Abstract
Introduction
Subjective cognitive decline (SCD) can represent a preclinical stage of Alzheimer’s disease. Diffusion tensor imaging (DTI) could aid an early diagnosis, yet only few monocentric DTI studies in SCD have been conducted, reporting heterogeneous results. We investigated microstructural changes in SCD in a larger, multicentric cohort.
Methods
271 participants with SCD, mild cognitive impairment (MCI) or Alzheimer’s dementia (AD) and healthy controls (CON) were included, recruited prospectively at nine centers of the observational DELCODE study. DTI was acquired using identical protocols. Using voxel-based analyses, we investigated fractional anisotropy (FA), mean diffusivity (MD) and mode (MO) in the white matter (WM). Discrimination accuracy was determined by cross-validated elastic-net penalized regression. Center effects were explored using variance analyses.
Results
MO and FA were lower in SCD compared to CON in several anterior and posterior WM regions, including the anterior corona radiata, superior and inferior longitudinal fasciculus, cingulum and splenium of the corpus callosum (p < 0.01, uncorrected). MD was higher in the superior and inferior longitudinal fasciculus, cingulum and superior corona radiata (p < 0.01, uncorrected). The cross-validated accuracy for discriminating SCD from CON was 67% (p < 0.01). As expected, the AD and MCI groups had higher MD and lower FA and MO in extensive regions, including the corpus callosum and temporal brain regions. Within these regions, center accounted for 3–15% of the variance.
Conclusions
DTI revealed subtle WM alterations in SCD that were intermediate between those in MCI and CON and may be useful to detect individuals with an increased risk for AD in clinical studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diffusion tensor imaging (DTI) is useful for the diagnosis of Alzheimer’s disease (AD) at the prodromal stage (mild cognitive impairment; MCI) and at the stage of manifest dementia, as shown in monocentric [4, 7, 8, 12, 29, 37] and multicentric studies [9, 10, 38]. It may especially aid the diagnosis at an early stage, as the DTI parameters mean diffusivity (MD), fractional anisotropy (FA) and mode of anisotropy (MO) indicate neuronal dysfunction at a microstructural level that are assumed to precede macroscopic atrophic changes in AD pathogenesis [6, 8].
Recently, evidence has accumulated indicating that subjective cognitive decline (SCD) could represent the earliest symptomatic stage of AD [21, 26]. To date, only few monocentric DTI studies with varying acquisition parameters and analysis methods investigated microstructural changes in SCD. Compared to cognitively healthy control (CON) participants, these studies reported lower FA and higher MD mainly in the corpus callosum and cingulum, the white matter (WM) of the medial temporal lobe (including parahippocampal WM and WM underlying the entorhinal cortex) and of the medial parietal lobe (including the WM underlying the precuneus as well as the retrosplenial cortex) [19, 25, 30, 33, 40]. Another study found changes in widespread WM regions across the brain [25]. Some of the WM changes predicted subsequent hippocampus atrophy and memory decline [32]. Moreover, altered diffusivity in the posterior cingulum was associated with tau pathology in a sample of participants with SCD or MCI [35].
However, other studies did not detect deviant MD or FA in SCD [23, 40]. The different results could partly be due to the high variability of DTI across different scanners [38]. Obviously, a post hoc comparison of outcomes of different monocentric studies cannot explicitly assess a multicenter effect. In a previous multicentric sample with retrospectively collected data, center accounted for 56–75% of the FA and MD variance [39]. This emphasizes the relevance to investigate the performance of DTI in a multicentric SCD sample. So far, the usefulness of DTI for capturing WM alterations in a multicentric sample has only been addressed in people with MCI who were β-amyloid positive. The results of this multicentric study were promising, revealing a significantly higher accuracy of the DTI parameters compared to the accuracy of volume markers for detecting prodromal AD [9].
Besides the evaluation of group differences, the application of a future biomarker in a multicenter data set provides a more realistic estimate of its potential diagnostic use in the presence of multicenter variability than its evaluation in a monocenter study. Using imaging markers for risk stratification in future clinical trials will likely involve multiple scanners. Therefore, an estimate of the effect of multicenter variance on the achievable levels of accuracy is of high interest.
In the present study, we aimed to assess if microstructural neuronal integrity as measured by DTI is altered at the stage of subjectively impaired cognitive functioning, i.e., before cognitive symptoms are measurable. To investigate this, we used a prospective sample from a multicentric longitudinal study with scan parameters and procedures that were matched across the centers [22]. We hypothesized that DTI could differentiate the diagnostic groups SCD, MCI and AD dementia from CON participants. Secondly, we hypothesized that the variance due to center would be reduced compared to a previous multicentric study that included data that were obtained with varying acquisition parameters [39].
Methods
Participants
We used data from the interim baseline data set of the multicenter DZNE-longitudinal Cognitive Impairment and Dementia Study (DELCODE), conducted by the German Center for Neurodegenerative Diseases (DZNE) [22]. After excluding all cases with insufficient image quality or neurologic conditions, DTI data from 271 participants from nine centers were included (35 AD, 45 MCI, 98 SCD and 93 CON; n = 2–76 participants per center; Supplementary Table S2). The participants underwent a clinical assessment of their cognitive status, including the Mini Mental State Examination (MMSE) [13] and an extensive neuropsychological testing battery [22]. Depressive symptoms were assessed by means of the Geriatric Depression Scale (GDS) [15]. The DELCODE exclusion criteria ensured that no persons were included who had a current major depressive episode, past or present major psychiatric disorders, neurological diseases other than AD, or unstable medical conditions [22].
SCD was defined as a persistent self-perceived cognitive impairment in the absence of objective cognitive impairment, lasting at least 6 months and being unrelated to an acute event [21]. The MCI patients met the core clinical criteria for MCI according to NIA-AA workgroup guidelines [1]. The AD patients had a clinical diagnosis of probable AD dementia according to the National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroups guidelines [27]. The CON participants had no objective cognitive impairment in cognitive tests, no history of neurological or psychiatric disease and did not report self-perceived cognitive decline. All participants or their representatives provided written informed consent. The study protocol was approved by the local institutional review boards and ethical committees of the participating centers. It was conducted in accord with the Helsinki Declaration of 1975.
Image acquisition and preprocessing
The data were acquired from nine Siemens 3.0 Tesla MRI scanners (four Verio, one Skyra, three TimTrio and one Prisma system) using identical acquisition parameters and harmonized instructions. To ensure high image quality throughout the acquisition phase, all scans had to pass a semiautomated quality check during the study conduction, so that protocol deviations could be reported to the study sites, and the acquisition at the respective site could be adjusted. An axial diffusion sequence was measured based on a single-shot echo planar imaging sequence (field of view 240 × 240 mm, matrix size 120 × 120, isotropic voxel size 2 mm, repetition time 12,100 ms, echo time 88 ms, flip angle 90°, number of gradients 60, b values 700 s/mm2 and 1000 s/mm2, number of slices 72, parallel imaging acceleration factor 2). High-resolution T1-weighted anatomical images were obtained using a sagittal magnetization-prepared rapid gradient echo (MPRAGE) sequence (field of view 256 × 256 mm, matrix size 256 × 256, isotropic voxel size 1 mm, echo time 4.37 ms, flip angle 7°, repetition time 2500 ms, number of slices 192, parallel imaging acceleration factor 2).
The T1-weighted anatomical images were segmented into gray matter (GM), WM, and cerebrospinal fluid (CSF) using the Statistical Parametric Mapping (SPM12) (Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/) New Segment toolbox implemented in Matlab 2015a (Mathworks, Natwick). The T1-weighted GM and WM partitions were normalized to the Montreal Neurological Institute (MNI) reference coordinate system using the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) algorithm [2] and the default brain template included in CAT12 [24] as target. We created a WM mask by averaging the normalized WM partitions of 45 randomly selected participants equally distributed across all diagnostic groups and applying a threshold of 50% WM density. The DTI scans were preprocessed using the FSL diffusion toolbox (Version 5.0.9, FMRIB, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl/). The images were corrected for eddy currents and head motion. Skull stripping was performed using the Brain Extraction Tool. DTIfit was used to fit the diffusion tensors at each voxel and calculate the MD, FA and MO maps. The DTI maps were then co-registered to the T1-weighted anatomical images. Using the deformation fields that originated from the normalization of the T1-weighted images, the DTI maps were normalized to the MNI reference coordinate system. The normalization quality was checked visually and the scan homogeneity was determined using the standard deviation across the sample. As a last step, we smoothed the DTI maps with a Gaussian kernel of 8 mm. Within the WM mask, we calculated mean FA and MD and MO indices for each participant. Hippocampal GM volume was calculated for each participant by summing up the modulated grey matter voxel values within a mask that had been manually delineated in the reference space according to the international harmonized protocol for hippocampus segmentation [14]. The raw hippocampal volume was proportionally scaled to the total intracranial volume to adjust for head size. For the elastic-net penalized regression, regional DTI mean values were calculated for all 48 major WM tracts of the John Hopkins University-International Consortium of Brain Mapping (JHU-ICBM) DTI atlas [28].
Statistics
Statistical analyses were performed using SPM12 and SPSS (IBM; Version 23). Kruskal–Wallis tests were used to compare age, years of education, MMSE and GDS between the diagnostic groups. The distribution of sex was compared by means of the Chi-square test.
Two-sample t tests were used to compare the WM FA, MD and MO values voxel-wise across the diagnostic groups. The directions of the comparisons were hypothesis-driven (FA: SCD/MCI/AD < CON, MO: SCD/MCI/AD < CON, MD: SCD/MCI/AD > CON). Additional contrasts in the opposite directions were also tested (Supplementary Fig. S4). The variance explained by center (partial η2) was calculated using univariate analyses of variance, controlled for diagnosis, age, gender, years of education. For exploratory purposes, a liberal statistical significance level of p < 0.01 was used, uncorrected for multiple comparisons. Masks were derived that included the voxel clusters (k ≥ 20) that had reached significance (p < 0.01) in the AD vs. CON comparison of FA, MD or MO, respectively. The inclusive masks entered the univariate analyses of variance to assess the center effect within the regions of significant group effects.
We chose elastic net regression to account for multicollinearity among the predictor variables, which would inflate the variance of the estimated regression coefficients [42]. We used the R package “glmnet” to determine the group discrimination accuracy (http://cran.r-project.org/web/packages/glmnet/). Individual analyses were performed for each group of participants with cognitive deficits versus CON. The regional mean values for all three DTI measures FA, MD and MO within the 48 WM tracts of the JHU-ICBM DTI atlas were combined and entered in the analyses. The mixing parameter alpha was set to α = 0.5, specifying the balance of regularization between the ridge penalty and the least absolute shrinkage and selection operator (LASSO) penalty [43]. To define the strength of the regularization, the parameter lambda was determined as the mean of 100 iteratively determined values in a grid search with tenfold cross-validation. The average area under the receiver operating characteristic curve (AUC) was obtained from all runs of a tenfold cross-validation that was repeated 1000 times. Within the cross-validation procedure, the effect of the covariates age, gender and site was partialed out using a linear regression model. The residuals were then entered to estimate the penalized regression model for group separation. Confidence intervals for the AUC were estimated by determining the 2.5 and 97.5 percentiles across all iterations of the cross-validation. The diagnostic accuracy of DTI measures was compared to the accuracy of hippocampus volume as a reference. For obtaining p values, the proportion of iterations with AUC ≤ 0.5 (corresponding to chance level) was determined. For creating boxplots, a cubical region of interest (8 × 8 × 8 mm) was placed in the region that showed the most consistent results across the diagnostic groups and DTI parameters, i.e., the left part of the splenium of the corpus callosum (MNI: x = − 20, y = − 50, z = 13) (Supplementary Fig. S1). Analyses were repeated excluding individuals who showed outlier values in these boxplots. For subjects with CSF amyloid status available, we repeated the analyses including only amyloid-positive AD and MCI subjects and amyloid-negative controls. Amyloid-positivity was defined as Aß42/40 < 0.09.
Results
The groups differed significantly regarding age (the CON group being youngest on average), but they did not differ significantly with respect to sex and years of education (Table 1). The MMSE scores differed significantly across all diagnostic groups (p < 0.001) but not between the CON and SCD groups. The GDS scores differed significantly between groups, with the lowest mean score in the CON group, but did not reach a clinically relevant level in any group.
Group comparisons in whole brain WM
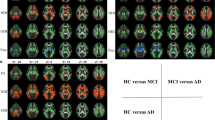
The voxel-based two-sample t tests revealed significantly higher MD in mainly anterior regions in the SCD group compared to the CON group (Cohen’s d: 0.4–0.5, p < 0.01, uncorrected) (Fig. 1; Supplementary Table S1). No clusters remained significant at a statistical threshold of p < 0.001 (uncorrected). In the AD as well as in the MCI group compared to the CON group, there was higher MD in several regions, including the genu, body and splenium of the corpus callosum, the anterior and superior corona radiata, posterior thalamic radiation, and sagittal stratum, which also remained significant at p < 0.05, FWE corrected.
FA was significantly lower in the SCD group compared to the CON group, mainly in the anterior corona radiata, inferior fronto-occipital fasciculus and splenium of the corpus callosum (Cohen’s d: 0.3–0.6, p < 0.01, uncorrected) (Fig. 2; Supplementary Table S1). At p < 0.001 (uncorrected), bilateral clusters in the inferior fronto-occipital fasciculus and splenium of the corpus callosum remained significant (Supplementary Table S1). In the AD group and in the MCI group compared to the CON group, FA was lower in a number of regions, including the genu and splenium of corpus callosum, the anterior corona radiata, and the posterior thalamic radiation, remaining statistically significant at p < 0.05, FWE corrected.
MO was significantly lower in the SCD group compared to the CON group in the anterior thalamic radiation, superior longitudinal fasciculus, cingulum and the splenium of the corpus callosum (Cohen’s d: 0.4–0.6, p < 0.01, uncorrected) (Fig. 3; Supplementary Table S1). Clusters in the anterior thalamic radiation, cingulum and splenium of the corpus callosum remained significant at p < 0.001 (uncorrected; Supplementary Table S1). In the AD group and in the MCI group compared to the CON group, MO was mainly lower in the genu and splenium of the corpus callosum and cingulum, remaining significant at p < 0.05, FWE corrected. For SCD vs. CON comparisons, no effects remained statistically significant when correcting for multiple comparisons. Supplementary Fig. S2 shows the distribution of MO across the diagnostic groups (ROI based).
Conducting analyses with contrasts in the non-hypothesized directions (i.e., MD: SCD < CON, FA: SCD > CON, MO: SCD > CON) did not result in significant group effects, except for small distributed clusters of significant MO differences, e.g., in the right inferior fronto-occipital fasciculus (T = 2.3, k = 75; Supplementary Fig. S4).
Group discrimination
For the discrimination of the diagnostic groups based on the DTI data, a cross-validated AUC of 67% was reached for separating SCD and CON (p < 0.01), 79% for MCI and CON (p < 0.01), and 93% for AD and CON (p < 0.01) (Fig. 4). When considering a subsample of CSF β-amyloid-positive SCD participants (n = 17) and β-amyloid-negative CON (n = 25), the AUC was 63% (p = 0.11). A subsample of CSF β-amyloid-positive MCI participants (n = 20) versus the β-amyloid-negative CON group reached an AUC of 91% (p < 0.01). A subsample of β-amyloid-positive AD participants resulted in an AUC of 88% (p = 0.01). For hippocampal volume, the AUC for SCD vs. HC was 57% (p = 0.08), 59% for CSF β-amyloid-positive SCD (p = 0.4), 77% for MCI (p = 0.01), 84% for β-amyloid-positive MCI (p < 0.01), 95% for AD (p < 0.01) and 94% for β-amyloid-positive AD (p < 0.01) (Fig. 4). A sensitivity analysis without outliers resulted in comparable AUC values (Supplementary Table S3, Supplementary Fig. S3).
Multicentric variability
Within the whole-brain WM of all participants combined, center explained 10% of the FA variance, 15% of the MD variance and 7% of the MO variance. When restraining the regions of interest to the voxel clusters that were significantly different between the AD group and the CON group (p < 0.01, uncorrected), center explained 10% of the FA, 14% of the MD and 3% of the MO variance (Fig. 5).
Discussion
We investigated if DTI revealed deviant brain WM diffusivity in SCD. Compared to CON participants, we found subtle diffusivity differences that were lower than the diffusivity alterations in MCI patients. DTI distinguished SCD from CON participants with a classification accuracy of 67% (p < 0.01).
In addition to FA and MD changes, which have previously been reported in SCD [19, 25, 30, 33, 40], our analyses revealed MO differences. MO has previously been used to assess WM alterations in MCI and AD [7,8,9, 18, 36]. It reflects the diffusion of water molecules, which is restricted by myelinated axons of neurons [41]. It has the advantage of capturing subtle WM changes even in regions with more than one fiber direction [7]. For example, MO reflects linear anisotropy in the corpus callosum and more planar anisotropy in regions with crossing fibres such as the boundary between two major white matter tracts. The diffusion tensor in DTI analyses is a symmetric 3 × 3 matrix that can be described by its eigenvalues and eigenvectors, reflecting the magnitude and direction of diffusion [34]. From this, the scalar indices MO, FA, and MD are derived. MO specifies the shape of diffusion, from planar (MO = − 1) to linear (MO = 1), so that a decrease in MO reflects a more planar shape of the diffusion tensor [11]. FA corresponds to the degree of directionality. Reduced FA is assumed to reflect a disrupted WM organization caused by microstructural damage, such as axonal degradation [34]. In contrast, MD is the mean of the three eigenvalues and informs about the diffusion rate. An increase in MD is assumed to reflect damage to the membrane integrity [34]. MD is also increased in regions affected by neurodegeneration, as this leads to an expansion of the regions filled with cerebrospinal fluid which are isotropic.
The AD group and the MCI group exhibited significantly higher MD and significantly lower FA and MO in extensive regions, including the corpus callosum and the anterior and superior corona radiata, as expected from previous monocentric [4, 7, 8, 12, 29, 37] and multicentric studies [9, 38].
Some of the monocentric studies on SCD reported slightly stronger diffusivity alterations than our study [19, 25, 30, 33]. In addition to methodical deviances such as using ROI-based approaches, differences may be due to the fact that the definition of the SCD group varied, as these studies were conducted before the conceptual framework for research on SCD was published [21]. The average MMSE scores of the SCD samples in these monocentric studies tended to be lower compared to our study, suggesting a stronger cognitive decline. Notably, the sample sizes of previous studies were considerably smaller than that of the present study (ranging from 16 to 28 SCD participants) and samples may have overlapped across reports from the same group [17, 32, 33, 35].
A multicentric design is necessary for evaluating the use of DTI across several settings. Because homogenized scanning procedures and parameters were applied, we expected stable DTI results across the centers. When we investigated the impact of the multicentric acquisition, we found that significant center effects were still present, yet markedly reduced compared to a previous multicentric study with a naturalistic design [3, 39]. The DTI parameter MO was least affected by center.
In the present study, we reported first results for the cross-sectional baseline data available in DELCODE. The results suggest that subtle white matter changes are present at the very early stage of SCD. These brain changes may be measurable earlier than hippocampal atrophy, which was shown to have a lower AUC for discriminating SCD and β-amyloid-positive SCD from CON. However, it must be kept in mind that the uncorrected significance levels of the voxel-based effects are prone to false-positive effects. The AUC of DTI for detecting SCD (67%, p < 0.01) was higher than that of hippocampal volume (57%, p = 0.08). Its discriminative accuracy was still below the benchmark for a useful biomarker, suggesting that DTI measures alone do not provide a useful early marker of preclinical AD in SCD cases. Subtle white matter differences could (for instance in combination with other markers) aid the identification of individuals with slight cognitive changes who have an increased risk for AD, but not as a stand-alone marker.
As a next step, analyses of the longitudinal data that are currently acquired will be performed to examine the value of DTI as a prognostic marker for the conversion of SCD to MCI and AD. Further studies should also address the relationship of DTI markers and the severity of self-reported cognitive impairment in addition to objectively measured memory change. As a limitation, the current sample size did not allow for matching age and gender across centers. This is especially relevant as age is related to white matter changes [16, 31] and was significantly different between the groups; on average, SCD participants were 2.8 years older than the CON participants. As matching was not possible, we controlled for gender and age in the statistical models. In the progress of the longitudinal DELCODE study, a future sample will allow for more homogeneous subsamples to be analyzed.
DTI can detect microstructural changes before substantial atrophy has taken place. It is a promising imaging marker for detecting early AD [5, 9] and has been shown to be highly sensitive to β-amyloid pathology [20]. In our study, we reported mild DTI WM deviations in SCD. Given the replication in larger longitudinal studies, these WM changes could—in combination with other markers—aid the identification of individuals with a higher risk for AD in clinical dementia studies.
References
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279
Ashburner J (2007) A fast diffeomorphic image registration algorithm. NeuroImage 38:95–113
Brueggen K, Grothe MJ, Dyrba M, Fellgiebel A, Fischer F, Filippi M, Agosta F, Nestor P, Meisenzahl E, Blautzik J, Frölich L, Hausner L, Bokde ALW, Frisoni G, Pievani M, Klöppel S, Prvulovic D, Barkhof F, Pouwels PJW, Schröder J, Hampel H, Hauenstein K, Teipel S (2017) The European DTI study on dementia—a multicenter DTI and MRI study on Alzheimer’s disease and mild cognitive impairment. NeuroImage 144:305–308
Cherubini A, Péran P, Spoletini I, Di Paola M, Di Iulio F, Hagberg GE, Sancesario G, Gianni W, Bossù P, Caltagirone C, Sabatini U, Spalletta G (2010) Combined volumetry and DTI in subcortical structures of mild cognitive impairment and Alzheimer’s disease patients. J Alzheimers Dis 19:1273–1282
Clerx L, Visser PJ, Verhey F, Aalten P (2012) New MRI markers for Alzheimer’s disease: a meta-analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. J Alzheimers Dis 29:405–429
Delbeuck X, van der Linden M, Collette F (2003) Alzheimer’s disease as a disconnection syndrome? Neuropsychol Rev 13:79–92
Douaud G, Jbabdi S, Behrens TE, Menke RA, Gass A, Monsch AU et al (2011) DTI measures in crossing-fibre areas: increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. NeuroImage 55:880–890
Douaud G, Menke RAL, Gass A, Monsch AU, Rao A, Whitcher B, Zamboni G, Matthews PM, Sollberger M, Smith S (2013) Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer’s disease. J Neurosci Off J Soc Neurosci 33:2147–2155
Dyrba M, Barkhof F, Fellgiebel A, Filippi M, Hausner L, Hauenstein K, Kirste T, Teipel SJ (2015) Predicting prodromal Alzheimer’s disease in subjects with mild cognitive impairment using machine learning classification of multimodal multicenter diffusion-tensor and magnetic resonance imaging data. J Neuroimaging Off J Am Soc Neuroimaging 25:738–747
Dyrba M, Ewers M, Wegrzyn M, Kilimann I, Plant C, Oswald A, Meindl T, Pievani M, Bokde ALW, Fellgiebel A, Filippi M, Hampel H, Klöppel S, Hauenstein K, Kirste T, Teipel SJ (2013) Robust automated detection of microstructural white matter degeneration in Alzheimer’s disease using machine learning classification of multicenter DTI data. PLoS ONE 8:e64925
Ennis DB, Kindlmann G (2006) Orthogonal tensor invariants and the analysis of diffusion tensor magnetic resonance images. Magn Reson Med 55:136–146
Fellgiebel A, Wille P, Müller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P (2004) Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord 18:101–108
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state. J Psychiatr Res 12:189–198
Frisoni GB, Jack CR Jr, Bocchetta M, Bauer C, Frederiksen KS, Liu Y, Preboske G, Swihart T, Blair M, Cavedo E, Grothe MJ, Lanfredi M, Martinez O, Nishikawa M, Portegies M, Stoub T, Ward C, Apostolova LG, Ganzola R, Wolf D, Barkhof F, Bartzokis G, DeCarli C, Csernansky JG, deToledo-Morrell L, Geerlings MI, Kaye J, Killiany RJ, Lehericy S, Matsuda H, O'Brien J, Silbert LC, Scheltens P, Soininen H, Teipel S, Waldemar G, Fellgiebel A, Barnes J, Firbank M, Gerritsen L, Henneman W, Malykhin N, Pruessner JC, Wang L, Watson C, Wolf H, deLeon M, Pantel J, Ferrari C, Bosco P, Pasqualetti P, Duchesne S, Duvernoy H, Boccardi M, Volumetry E-AWGoTHPfMH, for the Alzheimer’s Disease Neuroimaging I (2015) The EADC-ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: evidence of validity. Alzheimers Dement 11:111–125
Gauggel S, Birkner B (1999) Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS). Zeitschrift für linische Psychologie 28:18–27
Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, Stefano ND, Johansen-Berg H (2010) Age-related changes in grey and white matter structure throughout adulthood. NeuroImage 51:943–951
Grambaite R, Stenset V, Reinvang I, Walhovd KB, Fjell AM, Fladby T (2010) White matter diffusivity predicts memory in patients with subjective and mild cognitive impairment and normal CSF total tau levels. J Int Neuropsychol Soc 16:58–69
Groot Md, Ikram MA, Akoudad S, Krestin GP, Hofman A, van der Lugt A, Niessen WJ, Vernooij MW (2015) Tract-specific white matter degeneration in aging: the Rotterdam Study. Alzheimers Dement J Alzheimers Assoc 11:321–330
Hong YJ, Yoon B, Shim YS, Ahn KJ, Yang DW, Lee J-H (2015) Gray and white matter degenerations in subjective memory impairment: comparisons with normal controls and mild cognitive impairment. J Korean Med Sci 30:1652–1658
Hoy AR, Ly M, Carlsson CM, Okonkwo OC, Zetterberg H, Blennow K, Sager MA, Asthana S, Johnson SC, Alexander AL, Bendlin BB (2017) Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PLoS ONE 12:e0173982
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, Leon MJD, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de La Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 10:844–852
Jessen F, Spottke A, Boecker H, Brosseron F, Buerger K, Catak C, Fliessbach K, Franke C, Fuentes M, Heneka MT, Janowitz D, Kilimann I, Laske C, Menne F, Nestor P, Peters O, Priller J, Pross V, Ramirez A, Schneider A, Speck O, Spruth EJ, Teipel S, Vukovich R, Westerteicher C, Wiltfang J, Wolfsgruber S, Wagner M, Düzel E (2018) Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res Ther 10:15
Kiuchi K, Kitamura S, Taoka T, Yasuno F, Tanimura M, Matsuoka K, Ikawa D, Toritsuka M, Hashimoto K, Makinodan M, Kosaka J, Morikawa M, Kichikawa K, Kishimoto T (2014) Gray and white matter changes in subjective cognitive impairment, amnestic mild cognitive impairment and Alzheimer’s disease: a voxel-based analysis study. PLoS ONE 9:e104007
Kurth F, Gaser C, Luders E (2015) A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat Protoc 10:293–304
Li X-Y, Tang Z-C, Sun Y, Tian J, Liu Z-Y, Han Y (2016) White matter degeneration in subjective cognitive decline: a diffusion tensor imaging study. Oncotarget 7:54405–54414
Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, Garaci F, Toschi N, Habert M-O, Blennow K, Zetterberg H, O’Bryant SE, Johnson L, Galluzzi S, Bokde ALW, Broich K, Herholz K, Bakardjian H, Dubois B, Jessen F, Carrillo MC, Aisen PS, Hampel H (2015) Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis 48(Suppl 1):S171–S191
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 40:570–582
Müller MJ, Greverus D, Weibrich C, Dellani PR, Scheurich A, Stoeter P, Fellgiebel A (2007) Diagnostic utility of hippocampal size and mean diffusivity in amnestic MCI. Neurobiol Aging 28:398–403
Ryu SY, Lim EY, Na S, Shim YS, Cho JH, Yoon B, Hong YJ, Yang DW (2017) Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int Psychogeriatr 29:1–8
Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM (2005) Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci 1064:37–49
Selnes P, Aarsland D, Bjørnerud A, Gjerstad L, Wallin A, Hessen E, Reinvang I, Grambaite R, Auning E, Kjærvik VK, Due-Tønnessen P, Stenset V, Fladby T (2013) Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis 33:723–736
Selnes P, Fjell AM, Gjerstad L, Bjornerud A, Wallin A, Due-Tonnessen P, Grambaite R, Stenset V, Fladby T (2012) White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement J Alzheimers Assoc 8:S112–S121
Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci 7:31
Stenset V, Bjørnerud A, Fjell AM, Walhovd KB, Hofoss D, Due-Tønnessen P, Gjerstad L, Fladby T (2011) Cingulum fiber diffusivity and CSF T-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging 32:581–589
Teipel SJ, Grothe MJ, Filippi M, Fellgiebel A, Dyrba M, Frisoni GB, Meindl T, Bokde ALW, Hampel H, Klöppel S, Hauenstein K, The EDSD Study Group (2014) Fractional anisotropy changes in Alzheimer’s disease depend on the underlying fiber tract architecture: a multiparametric DTI study using joint independent component analysis. J Alzheimers Dis 41:69–83
Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein K-H, Filippi M, Ernemann U, Reiser MF, Hampel H (2010) Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis 22:507–522
Teipel SJ, Reuter S, Stieltjes B, Acosta-Cabronero J, Ernemann U, Fellgiebel A, Filippi M, Frisoni G, Hentschel F, Jessen F, Klöppel S, Meindl T, Pouwels PJW, Hauenstein K-H, Hampel H (2011) Multicenter stability of diffusion tensor imaging measures: a European clinical and physical phantom study. Psychiatry Res 194:363–371
Teipel SJ, Wegrzyn M, Meindl T, Frisoni G, Bokde ALW, Fellgiebel A, Filippi M, Hampel H, Kloppel S, Hauenstein K, Ewers M (2012) Anatomical MRI and DTI in the diagnosis of Alzheimer’s disease: a European multicenter study. J Alzheimers Dis 31(Suppl 3):S33–S47
Wang Y, West JD, Flashman LA, Wishart HA, Santulli RB, Rabin LA, Pare N, Arfanakis K, Saykin AJ (2012) Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta 1822:423–430
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265
Zhou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Stat Soc B 67(part 2):301–320
Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Stat Soc Ser B (Stat Methodol) 67:301–320
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical statement
The study has been approved by the local institutional review boards and ethics committees of the participating centers. It has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brueggen, K., Dyrba, M., Cardenas-Blanco, A. et al. Structural integrity in subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease based on multicenter diffusion tensor imaging. J Neurol 266, 2465–2474 (2019). https://doi.org/10.1007/s00415-019-09429-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09429-3