neurons in larvae compared to those of adult frogs. Despite the absence of central Kv1.1 immuno-positive VOR neurons in tadpoles, the impact of 4-AP on a subset of vestibular afferents has a sufficiently profound impact on the spontaneous and motion-evoked extraocular motor spike discharge (Figs. 1, 2). This confirms the dominating role of afferent inputs for sensory-motor transformations of vestibular signals [25].
Increased SO nerve resting rates
Extraocular motor discharge depends on the integrity of vestibular sensory inputs, and thus on the firing rate of semicircular canal and otolith afferent nerve fibers as indicated by imbalanced SO motoneuronal spike discharge after unilateral transection of the VIIIth nerve [25]. Moreover, the localization of Kv1.1 channels in thick vestibular afferents suggests that the augmented SO spike discharge in the presence of 4-AP derives exclusively from a firing rate increase of the latter fibers. This complies with the observation that bath application of similar concentrations of 4-AP in larval Xenopus causes an increase and regularization of the spontaneous firing rate as well as an augmentation and phase shift of motion-evoked responses exclusively in thick, irregular but not in thinner regular firing vestibular afferent fibers [26], compatible with their Kv1.1 immuno-positivity (Fig. 3a, b). Thus, based on the organization of VOR circuits as frequency-tuned channels and the exclusive Kv1.1 immuno-labeling of thick vestibular afferents, the 4-AP-provoked phase shift of cyclic extraocular motor responses directly derives from a block of the transient response behavior and consequent temporal extension of the spike firing of phasic, irregularly firing afferents [26]. This afferent firing rate alteration propagates through central vestibular neurons onto extraocular motoneurons where corresponding changes were encountered (Figs. 1, 2). A firing rate increase and regularization of Purkinje cell spike discharge was also observed in mutant mice with episodic ataxia type 2 following 4-AP application [27]. This latter finding, however, does not exclude that the observed effect is only indirect and due to the alteration of the discharge pattern of irregular vestibular afferents that as mossy fibers represent a major source of synaptic input to the cerebellum (Fig. 3d). Interestingly, however, the 4-AP-induced discharge regularization of irregular firing vestibular afferents is not mirrored by extraocular motoneurons. Rather, the occurrence of repetitious spike bursts suggests additional synaptic modifications of the regularized vestibular afferent input along the VOR circuitry.
4-AP-induced extraocular motor spike bursts
A likely synaptic substrate for generating repetitive spike bursts following application of 4-AP is a cyclic truncation of the excitation of central vestibular neurons by an inhibition that derives from local vestibular side loops [28] and cerebellar Purkinje cells (Fig. 3d) [29]. Both circuits are activated by irregular vestibular afferent fibers [9, 28], which in the presence of the Kv1.1 channel blocker increase the synaptic drive of these networks but also facilitate the feed-forward synaptic inhibition. These inhibitory side loops are capable of truncating the direct monosynaptic excitation that is mediated from irregular afferents onto central vestibular neurons (Fig. 3d). This creates more or less rhythmic spike bursts that are interrupted by variably sized and timed inhibition. Thus, while 4-AP regularizes the discharge of irregular firing vestibular afferents and that of directly connected postsynaptic elements, additional inhibitory circuits shape the firing pattern to generate oscillating spike bursts.
Clinical implications
The observed changes by the Kv1.1 blocker 4-AP in Xenopus tadpoles predict that 4-AP-induced increased vestibular afferent discharge—as the main driving force for all vestibular circuits—can contribute to the improvement of symptoms in patients treated with 4-AP for various diseases, such as downbeat and upbeat nystagmus or episodic ataxia type 2. This assumption is further supported by the fact that irregular firing vestibular afferents in amphibians and mammals have similar conductances [8, 26] and central connectivity [29]. However, 4-AP-related improvements of gaze and posture deficits in patients might be dominated by drug effects at multiple sites including central vestibular [9] and cerebellar neurons [27], potentially explaining the range of ameliorated behaviors and response patterns. The 4-AP sensitivity of multiple central areas is in fact supported by the abundance of Kv1.1 immuno-positive neurons in cerebellar and vestibular circuits in mice [30]. Nonetheless, the current results suggest that part of the reported 4-AP-induced improvements of clinical symptoms might also derive from a firing rate increase of vestibular nerve afferents, which so far has been underestimated as a potential target for this drug and the origin of improved symptoms. A contribution of afferent fibers is supported by the fact that the morpho-physiological organization of the peripheral and central vestibular system is highly conserved across vertebrates [2] including the presence of Kv1.1 channels in vestibular afferents of rodents [8], primates and humans (Mayadali and Horn, personal communication).
A large fraction of firing rate regularization of vestibular and cerebellar neurons in mammals [17] as well as the improved vestibular reflexes in patients [31] in the presence of 4-AP thus potentially depends on a firing rate increase in vestibular afferents. This can augment the direct effects of 4-AP on cerebellar Purkinje cells whose resting discharge rate and excitability are also increased by 4-AP at the same concentrations as used in the current and an earlier study [32]. In addition, in an animal model of episodic ataxia type 2, the tottering mouse, 4-AP reduced the irregularity of spontaneous firing of cerebellar Purkinje cells. In conclusion, the 4-AP-induced increase of peripheral vestibular input under static and dynamic conditions in the current study might explain at least in part the therapeutic effects of 4-AP in downbeat nystagmus and cerebellar ataxia. Given the robust increase and regularization of the firing rate in irregular/phasic vestibular afferents, it is possible that 4-AP might also have beneficial effects for peripheral vestibular disorders through a partial or complete rescue of spontaneous afferent activity.
References
Angelaki DE, Cullen KE (2008) Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci 31:125–150
Straka H, Fritzsch B, Glover JC (2014) Connecting ears to eye muscles: evolution of a 'simple' reflex arc. Brain Behav Evol 83:162–175
Straka H, Lambert FM, Pfanzelt S, Beraneck M (2009) Vestibulo–ocular signal transformation in frequency-tuned channels. Ann NY Acad Sci 1164:37–44
Carriot J, Jamali M, Chacron MJ, Cullen KE (2014) Statistics of the vestibular input experienced during natural self-motion: implications for neural processing. J Neurosci 34:8347–8357
Hänzi S, Straka H (2017) Developmental changes in head movement kinematics during swimming in Xenopus laevis tadpoles. J Exp Biol 220:227–236
Beraneck M, Straka H (2011) Vestibular signal processing by separate sets of neuronal filters. J Vestib Res 21:5–19
Goldberg JM (2000) Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130:277–297
Eatock RA, Songer JE (2011) Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34:501–534
Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB (2005) Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol 76:349–392
Beraneck M, Pfanzelt S, Vassias I, Rohregger M, Vibert N, Vidal PP, Moore LE, Straka H (2007) Differential intrinsic response dynamics determine synaptic signal processing in frog vestibular neurons. J Neurosci 27:4283–4296
Strupp M, Schüler O, Krafczyk S, Jahn K, Schautzer F, Büttner U, Brandt T (2003) Treatment of downbeat nystagmus with 3,4-diaminopyridine: a placebo-controlled study. Neurology 61:165–170
Claassen J, Spiegel R, Kalla R, Faldon M, Kennard C, Danchaivijitr C, Bardins S, Rettinger N, Schneider E, Brandt T, Jahn K, Teufel J, Strupp M, Bronstein A (2013) A randomised double-blind, cross-over trial of 4-aminopyridine for downbeat nystagmus—effects on slowphase eye velocity, postural stability, locomotion and symptoms. J Neurol Neurosurg Psychiatry 84:1392–1399
Strupp M, Teufel J, Zwergal A, Schniepp R, Khodakhah K, Feil K (2017) Aminopyridines for the treatment of neurologic disorders. Neurol Clin Pract 7:65–76
Strupp M, Kalla R, Dichgans M, Freilinger T, Glasauer S, Brandt T (2004) Treatment of episodic ataxia type 2 with the potassium channel blocker 4-aminopyridine. Neurology 62:1623–1625
Strupp M, Kalla R, Claassen J, Adrion C, Mansmann U, Klopstock T, Freilinger T, Neugebauer H, Spiegel R, Dichgans M, Lehmann-Horn F, Jurkat-Rott K, Brandt T, Jen JC, Jahn K (2011) A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias. Neurology 77:269–275
Glasauer S, Kalla R, Büttner U, Strupp M, Brandt T (2005) 4-Aminopyridine restores visual ocular motor function in upbeat nystagmus. J Neurol Neurosurg Psychiatry 76:451–453
Glasauer S, Rössert C, Strupp M (2011) The role of regularity and synchrony of cerebellar Purkinje cells for pathological nystagmus. Ann N Y Acad Sci 1233:162–167
Strupp M, Querner V, Eggert T, Straube A, Brandt T (2003) 3,4-Diaminopyridine improves head-shaking nystagmus caused by neurovascular cross-compression. Ann N Y Acad Sci 1004:506–510
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin): a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Publishing, New York
Dietrich H, Glasauer S, Straka H (2017) Functional organization of vestibulo–ocular responses in abducens motoneurons. J Neurosci 37:4032–4045
Soupiadou P, Branoner F, Straka H (2018) Pharmacological profile of vestibular inhibitory inputs to superior oblique motoneurons. J Neurol 265(Suppl 1):S18–S25
Branoner F, Straka H (2015) Semicircular canal-dependent developmental tuning of translational vestibulo–ocular reflexes in Xenopus laevis. Dev Neurobiol 75:1051–1067
Lambert FM, Beck JC, Baker R, Straka H (2008) Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus. J Neurosci 28:8086–8096
Beraneck M, Lambert FM, Straka H (2008) Membrane properties of central vestibular neurons in larval Xenopus: eco-physiological adaptations to locomotor strategy. Soc Neurosci Abstr 34(169):12
Branoner F, Straka H (2018) Semicircular canal influences on the developmental tuning of the translational vestibulo–ocular reflex. Front Neurol 9:404
Gensberger KD, Wühr M, Hoffman LF, Paulin MG, Straka H (2017) Spike time regularity of horizontal canal afferent fibers as decisive factor for motion encoding in Xenopus laevis tadpoles. Soc Neurosci Abstr 43(225):11
Alviña K, Khodakhah K (2010) The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci 30:7258–7268
Straka H, Dieringer N (2000) Convergence pattern of uncrossed excitatory and inhibitory semicircular canal-specific inputs onto second-order vestibular neurons of frogs. Exp Brain Res 135:462–473
Straka H, Dieringer N (2004) Basic organization principles of the VOR: lessons from frogs. Prog Neurobiol 73:259–309
Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL (1994) Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci 14:4588–4599
Kalla R, Teufel J, Feil K, Muth C, Strupp M (2016) Update on the pharmacotherapy of cerebellar and central vestibular disorders. J Neurol 263(Suppl 1):S24–S29
Etzion Y, Grossman Y (2001) Highly 4-aminopyridine sensitive delayed rectifier current modulates the excitability of guinea pig cerebellar Purkinje cells. Exp Brain Res 139:419–425
Acknowledgements
The authors acknowledge financial support from the German Science Foundation (CRC 870; STR 478/3-1; RTG 2175) and the German Federal Ministry of Education and Research under the Grant code 01 EO 0901.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing financial interests.
Ethical standards
All studies have been approved by the appropriate ethics committee (ROB-55.2-2532.Vet_03-17-24) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
This manuscript is part of a supplement sponsored by the German Federal Ministry of Education and Research within the funding initiative for integrated research and treatment centers.
Rights and permissions
About this article
Cite this article
I Gusti Bagus, M., Gordy, C., Sanchez-Gonzalez, R. et al. Impact of 4-aminopyridine on vestibulo–ocular reflex performance. J Neurol 266 (Suppl 1), 93–100 (2019). https://doi.org/10.1007/s00415-019-09452-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09452-4